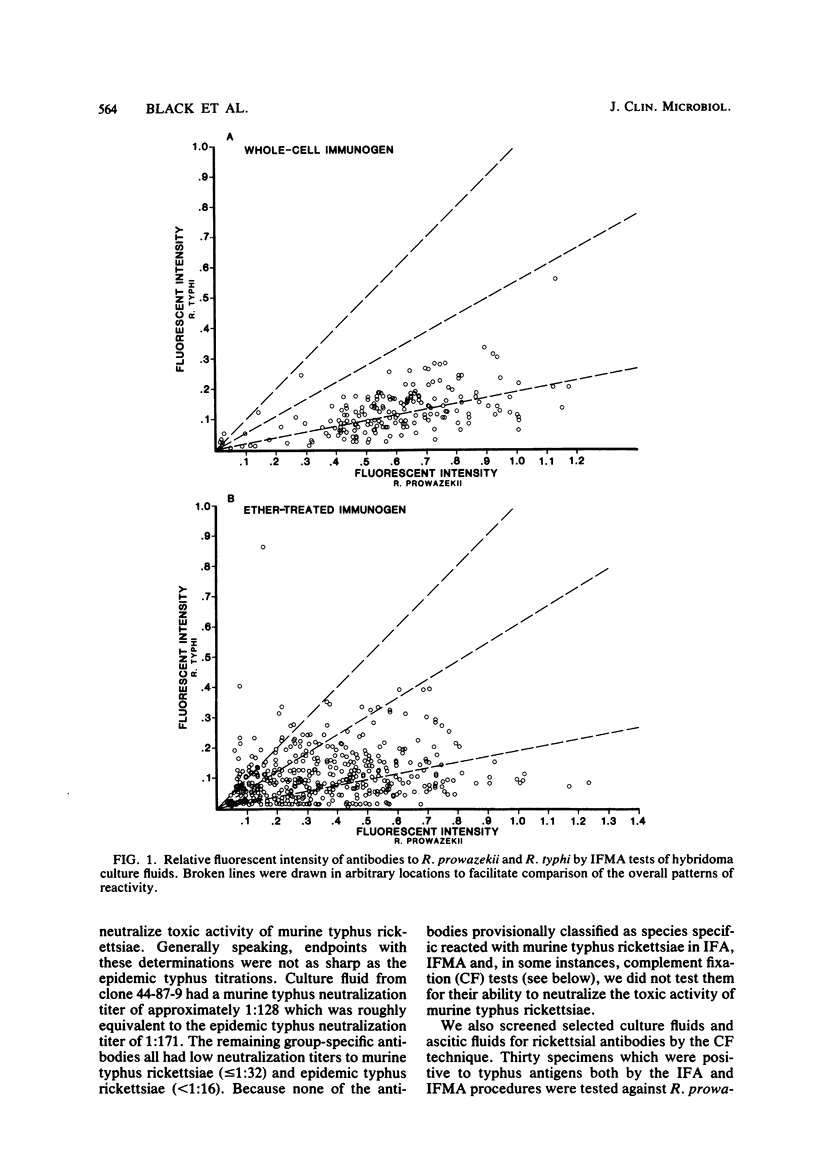
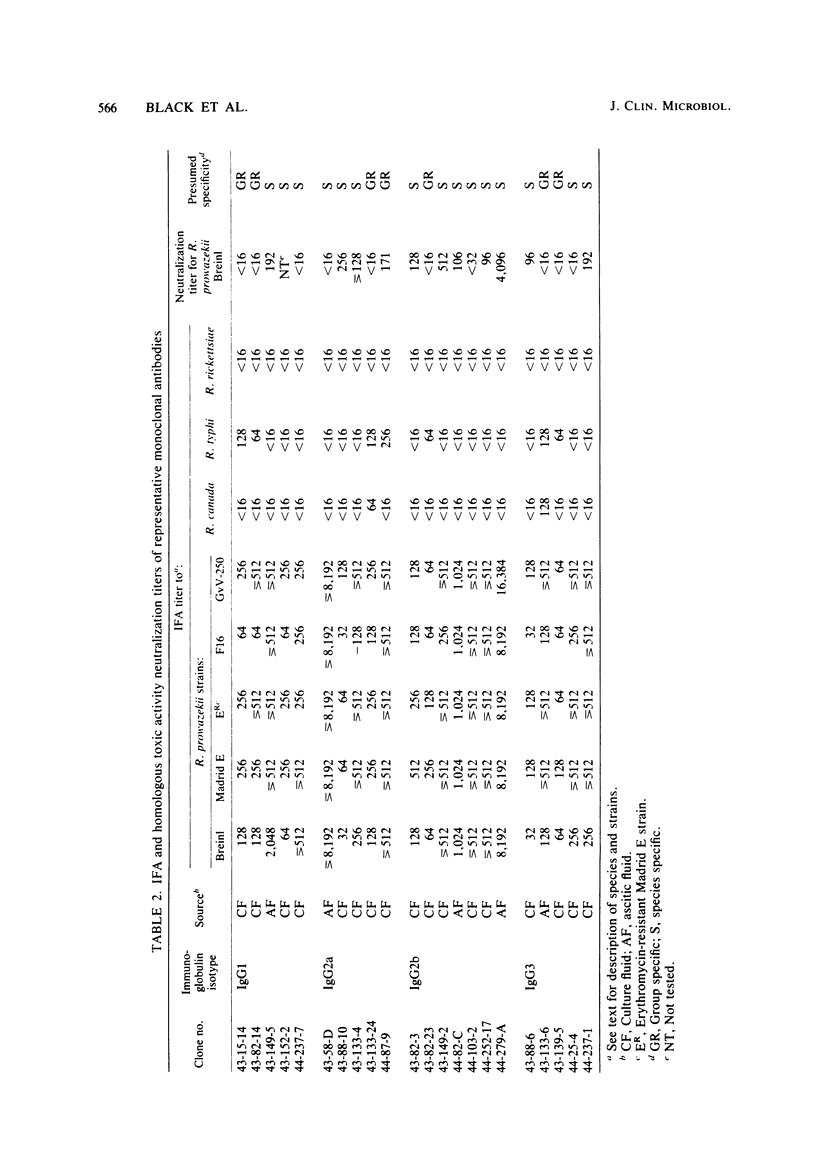
Abstract
A solid-phase immunofluorometric assay was used to detect mouse monoclonal antibodies to epidemic typhus rickettsiae, Rickettsia prowazekii (the immunizing antigen), and to murine typhus rickettsiae, Rickettsia typhi, a related antigen. Of the 649 hybridoma cultures obtained, 628 contained antibodies either to R. prowazekii or to both R. prowazekii and R. typhi. A total of 72 cultures were cloned by limiting dilution and yielded 137 antibody-producing clones. Of these, 104 produced antibodies specific for R. prowazekii, 22 produced antibodies that reacted with R. prowazekii and R. typhi, and 11 produced antibodies that reacted with R. prowazekii, R. typhi, and R. canada. The immunoglobulin isotypes of the mouse monoclonal antibodies produced were identified by a related indirect immunofluorometric assay technique with fluorescein isothiocyanate-conjugated antisera specific for each isotype. Antibodies were also evaluated by indirect fluorescent antibody tests, and antibodies from selected clones were found to neutralize rickettsial toxic activity in mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black C. M., Pine L., Reimer C. B., Benson R. F., Wells T. W. Characterization of antibody responses in legionellosis with an immunofluorometric assay. J Clin Microbiol. 1982 Jun;15(6):1077–1084. doi: 10.1128/jcm.15.6.1077-1084.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeman F. M., Masiello S. A., Williams M. S., Elisberg B. L. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975 Jun 12;255(5509):545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- Brusman H. P. Spectrophotometric determination of fluorescein-protein ratios: some theoretical and practical considerations. Anal Biochem. 1971 Dec;44(2):606–611. doi: 10.1016/0003-2697(71)90249-1. [DOI] [PubMed] [Google Scholar]
- Burgett M. W., Fairfield S. J., Monthony J. F. A solid phase fluorescent immunossay for the quantitation of the C4 component of human complement. J Immunol Methods. 1977;16(3):211–219. doi: 10.1016/0022-1759(77)90199-5. [DOI] [PubMed] [Google Scholar]
- Dasch G. A., Samms J. R., Weiss E. Biochemical characteristics of typhus group rickettsiae with special attention to the Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1978 Feb;19(2):676–685. doi: 10.1128/iai.19.2.676-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelder A. M., Ploem J. S. An immunofluorescence reaction for Schistosoma manosoni using the defined antigen substrate spheres (DASS) system. J Immunol Methods. 1974 Mar;4(2):239–251. doi: 10.1016/0022-1759(74)90067-2. [DOI] [PubMed] [Google Scholar]
- Gonchoroff N. J., Kendal A. P., Phillips D. J., Reimer C. B. Immunoglobulin M and G antibody response to type- and subtype-specific antigens after primary and secondary exposures of mice to influenza A viruses. Infect Immun. 1982 May;36(2):510–517. doi: 10.1128/iai.36.2.510-517.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C., Redus M. A., Newhouse V. F., Smith J. D. Evidence of Rickettsia prowazekii infections in the United States. Am J Trop Med Hyg. 1980 Mar;29(2):277–284. doi: 10.4269/ajtmh.1980.29.277. [DOI] [PubMed] [Google Scholar]
- Newhouse V. F., Shepard C. C., Redus M. D., Tzianabos T., McDade J. E. A comparison of the complement fixation, indirect fluorescent antibody, and microagglutination tests for the serological diagnosis of rickettsial diseases. Am J Trop Med Hyg. 1979 Mar;28(2):387–395. doi: 10.4269/ajtmh.1979.28.387. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Phillips D. J., Galland G. G., Reimer C. B., Kendal A. P. Evaluation of a solid-phase immunoassay with fluorescein isothiocyanate-conjugated heterogeneous or monoclonal antibodies for identification of virus isolates, with influenza virus as a model. J Clin Microbiol. 1982 May;15(5):931–937. doi: 10.1128/jcm.15.5.931-937.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. J., Kendal A. P., Webster R. G., Feorino P. M., Reimer C. B. Detection of monoclonal influenza antibodies synthesized in culture by hybridoma cells with a solid-phase indirect immunofluorometric assay. J Virol Methods. 1980;1(5):275–283. doi: 10.1016/0166-0934(80)90024-5. [DOI] [PubMed] [Google Scholar]
- Sundeen J. T., Krakauer R. S. A quantitative assay for low levels of IgM by solid-phase immunofluorescence. J Immunol Methods. 1979;26(3):229–244. doi: 10.1016/0022-1759(79)90248-5. [DOI] [PubMed] [Google Scholar]
- Woodman D. R., Weiss E., Dasch G. A., Bozeman F. M. Biological properties of Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1977 Jun;16(3):853–860. doi: 10.1128/iai.16.3.853-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


