Abstract
“Cluster 9” family lipoproteins function as ligand-binding subunits of ABC-type transporters in maintaining transition metal homeostasis and have been implicated in the virulence of several bacteria. While these proteins share high similarity, the specific metal that they recognize and whether their role in virulence directly involves metal homeostasis cannot be reliably predicted. We examined the cluster 9 protein Lsp of Streptococcus pyogenes and found that specific deletion of lsp produced mutants highly attenuated in a murine model of soft tissue infection. Under standard in vitro conditions, growth of the Lsp− mutant was indistinguishable from that of the wild type, but growth was defective under zinc-limited conditions. The growth defect could be complemented by plasmids expressing wild-type Lsp but not Lsp engineered to lack its putative lipidation residue. Furthermore, Zn2+ but not Mn2+ rescued Lsp− growth, implicating Zn2+ as the physiological ligand for Lsp. Mutation of residues in the putative Zn2+-binding pocket generated variants both hypo- and hyperresistant to zinc starvation, and both mutant classes displayed attenuated virulence. Together, these data suggest that Lsp is a ligand-binding component of an ABC-type zinc permease and that perturbation of zinc homeostasis inhibits the ability of S. pyogenes to cause disease in a zinc-limited host milieu.
Transition metals, including iron, zinc, manganese, nickel, and copper, participate in many structural and catalytic functions that are necessary to support the pathogenesis of bacterial infections. However, the ease with which these metals promote cellular electron trafficking also gives them potential to engage in destructive metal-based reactions (25, 59). Thus, a successful pathogen must evolve mechanisms to control the availability and distribution of individual metals within the bacterial cell while exposed to a dynamic host environment with profoundly fluctuating transition metal abundance (13, 15, 41, 62).
Evidence is emerging to suggest that the mechanisms by which Streptococcus pyogenes (group A streptococcus) interacts with transition metals play an important role in its ability to cause disease. This gram-positive bacterium is the causative agent of numerous diseases of soft tissue, ranging from self-limiting (e.g., pharyngitis) to destructive and life-threatening (e.g., necrotizing fasciitis), as well as serious postinfectious sequelae such as rheumatic fever and acute glomerulonephritis. A number of metal transporters of S. pyogenes have been characterized, and several of these have been shown to play roles in the virulence of streptococcal infection in various animal models (3, 4, 40, 41, 57, 70). However, the full repertoire of metal transporters and regulatory elements that comprise the metalloregulatory network of S. pyogenes has not been characterized. In particular, the import and export proteins specific for each of the important transition metals have not been identified. Consequently, global understanding of S. pyogenes transition metal metabolism is not well developed, especially for the situation when S. pyogenes is exposed to and influenced by host defenses. This is particularly true for zinc.
Zinc is the second-most-abundant transition metal in humans, and it serves as a structural element or cofactor for numerous surface proteins, enzymes, and regulatory proteins (22, 76). Since high concentrations of zinc can be toxic, most bacterial species maintain zinc homeostasis using transcriptional regulatory controls to balance the expression of genes encoding both zinc import and zinc export proteins. The result is that the intracellular zinc concentration is maintained in a narrow range. For example, analysis of Escherichia coli has shown that the intracellular concentration of zinc varies only over a femtomolar range (25, 62). The S. pyogenes genome lacks the transcription regulator Zur, which plays an important role in regulation of zinc homeostasis in other gram-positive pathogens (63). However, the S. pyogenes genome does encode PerR, which along with Zur is a member of the Fur family of metal-binding regulators (38, 70). Acting primarily as a repressor, PerR regulates expression of genes involved in oxidative stress in S. pyogenes and other gram-positive species (10, 32, 38, 44, 70, 81). Deletion mutants that lack PerR are highly attenuated in models of soft tissue infection, despite their hyperresistance to oxidative stress (6, 70). Transcription profiling of the HSC5 strain in mid-logarithmic phase has revealed that deletion of perR results in highly altered levels of expression of six genes (8). In HSC5, only the promoter of pmtA, which encodes a putative P-type zinc efflux pump, contained a binding site for PerR (8). Of the remainder, four genes were only indirectly regulated by PerR but were under the direct control of the repressor AdcR (8). This regulator was originally identified as a repressor of the Adc operon, which encodes the components of an ABC-type metal permease that functions in the importation of zinc (8, 63). Another recent study using a different strain of S. pyogenes reported five genes with greater than threefold differences in transcription at the comparable growth phase, including pmtA and two from the AdcR operon, rpsN.2 and adcA (32). Taken together, these data suggest that there is overlap between how S. pyogenes maintains zinc homeostasis and how it adapts to oxidative stress. However, since none of the proteins encoded by the four AdcR-regulated genes were found to contribute to resistance to oxidative stress (8), the significance of this relationship is not clear.
An additional complication is that the functions of the polypeptides encoded by several of the AdcR-regulated genes are not known. For example, the protein Lsp (23) was originally identified on the basis of its homology to a laminin-binding adhesin of Streptococcus agalactiae (79). Further, an Lsp mutant was found to have defective ability to adhere to epithelial cells (23). It was also recognized that Lsp had a high degree of similarity to PsaA of Streptococcus pneumoniae, a member of the cluster 9 family of ligand-binding components of the ATP-binding cassette (ABC-type) transporter superfamily (16, 21). Members of the cluster 9 family are involved in metal transport, most commonly with Zn2+ and Mn2+ (2, 16, 34, 35, 48, 67). While PsaA is a protective antigen of S. pneumoniae (9, 77) and PsaA-deficient mutants also show an adherence defect, it appears that adherence is affected indirectly via a reduction in the ability of the mutants to transport Mn2+ (43, 56, 71). Thus, PsaA may make its principal contribution to virulence as a component of an ABC-type Mn2+ transporter. The similarity between PsaA and Lsp suggests that Lsp also may be involved in Mn2+ transport. However, the heterogeneity in binding pocket architecture that has been revealed by structural analyses of multiple cluster 9 polypeptides has shown that binding site homology alone does not prove to be a reliable predictor of which metal(s) can be bound by any particular family member, especially in the setting of physiological metal concentrations (2, 35, 53).
Our previous studies using a murine soft tissue infection model have shown that lsp is upregulated several hundredfold during infection compared with culture under standard in vitro conditions (8). Thus, further characterization of the function of Lsp has the potential to provide significant insight into streptococcal pathogenesis and especially into the nature of the tissue environment to which S. pyogenes must rapidly adapt. In the present study, we probed the function of Lsp through the construction of both a deletion mutant and mutants that express Lsp polypeptides that were altered in specific functional residues. The latter included those which contributed to the architecture of its putative metal-binding site. These mutants were then characterized to test: (i) the role of Lsp in Zn2+ and Mn2+ homeostasis, (ii) whether Lsp contributes to the ability of S. pyogenes to cause disease in soft tissue, and (iii) whether the role of Lsp in virulence is specifically linked to a role in metal homeostasis.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Molecular cloning experiments routinely utilized E. coli TOP10 (Invitrogen, Carlsbad, CA). The construction of an Lsp− mutant (AB103) containing an in-frame deletion of lsp from the chromosome of wild-type strain HSC5 (33) was described in a previous study (8). Other mutant derivatives of HSC5 were constructed as described below. Unless otherwise indicated, S. pyogenes was cultured in Todd-Hewitt broth (BBL) supplemented with 0.2% yeast extract (Difco) (THY medium) under conditions described previously (80). To produce solid medium, Bacto agar (Difco) was added to THY medium to a final concentration of 1.4%, and all solid cultures were incubated under anaerobic conditions using a commercial gas generator (GasPak; BBL catalog number 260678). Culture for infection of mice was conducted as described previously (7). Strains of E. coli were cultured in Luria-Bertani broth (75). Where appropriate, antibiotics were used to supplement media at the following concentrations: kanamycin, 50 μg ml−1 for E. coli; erythromycin, 750 μg ml−1 for E. coli and 1 μg ml−1 for S. pyogenes, and chloramphenicol, 3 μg ml−1 for S. pyogenes.
DNA techniques.
Plasmid DNA was isolated by standard methods and used to transform E. coli by a standard method (47). Transformation of S. pyogenes was performed by electroporation as previously described (12). Restriction endonucleases, ligases, kinases, and polymerases were used according to the manufacturer's recommendations. Chromosomal DNA was purified from S. pyogenes as described previously (12). Rapid PCR amplification of streptococcal DNA from crude lysates was performed using a standard method (39). The site-directed mutations described below were generated by PCR using a commercial kit (QuikChange; Stratagene) following the protocols recommended by the manufacturer. The fidelity of all plasmid constructs was confirmed by DNA sequencing, which was performed by a commercial service (SeqWright, Houston, TX).
Construction of mutants.
For consistency with our prior publications, unless otherwise indicated, all references to genomic loci and the sequences presented are derived from the genome of S. pyogenes SF370 (24). Sequence analysis revealed that lsp in HSC5 encodes a predicted protein that is identical to that encoded by lsp in the reference genome. Construction of the in-frame deletion mutant (AB103) of lsp (SPy_2007) has been described previously (8, 72). Additional mutants that expressed Lsp polypeptides altered by the replacement of selected histidine residues by alanines were constructed. The mutant lsp alleles were constructed using the primers listed in Table 1 and the method described in detail below and elsewhere (54). The resulting alleles were then used to replace the chromosomally encoded lsp by the method of Ji et al. (42). The correct chromosomal sequence for all mutants was confirmed by DNA sequence analysis of PCR products generated using the appropriate primers (Table 1). The resulting strains are BFW100 (HSC5 lspH66A) and BFW101 (HSC5 lspH142A).
TABLE 1.
Primers used in this study
| Primer | Sequencea | Descriptionb |
|---|---|---|
| BFW050 | CCGGAATTCCGGCGAAATTAGAAAAGAGGACAAGCATATGAAAAAAGGTTTTTTTCTCATGGCTATGGTCGTGAGT | Reverse amplification primer for lsp |
| BFW052 | CCGCTGCAGTTACTTCAACTGTTGATAGAGCACTTCCAAATTTGCTCTAAGATTTTCTAGATATGTCTTGTTTCCG | Forward amplification primer for lsp |
| BFW053 | GTGAGTTTAGTAATGATAGCAGGGGCTGATAAGTCAGCAAACCCCAAACAG | Reverse deletion primer for lspC20A |
| BFW054 | CTGTTTGGGGTTTGCTGACTTATCAGCCCCTGCTATCATTACTAAACTCAC | Forward deletion primer for lspC20A |
| BFW055 | GATGATCCAATCAGGTGCAGGCATTGCTTCCTTTGAACCGTCTGTAAATGATG | Reverse deletion primer for lspH66A |
| BFW056 | CATCATTTACAGACGGTTCAAACCAAGCAATGCCTGCACCTGATTGGATCATC | Forward deletion primer for lspH66A |
| BFW057 | TGATCCTGCGACACTTTATGACCCAGCTACCTGGACAGATCCCGTTTTAGCTG | Reverse deletion primer for lspH142A |
| BFW058 | CAGCTAAAACGGGATCTGTCCAGGTAGCTGGGTCATAAAGTGTCGCAGGATCA | Forward deletion primer for lspC20A |
| BFW068 | GCGCGGTACCAACTAAACCAGAAAAAGATAGTTC | Reverse amplification primer paired with BFW052 for pJRS233 cloning of lspH66A |
| BFW069 | GCGCGGTACCAGTTGCCATTGACAAAGCATTGAT | Reverse amplification primer paired with BFW052 for pJRS233 cloning of lspH142A |
Restriction sites engineered for cloning purposes are underlined.
Primers are described according to their purposes as follows: “amplification” primers amplify the region containing the gene of interest for construction of mutant S. pyogenes strains, and “deletion” primers were used to amplify genes of interest for cloning into a streptococcal expression vector as described in Materials and Methods.
Plasmids for expression of lsp derivatives.
A plasmid for expression of lsp was constructed as follows. Primers BFW050 and BFW052 (Table 1) were used to amplify a fragment from HSC5 chromosomal DNA that contained lsp along with a streptococcal ribosome-binding site and start codon derived from the gene encoding mitogenic factor (28). The fragment was digested with EcoRI and PstI and inserted between the compatible EcoRI and PstI sites of pABG5, a derivative of the E. coli-streptococcal shuttle vector pLZ12 (31). As a result, lsp is placed under the control of the well-characterized rofA promoter (31). The DNA sequence of the resulting plasmid, pLsp, was confirmed and was then used as a template for site-specific mutagenesis with the primers described in Table 1 and the method described above to change selected codons to encode alanine residues. These included C20A, H66A, and H142A, and the resulting plasmids (pLspC20A, pLspH66A, pLspH142A, respectively) were used to transform HSC5 or AB103 as described below.
Metal chelation growth yield assays.
The abilities of various S. pyogenes strains to adapt to environments restricted for zinc were quantitatively compared by a growth yield method that was derived from a metal sensitivity assay described previously (27, 58). Standard THY medium was supplemented with the zinc-specific chelating agent N,N,N′,N′-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (Sigma) over a range of concentrations as noted below. Various strains of S. pyogenes cultured overnight in unsupplemented THY medium were used to inoculate 3 ml of TPEN-containing medium contained in 15-ml polyethylene conical tubes at a final ratio of 0.1% (vol/vol). The tube caps were then tightly sealed and the cultures incubated overnight at 37°C. Growth yield was then determined by measurement of optical density at 600 nm and reported relative to that of the wild-type reference strain grown in unsupplemented medium. In order to ensure that relative growth yields were not influenced by any zinc-independent effects of plasmid maintenance, the plasmid vector pABG5 was introduced into wild-type and Lsp− mutant strains and culture of all strains was conducted in the presence of chloramphenicol. The specificity of the effect of treatment on growth yield was assessed at selected concentrations of TPEN by the addition of equimolar concentrations of either ZnCl2 or MnCl2. Unless otherwise indicated, data presented represent the mean value and standard error of the mean derived from at least two independent experiments.
Murine subcutaneous ulcer model.
The method of Bunce et al. (11) as modified by others (52, 74) was used to assess the ability of various S. pyogenes strains to cause disease in soft tissue as described in detail elsewhere (7). Data presented are pooled from two independent experiments, in which each experimental group consisted of five SKH1 hairless mice (Charles River Labs) between 5 and 8 weeks of age.
Statistical analyses.
The difference between the numbers of mice developing an ulcer following subcutaneous challenge with the wild-type and mutant strains was tested for significance by the chi-square test with Yates' correction (30), and differences in the areas of the resulting ulcers were tested by the Mann-Whitney U test (30). Differences in growth yields of various strains under conditions of zinc limitation were tested for significance with the chi-square test as described above. For all test statistics, the null hypothesis was rejected when the P value was <0.05.
RESULTS
An Lsp deletion mutant is highly attenuated.
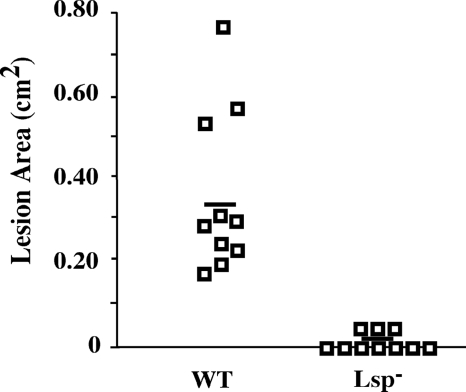
We previously reported that lsp and the other AdcR regulon genes are profoundly derepressed during infection of soft tissue by a wild-type strain in the murine subcutaneous ulcer model of S. pyogenes infection (8). In order to assess the overall contribution of lsp to virulence, the ability of an Lsp-deficient mutant to cause disease in the murine model was assessed. Because lsp (SPy_2007) is cotranscribed with an additional member of the Adc regulon (phtD, SPy_2006), we utilized a previously characterized mutant constructed to contain an in-frame deletion of the lsp open reading frame (7). No differences in growth rate and yield were noted for in vitro growth in several different standard culture media (8). For the wild-type strain used in this study (HSC5), injection into the subcutaneous tissue of an SKH1 hairless mouse results in a local draining ulcer that attains a maximum size at 3 days postinfection. Thereafter, the ulcer heals during the ensuing 10 to 14 days (7). It was found that the mutant was significantly attenuated in its ability to cause lesions (Fig. 1). When examined at the time of maximal lesion size caused by the wild type (3 days), only 30% of the mice infected with the mutant strain had developed a visible lesion, compared with 100% of the mice infected by the wild type (P = 0.005). These results did not change over the duration of the experiment (5 days), and for those few mice that did develop lesions, these lesions were significantly smaller than those produced by the wild-type strain (P < 0.001). These data indicate that upregulation of lsp expression during infection correlates with the virulence of S. pyogenes to cause disease in this soft tissue infection model.
FIG. 1.
Lsp− mutants are attenuated. The virulence of a strain lacking Lsp (Lsp−) was compared with that of its wild-type parent (WT) in the murine model of subcutaneous infection. The data shown are pooled from two independent experiments. Each symbol represents the area of ulcer observed in an individual mouse, and the horizontal bar indicates the mean value obtained for the set of 10 mice. Symbols that bisect the x axis represent animals that failed to develop any discernible lesions. The Lsp− mutant was significantly less virulent than the wild type compared both on the basis of lesion area (P = 0.005) and in terms of the number of animals that developed lesions (P < 0.001).
Lsp− mutants are more sensitive to zinc starvation.
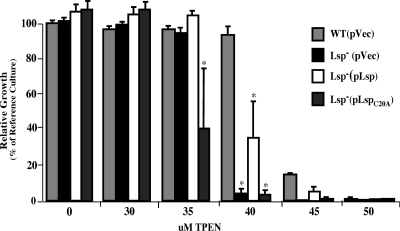
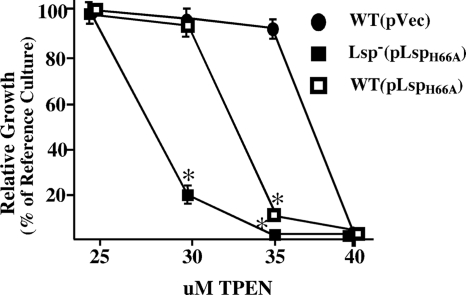
In considering the mechanism by which Lsp is required for virulence, the similarity between PsaA and Lsp suggests that the latter may also be a member of the cluster 9 family of the solute-binding components of an ABC transporter. Because this family is associated with transporting transition metals (16, 21) and since lsp is regulated by the putative zinc-responsive regulator AdcR (63), it was of interest to determine if Lsp contributed to biological zinc homeostasis. We adapted an assay that uses titration of the zinc-specific chelator TPEN in growth media (27, 58) to compare the abilities of mutant and wild-type strains to grow under conditions of zinc starvation. Growth of the wild type and the Lsp− mutant is similar when zinc is plentiful. One strength of the assay used for testing growth yield under conditions of zinc starvation is the ability to selectively chelate zinc in otherwise standard media. On the other hand, slight differences in zinc concentration exist between different preparations of media (within a 5 μM range), which is reflected in the figures, though the relationships are consistent. This analysis revealed that when measured over a range of TPEN concentrations, the Lsp− mutant demonstrated a hypersensitivity to zinc starvation compared to the wild-type strain [compare WT(pVec) to Lsp−(pVec) in Fig. 2]. This defect was associated with the loss of Lsp, since complementation with a plasmid-encoded copy of the wild-type lsp significantly improved the mutant's ability to grow in zinc-depleted medium [Lsp−(pLsp) in Fig. 2]. These data implicate Lsp as an important component of the Zn2+ homeostasis network of S. pyogenes.
FIG. 2.
Lsp mutants are sensitive to zinc starvation. Growth of the wild type (WT) was compared to that of the Lsp deletion mutant (Lsp−) as well as to the Lsp− strain complemented with a plasmid expressing either wild-type Lsp (pLsp) or Lsp modified to lack its putative lipidation residue (pLspC20A). Zinc starvation was imposed by the addition of increasing concentrations of TPEN as shown, and growth is presented relative to that of the wild type without vector cultured in the absence of TPEN or antibiotic (reference culture). To control for any zinc-independent effects on growth caused by maintenance of a plasmid, empty vector plasmid pABG5 (pVec) was introduced into the WT and Lsp− strains and all cultures conducted in medium supplemented with chloramphenicol. The data reported represent the means and standard errors of the means derived from at least two independent experiments. Asterisks indicate P values of <0.05.
A predicted lipidation residue is essential for Lsp function.
In gram-positive bacteria, the cluster 9 solute-binding proteins are anchored to the membrane as lipoproteins, presumably supporting their interaction with other components of the transporter complex. Consistent with this, a computational analysis predicted that Lsp possesses a canonical signal peptidase II-dependent lipoprotein signal sequence, with a predicted lipidated cysteine at residue 20. In order to test whether the ability of Lsp to support growth under zinc-restricted conditions is consistent with the behavior of a lipoprotein, site-directed mutagenesis was used to substitute an alanine residue at position 20 for the cysteine residue encoded on a plasmid-carried copy of lsp. The resulting plasmid (pLspC20A) was then introduced into the Lsp deletion strain for comparison of its growth phenotype to that of the wild type in the TPEN titration assay. The growth profile of the strain expressing this mutant Lsp allele was more sensitive to zinc starvation than both the wild-type and the complementation strains (Fig. 2). Thus, LspC20A was defective in its ability to support growth in medium in which zinc concentrations are limiting. These data implicate Lsp as a lipoprotein whose location at the membrane is important to support its role in zinc homeostasis.
Zinc, but not manganese, rescues growth of an Lsp− mutant in zinc-poor conditions.
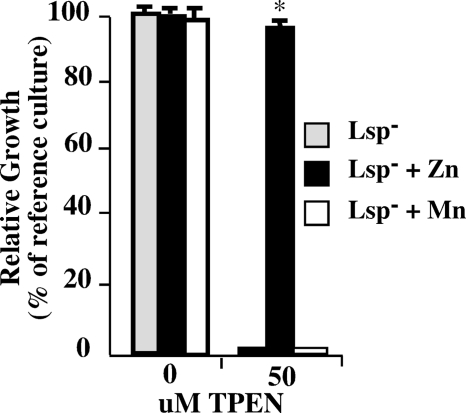
Similarities of binding site architecture make it difficult to predict the specific metals bound by cluster 9 proteins (2). As an example, PsaA was crystallized with what appears to be Zn2+ in its binding pocket; however, the growth of PsaA mutants under conditions of metal limitation can be rescued only by Mn2+ and not by Zn2+ (56). In the data presented above, the loss of Lsp was associated with a reduced ability to grow under conditions of zinc starvation. However, the limited ability of TPEN to chelate some Mn2+ may have induced manganese stress on Lsp− mutants. To examine this possibility, either 50 μM Zn2+ or Mn2+ was added to medium containing TPEN and the growth yield of the Lsp− mutant was compared with that in untreated medium. Consistent with the data presented above, the mutant was able to grow normally in medium that did not contain TPEN but was unable to grow in TPEN-treated medium (Fig. 3). In contrast, the addition of Zn2+ supported the growth of the mutant in medium containing 50 μM TPEN to produce a final yield that was indistinguishable from growth in untreated medium (Fig. 3). Growth could be rescued only by the addition of Zn2+ and supplementation, of TPEN-treated medium with Mn2+ failed to support growth (Fig. 3), even when examined over a broad range of Mn2+ and TPEN concentrations (data not shown). Thus, while it cannot be excluded that Lsp interacts biologically with both Mn2+ and Zn2+, these data do establish an important role for Lsp in zinc homeostasis in TPEN-treated medium.
FIG. 3.
Zinc rescues the Lsp− phenotype. Growth of the Lsp− mutant in unmodified and TPEN-treated media was compared with that in media supplemented with equimolar zinc and manganese, as indicated. Growth is presented relative to that of the mutant in unmodified medium (reference culture). For consistency with other experiments, empty plasmid vector pABG5 was introduced into the Lsp− mutant and all cultures were conducted in media supplemented with chloramphenicol. Data reported represent the means and standard errors of the means derived from at least two independent experiments. The asterisk indicates a P value of <0.05.
Mutations of residues in a predicted metal-binding site of Lsp alter growth in zinc-restricted media.
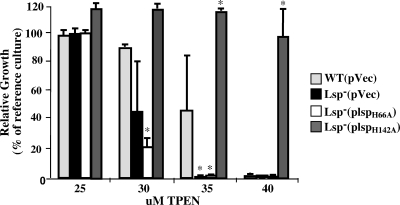
Similar to the case for PsaA− mutants of S. pneumoniae (5, 71), Lsp− mutants of S. pyogenes have a defect in their ability to adhere to cultured host cells (23). However, subsequent analyses of PsaA− mutants have suggested that the adherence defect is an indirect result of an imbalance in metal homeostasis promoted by the loss of PsaA (43). Thus, it was of interest to determine whether the contribution of Lsp to virulence is associated with its role in promoting metal homeostasis. The strategy was to construct mutant Lsp alleles altered in their abilities to promote growth under conditions of zinc limitation for subsequent evaluation in models of virulence. The T-COFFEE method (60, 66) was used to compare the Lsp amino acid sequence to those of several other cluster 9 family members for which three-dimensional structures have been determined, including PsaA (48) and AdcAII (53) from Streptococcus pneumoniae, TroA from Treponema pallidum (49, 50), and ZnuA from Synechocystis sp. strain 6903 (2). The alignment highlighted several conserved histidine residues involved in the Zn2+ coordination complex in the binding pockets of these proteins that are also conserved in Lsp (see Fig. S1 in the supplemental material). Two of these (H66 and H142) were targeted for site-specific substitution with an alanine residue, and the resulting mutant alleles were expressed from a plasmid in the Lsp− deletion strain. Analyses of the resulting mutants revealed that both produced strong phenotypes with respect to growth under conditions of zinc limitation. The H66A mutant manifested a growth yield phenotype under zinc starvation conditions that is indistinguishable from the defective phenotype observed with the Lsp− deletion strain (Fig. 4). In addition, introduction of the H66A expression plasmid into a wild-type background had a dominant-negative effect, as expression of H66A in a wild-type background consistently reduced the ability of the resulting strain to adapt to zinc starvation compared to the wild-type strain with the empty vector alone (Fig. 5). A dominant-negative effect is consistent with the behavior expected when expression of a nonfunctional polypeptide competes with the wild-type protein for assembly into a multisubunit transporter, in this case an ABC-type transporter. A markedly different effect resulted from the H142A substitution. Expression of this protein in the Lsp− mutant results in a strain that is hyperresistant to zinc limitation, as shown by the observation that the growth of the H142A mutant is virtually unaltered at TPEN concentrations that block essentially any growth by the wild-type strain (Fig. 4). It should be noted that the reference culture for Fig. 4 is the wild type without added vector or antibiotics and that the other strains are given for comparison, indicating the effect of the vector/antibiotic system upon overall growth. Wild-type and Lsp− strains grown in identical standard medium preparations do not show significant differences in growth, with the differences being observed only as zinc becomes scarce. Taken together, these data show that manipulation of Lsp residues implicated in binding metal produces mutants with altered abilities to grow under conditions of zinc limitation.
FIG. 4.
Hypo- and hyperresistance to zinc starvation results from mutation of binding site residues. The importance of several histidine residues of the predicted Lsp metal-binding site was evaluated by alanine substitution mutagenesis. Plasmids expressing the altered alleles (pLspH66A and pLspH142A) were introduced into the Lsp− mutant and their ability to promote growth following the addition of the indicated concentrations of TPEN then compared to growth of the wild-type (WT) or Lsp− strain, both of which contained the empty pABG5 vector (pVec). Culture for all assays was conducted in medium supplemented with chloramphenicol. Growth is presented relative to that of WT(pVec) grown in the absence of TPEN and antibiotic (reference culture). The data reported represent the means and standard errors of the means derived from at least two independent experiments. Asterisks represent P values of <0.05.
FIG. 5.
The LspH66A mutation exerts a dominant effect upon the wild-type strain. A plasmid expressing LspH66A was introduced into the wild-type (WT) and Lsp− strains and their abilities to grow in the presence of a range of TNEN concentrations compared with that of the WT strain containing the pABG5 vector (pVec). All cultures were conducted in medium supplemented with chloramphenicol. Growth is presented relative to WT(Vec) in the absence of TPEN and antibiotic (reference culture). The data shown are the means and standard deviations derived from a single experiment conducted in triplicate that is representative of at least two independent experiments conducted on different days. Asterisks indicate P values of <0.05.
Lsp mutants with altered metal phenotypes are attenuated in virulence.
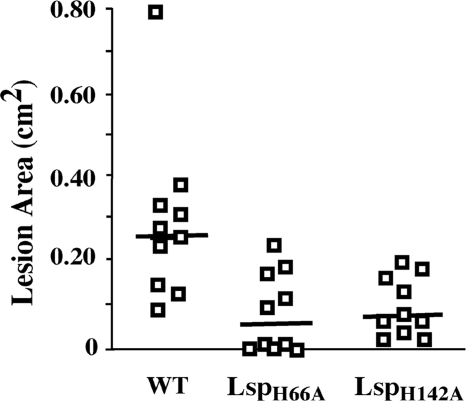
The availability of Lsp mutants with altered metal phenotypes allowed an assessment as to whether the effect of Lsp upon zinc homeostasis was also associated with an ability to support virulence in soft tissue infection. Each of the H66A and H142A lsp alleles were used to replace the chromosomally carried wild-type allele to construct strains whose virulence properties could be analyzed in the murine subcutaneous ulcer model in the absence of antibiotic selection. It was found that the H66A mutant, which has a reduced ability to promote growth under conditions of zinc starvation, was also highly attenuated in its ability to cause disease in cutaneous tissue. When examined at 3 days postinfection, fewer mice infected with the H66A mutant developed lesions than those infected with the wild type (P < 0.05), and for those mice in which lesions developed, these were significantly smaller (P = 0.005) (Fig. 6). Interestingly, the H142A mutant, which was relatively hyperresistant to zinc starvation, was also attenuated for soft tissue infection. Unlike the case for the H66A mutant, the number of mice that developed lesions following infection by the H142A mutant did not significantly differ from that for mice infected by the wild type (Fig. 6). However, the lesions produced by the H142A mutant were significantly smaller than those generated by the wild type (P = 0.0029) (Fig. 6). These data show that several site-specific modifications to Lsp that alter its function in zinc homeostasis are associated with an attenuated ability to cause disease in the murine model of soft tissue infection. Furthermore, they suggest that the primary contribution of Lsp to virulence is to promote zinc homeostasis in a zinc-restricted infectious milieu.
FIG. 6.
Both hypo- and hyperresistant Lsp− mutants are attenuated. The lspH66A and lspH142A alleles, hypo- and hyperresistant to zinc starvation, respectively, were used to replace the chromosomal copy of lsp in the wild-type strain (WT). The virulence of the resulting mutants (LspH66A and LspH142A) was then compared with that of the wild type in the murine model of subcutaneous infection. The data shown are from two independent experiments. Each symbol represents the area of ulcer observed in an individual mouse, and the horizontal bar indicates the mean value obtained for the set of 10 mice. The mutants were both significantly less virulent than the wild type compared on the basis of lesion area (P = 0.005 for LspH66A; P = 0.003 for LspH142A).
DISCUSSION
For most pathogens, metal homeostasis is accomplished through the action of a network of metal-sensing regulators of gene transcription that continually adjust the expression of genes encoding metal transporters. The transporters differ with respect to the species of metal transported, whether the metal is imported or exported, and the affinity at which the metal is recognized (14, 25, 29, 34, 64). In addition, pathogens often use the metal regulatory network to exploit differences in metal availability as a cue to distinguish between distinct host compartments for regulation of virulence gene expression (17, 26, 55). Thus, identification and characterization of the various transporters, metal-sensitive regulators, and other components of a pathogen's metal regulatory network provide important insight into how that pathogen interacts with specific host tissues during the infection process.
In this study, we offer evidence that zinc homeostasis is critical for the establishment of a successful infection by S. pyogenes in the murine skin infection model and is mediated at least in part by the lipoprotein Lsp, which likely functions as the substrate-binding subunit of an ABC-type transporter. Our data suggest that the major contribution of Lsp to virulence lies in its ability to function in zinc homeostasis, particularly under conditions in which the availability of zinc is limiting. Consistent with previous data showing that lsp is highly upregulated during infection of soft tissue in the murine ulcer model of streptococcal infection (8), our observation that Lsp− mutants are attenuated in this model suggests that this host compartment becomes restricted for zinc over the course of infection.
The conclusion that the principal role for Lsp in virulence is to promote zinc homeostasis is supported by our observation that mutation of each of two histidine residues predicted to be involved in binding zinc altered how Lsp promoted growth under zinc-limiting conditions in vitro and resulted in an attenuated ability to produce disease in soft tissue. It has been previously reported that an Lsp-null mutant had a reduced ability to adhere to epithelial cells, suggesting that Lsp may act directly as an adhesin to recognize a host cell receptor (23). It was also noted that the Lsp− mutant had reduced expression of several virulence factors, including PrtFII, a fibronectin-binding adhesin (23). Thus, in addition to an alteration in zinc homeostasis, several other factors may have contributed to the attenuation observed in the present study, including the loss of an adhesive function promoted by Lsp itself and/or an indirect effect on expression of other virulence factors. Similar questions have been raised in regard to the contributions of other members of the cluster 9 family of ligand-binding lipoproteins to virulence (43). In this regard, it is interesting to note that the S. pyogenes mutant expressing LspH142A is attenuated despite the fact that it is hyperresistant to zinc starvation. Since this mutant expresses a functioning protein, it is unlikely that attenuation could be the result of the loss of an adhesive function conferred by the direct recognition of Lsp by a host cell receptor, although the possibility of other zinc-independent effects for this mutant still exists. Similarly, because the LspH142A mutant can scavenge zinc under conditions that restrict growth of the wild-type strain, it is unlikely that attenuation resulted from a disruption of gene regulation caused by low intracellular levels of zinc. Rather, attenuation likely results from disruption of fine-tuned control over metal homeostasis. Studies of Escherichia coli have shown that bacterial cells accumulate zinc to concentrations in the millimolar range yet tolerate changes in the intracellular concentration of free zinc over only a femtomolar range (25, 62). The observation that mutations in Lsp that render S. pyogenes hypo- and hyperresistant to zinc limitation both result in attenuation of virulence suggests that this bacterium cannot tolerate disruption of metal homeostasis while growing in the metal-limited milieu of host tissue. A similar mechanism may underlie the observation that S. pyogenes mutants defective in expression of PerR, a transcription repressor involved in the adaptive response to oxidative stress, are attenuated despite the fact that they are hyperresistant to peroxide stress (6). Their attenuation is apparently the result of the dysregulation of zinc homeostasis caused by the derepression of pmtA, which encodes a membrane protein that functions to export zinc from the bacterial cell (6).
The mechanism by which the cluster 9 family of ABC-type permeases acquire and transport metal is not well understood, despite considerable study and the availability of several three-dimensional structures (2, 48-51, 53, 65). Thus, an interesting question concerns the molecular basis for the ability of LspH142A to promote hyperresistance to zinc starvation. The process of metal uptake requires that several steps be coordinated, including specific recognition of the metal ion by the ligand-binding protein, the docking of the ligand-binding protein with a transmembrane transporter complex, the release and transfer of the metal ion to the membrane complex, and the ATP-dependent transport of the metal ion across the membrane (18, 19, 36, 37). While understanding of the detailed mechanism of these steps is gradually growing, there is a particular lack of mutational analyses directed at understanding how the ligand-binding proteins of the cluster 9 family recognize and release their metal ion substrates. However, analyses of the available three-dimensional structures have revealed that these ligand-binding components adopt a general “C-clamp” configuration in which a molecule of zinc is coordinately bound by three histidine residues in the cleft between the two arms of the clamp (2). Comparison of metal-bound and apo forms of ZnuA indicate that release of zinc is accompanied by a rotation of two histidine residues out of the metal-binding pocket, while the third histidine residue remains in place (82). Since zinc can bind relatively well to two histidines, it is suggested that this rotation is required to facilitate zinc release (82). Interestingly, for the hyperresistant LspH142A mutant, H142 corresponds to the nonrotating residue of ZnuA. Since it seems unlikely that the loss of any residue that is involved in coordinating the bound zinc ion would result in a higher affinity of initial binding, the hyperresistance phenotype of the LspH142A mutant may be due to an enhanced rate of release under the in vitro conditions employed for analysis. It is also interesting to note that the analysis of structural data has not yet revealed any clear molecular basis for the specificity of metal binding. This is illustrated in the cases of PsaA and the recently described cluster 9 family member AdcAII of S. pneumoniae (53). Both of these proteins crystallized bound to zinc (48, 53, 65). However, phenotypically, PsaA has been shown to contribute to transport of manganese rather than zinc (56). While Lsp is structurally related to PsaA (see Fig. S1 in the supplemental material), the phenotypic data for Lsp presented in this study support zinc as a biological ligand. The totality of the evidence suggests zinc as the primary physiological ligand for Lsp, though interaction with other metals is still in question. The physiological ligand of AcdAII has not yet been determined.
The genomic locus encoding PsaA is typical of most ABC-type transporters and includes the genes encoding the ATP-binding protein and the integral membrane subunit (43). However, the locus encoding Lsp is unusual, in that it is located in an operon along with a gene encoding a surface protein of the histidine triad family (phtD, SPy_2006). The histidine triad proteins were first identified in S. pneumoniae, and structural studies of the signature “pneumococcal histidine triad” motif (HXXHXH) have revealed that this motif can bind zinc (68, 69). A recent report (61) shows a role in S. pneumoniae for Pht proteins in inhibiting surface deposition of complement involving interaction with complement factor H. An interesting possibility is that PhtD may interact with Lsp to promote zinc scavenging or perhaps may serve as an extracellular zinc reservoir. Future study should address the potential effects of lsp transcription upon phtD transcription and ultimate dependence upon interaction between the two for functional stability. Consequently, a portion of the phenotype observed for the Lsp− mutant could be the result of an effect upon PhtD, either in vitro or in vivo. For example, the partial complementation by ectopic wild-type Lsp in the Lsp-deficient mutant strains (Fig. 2) could be a manifestation of such an effect.
Another anomaly of the Lsp locus is that it also lacks the genes for the other subunits of an ABC-type transporter (8). Similarly, the S. pyogenes genome contains a locus with a high level of homology to the Adc locus of S. pneumoniae that encodes an ABC-type transporter involved in zinc transport (Spr_1975-1978) (20). Like in S. pneumoniae, the S. pyogenes Adc locus contains genes for the AdcR transcription regulator (SPy_0092) and the structural components of the transporter (SPy_0093 and SPy_0094), but unlike in S. pneumoniae, it lacks the gene for the cluster 9 family ligand-binding subunit. An open reading frame encoding a protein with high homology to AdcA of S. pneumoniae is found at a distal locus (SPy_0714, 61% identical and 76% similar) that also lacks any other components of an ABC-type transporter. This protein, in contrast to AdcA of S. pneumoniae, contains the N-terminal solute-binding domain and then an additional C-terminal fusion protein of unknown function, giving it a length of 515 amino acid residues compared to 311 for the AdcA protein. We have previously reported that these genes all have shared regulation as members of the AdcR regulon as confirmed by transcriptional studies (6). This raises the interesting possibility that this AdcA-like protein and Lsp share the Adc-like transporter components; perhaps they do so simultaneously or perhaps the two-ligand binding proteins bind and transport zinc with different affinities, allowing the bacterium to swap these domains to fine-tune zinc homeostasis to match zinc demand with the available external pools of zinc in the dynamic host milieu. A swapping mechanism may also explain the unusual structure of the recently described AdcII locus of S. pneumoniae. While this gene cluster differs from the Lsp locus in that it contains a total of five open reading frames, one of these has high homology to phtD (53). The locus also lacks any obvious candidates for the membrane components of an ABC-type transporter (53), suggesting that the AdcII cluster 9 family member may swap with another ligand-binding subunit to produce a functional transporter.
An adaptive response to alterations in the extracellular pool of zinc likely plays a key role in the ability of S. pyogenes to infect various tissues, as evidence is accumulating to suggest that the host actively modulates the concentration of free zinc in tissue compartments for defensive purposes. For example, human abscess fluid becomes limiting for the growth of Staphylococcus aureus and Candida albicans due to the restriction of zinc (1, 78). In innate immunity, Toll-like receptor 4 has a recently described regulatory function in zinc homeostasis of mammalian cells, suggesting that the immune response takes an active role both in regulation of zinc availability and in the adaptation of immune cells for functioning in a zinc-restricted environment (45). Also, the heterooligomer calprotectin, a human acute-phase reactant produced primarily by neutrophils, is an important proinflammatory protein that has been shown to bind zinc and may play a role in the regulation of zinc bioavailability (46, 73). Given the importance of zinc and the restriction of zinc in the host's response against pathogens, further analysis of S. pyogenes zinc homeostasis mechanisms, including the contribution of Lsp, will reveal important new insight into the role of transition metals in host-pathogen interactions.
Supplementary Material
Acknowledgments
This work was supported by Childhood Infection and Immunity training grant 5T32HD007507-10 and by Public Health Service grants AI046433, AI064721, and AI070759, all of which are from the National Institutes of Health.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 27 April 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bamberger, D. M., B. L. Herndon, and P. R. Suvarna. 1993. The effect of zinc on microbial growth and bacterial killing by cefazolin in a Staphylococcus aureus abscess milieu. J. Infect. Dis. 168893-896. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, S., B. Wei, M. Bhattacharyya-Pakrasi, H. B. Pakrasi, and T. J. Smith. 2003. Structural determinants of metal specificity in the zinc transport protein ZnuA from Synechocystis 6803. J. Mol. Biol. 3331061-1069. [DOI] [PubMed] [Google Scholar]
- 3.Bates, C. S., G. E. Montanez, C. R. Woods, R. M. Vincent, and Z. Eichenbaum. 2003. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 711042-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, C. S., C. Toukoki, M. N. Neely, and Z. Eichenbaum. 2005. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect. Immun. 735743-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 645255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenot, A., K. Y. King, and M. G. Caparon. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol. Microbiol. 55221-234. [DOI] [PubMed] [Google Scholar]
- 7.Brenot, A., K. Y. King, B. Janowiak, O. Griffith, and M. G. Caparon. 2004. Contribution of glutathione peroxidase to the virulence of Streptococcus pyogenes. Infect. Immun. 72409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenot, A., B. F. Weston, and M. G. Caparon. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 631185-1196. [DOI] [PubMed] [Google Scholar]
- 9.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bsat, N., H. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29189-198. [DOI] [PubMed] [Google Scholar]
- 11.Bunce, C., L. Wheeler, G. Reed, J. Musser, and M. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 602636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caparon, M. G., and J. R. Scott. 1991. Genetic manipulation of the pathogenic streptococci. Methods Enzymol. 204556-586. [DOI] [PubMed] [Google Scholar]
- 13.Changela, A., K. Chen, Y. Xue, J. Holschen, C. E. Outten, T. V. O'Halloran, and A. Mondragon. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 3011383-1387. [DOI] [PubMed] [Google Scholar]
- 14.Chen, P. R., and C. He. 2008. Selective recognition of metal ions by metalloregulatory proteins. Curr. Opin. Chem. Biol. 12214-221. [DOI] [PubMed] [Google Scholar]
- 15.Chivers, P. T., and R. T. Sauer. 2002. NikR repressor: high-affinity nickel binding to the C-terminal domain regulates binding to operator DNA. Chem. Biol. 91141-1148. [DOI] [PubMed] [Google Scholar]
- 16.Claverys, J. P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152231-243. [DOI] [PubMed] [Google Scholar]
- 17.Cromie, M. J., Y. Shi, T. Latifi, and E. A. Groisman. 2006. An RNA sensor for intracellular Mg(2+). Cell 12571-84. [DOI] [PubMed] [Google Scholar]
- 18.Davidson, A. L., E. Dassa, C. Orelle, and J. Chen. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72317-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson, R. J., K. Hollenstein, and K. P. Locher. 2007. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 65250-257. [DOI] [PubMed] [Google Scholar]
- 20.Dintilhac, A., G. Alloing, C. Granadel, and J.-P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC transporter. Mol. Microbiol. 25727-739. [DOI] [PubMed] [Google Scholar]
- 21.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148119-131. [DOI] [PubMed] [Google Scholar]
- 22.Eide, D. J. 2006. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763711-722. [DOI] [PubMed] [Google Scholar]
- 23.Elsner, A., B. Kreikemeyer, A. Braun-Kiewnick, B. Spelleberg, B. A. Buttaro, and A. Podbielski. 2002. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion an internalization. Infect. Immun. 704859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 984658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300931-936. [DOI] [PubMed] [Google Scholar]
- 26.Fischbach, M. A., H. Lin, D. R. Liu, and C. T. Walsh. 2006. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat. Chem. Biol. 2132-138. [DOI] [PubMed] [Google Scholar]
- 27.Gaballa, A., and J. D. Helmann. 2002. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45997-1005. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh, J., and M. G. Caparon. 2006. Specificity of Streptococcus pyogenes NAD(+) glycohydrolase in cytolysin-mediated translocation. Mol. Microbiol. 621203-1214. [DOI] [PubMed] [Google Scholar]
- 29.Giedroc, D. P., and A. I. Arunkumar. 2007. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 20073107-3120. [DOI] [PubMed] [Google Scholar]
- 30.Glantz, S. 2002. Primer of biostatistics, 5th ed. McGraw-Hill Co., New York, NY.
- 31.Granok, A. B., A. Claiborne, R. P. Ross, and M. G. Caparon. 2000. The RofA binding site in Streptococcus pygogenes is utilized in multiple transcriptional pathways. J. Bacteriol. 1821529-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gryllos, I., R. Grifantini, A. Colaprico, M. E. Cary, A. Hakansson, D. W. Carey, M. Suarez-Chavez, L. A. Kalish, P. D. Mitchell, G. L. White, and M. R. Wessels. 2008. PerR confers phagocytic killing resistance and allows pharyngeal colonization by group A Streptococcus. PLoS. Pathog. 4e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanski, E., and M. G. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus, Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 896172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hantke, K. 2005. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8196-202. [DOI] [PubMed] [Google Scholar]
- 35.Hazlett, K. R., F. Rusnak, D. G. Kehres, S. W. Bearden, C. J. La Vake, M. E. La Vake, M. E. Maguire, R. D. Perry, and J. D. Radolf. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J. Biol. Chem. 27820687-20694. [DOI] [PubMed] [Google Scholar]
- 36.Hollenstein, K., R. J. Dawson, and K. P. Locher. 2007. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 17412-418. [DOI] [PubMed] [Google Scholar]
- 37.Hollenstein, K., D. C. Frei, and K. P. Locher. 2007. Structure of an ABC transporter in complex with its binding protein. Nature 446213-216. [DOI] [PubMed] [Google Scholar]
- 38.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 693744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hynes, W. L., J. J. Ferretti, M. S. Gilmore, and R. A. Segarra. 1992. PCR amplification of streptococcal DNA using crude cell lysates. FEMS Microbiol. Lett. 94139-142. [DOI] [PubMed] [Google Scholar]
- 40.Janulczyk, R., J. Pallon, and L. Bjorck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificities for metal cations. Mol. Microbiol. 34596-606. [DOI] [PubMed] [Google Scholar]
- 41.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 712656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji, Y., L. McLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 725858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 1825290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitamura, H., H. Morikawa, H. Kamon, M. Iguchi, S. Hojyo, T. Fukada, S. Yamashita, T. Kaisho, S. Akira, M. Murakami, and T. Hirano. 2006. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 7971-977. [DOI] [PubMed] [Google Scholar]
- 46.Korndorfer, I. P., F. Brueckner, and A. Skerra. 2007. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 370887-898. [DOI] [PubMed] [Google Scholar]
- 47.Kushner, S. R. 1978. An improved method for transformation of Escherichia coli with ColE1-derived plasmids, p. 17-23. In H. W. Boyer and S. Micosia (ed.), Genetic engineering. Elsevier/North-Holland Biomedical Press, New York, NY.
- 48.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 61553-1561. [DOI] [PubMed] [Google Scholar]
- 49.Lee, Y. H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6628-633. [DOI] [PubMed] [Google Scholar]
- 50.Lee, Y. H., M. R. Dorwart, K. R. Hazlett, R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 2002. The crystal structure of Zn(II)-free Treponema pallidum TroA, a periplasmic metal-binding protein, reveals a closed conformation. J. Bacteriol. 1842300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li, H., and G. Jogl. 2007. Crystal structure of the zinc-binding transport protein ZnuA from Escherichia coli reveals an unexpected variation in metal coordination. J. Mol. Biol. 3681358-1366. [DOI] [PubMed] [Google Scholar]
- 52.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 686384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loisel, E., L. Jacquamet, L. Serre, C. Bauvois, J. L. Ferrer, T. Vernet, A. M. Di Guilmi, and C. Durmort. 2008. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J. Mol. Biol. 381594-606. [DOI] [PubMed] [Google Scholar]
- 54.Lyon, W. R., and M. G. Caparon. 2003. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J. Bacteriol. 1853661-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maresso, A. W., and O. Schneewind. 2006. Iron acquisition and transport in Staphylococcus aureus. Biometals 19193-203. [DOI] [PubMed] [Google Scholar]
- 56.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53889-901. [DOI] [PubMed] [Google Scholar]
- 57.Montanez, G. E., M. N. Neely, and Z. Eichenbaum. 2005. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology 1513749-3757. [DOI] [PubMed] [Google Scholar]
- 58.Moore, C. M., A. Gaballa, M. Hui, R. W. Ye, and J. D. Helmann. 2005. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 5727-40. [DOI] [PubMed] [Google Scholar]
- 59.Nelson, N. 1999. Metal ion transporters and homeostasis. EMBO. J. 184361-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302205-217. [DOI] [PubMed] [Google Scholar]
- 61.Ogunniyi, A. D., M. Grabowicz, L. K. Mahdi, J. Cook, D. L. Gordon, T. A. Sadlon, and J. C. Paton. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB. J. 23731-738. [DOI] [PubMed] [Google Scholar]
- 62.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2922488-2492. [DOI] [PubMed] [Google Scholar]
- 63.Panina, E. M., A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA 1009912-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pennella, M. A., and D. P. Giedroc. 2005. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals 18413-428. [DOI] [PubMed] [Google Scholar]
- 65.Pilling, P. A., M. C. Lawrence, A. M. Berry, A. D. Ogunniyi, R. A. Lock, and J. C. Paton. 1998. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr. D 541464-1466. [DOI] [PubMed] [Google Scholar]
- 66.Poirot, O., E. O'Toole, and C. Notredame. 2003. Tcoffee@igs: a web server for computing, evaluating and combining multiple sequence alignments. Nucleic. Acids. Res. 313503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 9610887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riboldi-Tunnicliffe, A., C. J. Bent, N. W. Isaacs, and T. J. Mitchell. 2004. Expression, purification and X-ray characterization of residues 18-230 from the pneumococcal histidine triad protein A (PhtA) from Streptococcus pneumoniae. Acta Crystallogr. D 60926-928. [DOI] [PubMed] [Google Scholar]
- 69.Riboldi-Tunnicliffe, A., N. W. Isaacs, and T. J. Mitchell. 2005. 1.2 Angstroms crystal structure of the S. pneumoniae PhtA histidine triad domain, a novel zinc binding fold. FEBS. Lett. 5795353-5360. [DOI] [PubMed] [Google Scholar]
- 70.Ricci, S., R. Janulczyk, and L. Björck. 2002. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect. Immun. 704968-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero-Steiner, S., T. Pilishvili, J. S. Sampson, S. E. Johnson, A. Stinson, G. M. Carlone, and E. W. Ades. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27337-346. [DOI] [PubMed] [Google Scholar]
- 73.Sampson, B., M. K. Fagerhol, C. Sunderkotter, B. E. Golden, P. Richmond, N. Klein, I. Z. Kovar, J. H. Beattie, B. Wolska-Kusnierz, Y. Saito, and J. Roth. 2002. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet 3601742-1745. [DOI] [PubMed] [Google Scholar]
- 74.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 981954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott, J. R. 1992. Sex and the single circle: conjugative transposition. J. Bacteriol. 1746005-6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekler, I., S. L. Sensi, M. Hershfinkel, and W. F. Silverman. 2007. Mechanism and regulation of cellular zinc transport. Mol. Med. 13337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seo, J. Y., S. Y. Seong, B. Y. Ahn, I. C. Kwon, H. Chung, and S. Y. Jeong. 2002. Cross-protective immunity of mice induced by oral immunization with pneumococcal surface adhesin A encapsulated in microspheres. Infect. Immun. 701143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sohnle, P. G., B. L. Hahn, and R. Karmarkar. 2001. Effect of metals on Candida albicans growth in the presence of chemical chelators and human abscess fluid. J. Lab. Clin. Med. 137284-289. [DOI] [PubMed] [Google Scholar]
- 79.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lutticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.VanHeyningen, T., G. Fogg, D. Yates, E. Hanski, and M. Caparon. 1993. Adherence and fibronectin-binding are environmentally regulated in the group A streptococcus. Mol. Microbiol. 91213-1222. [DOI] [PubMed] [Google Scholar]
- 81.van Vliet, A. H., M. L. Baillon, C. W. Penn, and J. M. Ketley. 1999. Campylobacter jejuni contains two Fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 1816371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei, B., A. M. Randich, M. Bhattacharyya-Pakrasi, H. B. Pakrasi, and T. J. Smith. 2007. Possible regulatory role for the histidine-rich loop in the zinc transport protein, ZnuA. Biochemistry 468734-8743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.