Abstract
An individual, human or animal, is defined to be in a conscious state empirically by the behavioral ability to respond meaningfully to stimuli, whereas the loss of consciousness is defined by unresponsiveness. PET measurements of glucose or oxygen consumption show a widespread ≈45% reduction in cerebral energy consumption with anesthesia-induced loss of consciousness. Because baseline brain energy consumption has been shown by 13C magnetic resonance spectroscopy to be almost exclusively dedicated to neuronal signaling, we propose that the high level of brain energy is a necessary property of the conscious state. Two additional neuronal properties of the conscious state change with anesthesia. The delocalized fMRI activity patterns in rat brain during sensory stimulation at a higher energy state (close to the awake) collapse to a contralateral somatosensory response at lower energy state (deep anesthesia). Firing rates of an ensemble of neurons in the rat somatosensory cortex shift from the γ-band range (20–40 Hz) at higher energy state to <10 Hz at lower energy state. With the conscious state defined by the individual's behavior and maintained by high cerebral energy, measurable properties of that state are the widespread fMRI patterns and high frequency neuronal activity, both of which support the extensive interregional communication characteristic of consciousness. This usage of high brain energies when the person is in the “state” of consciousness differs from most studies, which attend the smaller energy increments observed during the stimulations that form the “contents” of that state.
Keywords: anesthesia, awake, glutamate, neuroimaging, oxygen consumption
The excitement in modern neuroscience anticipates relating brain activity to mental processes of behavioral states, such as consciousness. In recent years, fMRI, 13C magnetic resonance spectroscopy (MRS), PET, and electrophysiology experiments have been directed toward these goals. These studies have measured brain energy production in the form of glucose oxidation in the resting awake state and the anesthetized state, and have followed regional changes during stimulation from these states. The most striking result is that the total energy consumption supporting neuronal firing in the conscious awake, baseline state is orders of magnitude larger than the energy changes during stimulation (1–3). Nonetheless, most research relating brain activity to mental processes has been based on the smaller fMRI or PET increments, which generally are interpreted as localizing psychological concepts. Early functional imaging studies by Posner and Raichle (4), using PET scans, posited that a connection between mental concepts and brain activities can be made by the difference in the images obtained when the assumed mental concept, or module, was or was not involved in a task (e.g., by comparing brain images of a subject reading proper and nonsense words). Differences between 2 functional images were interpreted as providing a quantitative map that localized the neuronal underpinnings of the mental modules; in the case at hand, that mental activity would be semantics. This experimental paradigm was soon adopted for fMRI studies, a technology which made functional brain imaging widely accessible.
However, it soon became clear that the brain response to cognitive subtraction did not follow the simplistic assumptions of “pure insertion,” but rather depended on the context of the task. The dependence of brain responses on their context created problems for cognitive psychology. Jerry Fodor, a founder of the field, concluded in 2000 that the mind does not work that way (5), because the dependence on context undercut the causality claimed for the concepts of cognitive neuroscience. Results, including the influence of context in psychologically-based fMRI or PET studies, led us, in 1996, to question the value of such concepts for functional brain imaging. We suggested that the field would be better served by using the functional imaging data to question cognitive concepts, rather than by considering those assumptions proven when a difference image is acquired (6).
However, prominent neuroimagers retained the potential value of the cognitive concepts, and considered that the loss of pure insertion might arise from the nonlinear nature of brain responses (7). Parametric methods were designed to overcome brain nonlinearity and to more closely relate brain responses to the input activities (8). However, in these studies, the search for brain responses to psychological concepts remained the goal. A strong version of that goal, that represents the popular position of pure insertion despite empirical set-backs, was described by Gazzaniga (9), who says that “we now understand that changes in our brain are both necessary and sufficient for changes in our mind,” and continues by praising cognitive neuroscience for studying the mechanisms of cognitive phenomena.
This report studies the support of behavior by brain activity without making mentalistic/psychological assumptions about the unobserved processes presumably involved in observed behaviors or behavioral states and finds a necessary role for the unassigned high baseline energy. In both of these respects, it offers a previously untried methodology for relating brain activities to mental processes. We believe that brain activities provide necessary support for behavioral processes that are performed and experienced by the human (or rodent) in the state of consciousness. In our study, brain experiments are used to determine neuronal and energetic properties of a behavioral state, as distinguished from the claims that imaging results localize in the brain the mental processes that cause a cognitive conceptualization of behavior. Brain activities support and are properties of a person in a behavioral state, such as consciousness, and they, thus, enable mental processes such as remembering or intending, all of which contribute to being conscious. Instead of aiming to localize assumptions about the nature of a mental process, we start with a behavioral index that the individual is in the conscious state and then measure neuronal properties of that state. Rather than localizing psychological assumptions which, in Zeman's description (10), would form the contents of consciousness, the subjects (rat or human) are defined as being in a conscious state by observations of reproducible behavior.
The state of consciousness is defined by the subject's ability to respond to stimuli using the criteria established in anesthesia (11). The object of study should be the person, an entity which includes brain, body, and mental processes, that cooperate to interact with the environment. This position has been championed by Antonio Damasio who, as a neurologist and neuroscientist, has written compelling books in support of the idea that the study of “mental activity, from its simplest aspects to its most sublime, requires both brain and body” (12). The neurophysiological basis of his belief is documented in an account of how body and brain are in continual back and forth interactions via chemical and neuronal impulses. Our position also resonates with a comprehensive analysis of cognitive neuroscience developed by Bennett and Hacker (13). Their criticism is that mental functions are performed by the person; therefore, they are not located, represented, or encrypted in a brain. A person adds, subtracts, feels pain or decides to marry, not the brain. In this interpretation, brain activities can be necessary for a person's behavior, but they are not sufficient to explain it.
We propose that high baseline energy (therefore, the neuronal activity) in the awake state is a necessary property of the conscious state; when the energy is reduced sufficiently, there is loss of consciousness. Two additional brain properties measured are the significant changes in fMRI activation patterns and the neuronal population activity at different baseline energy states. Implications for philosophies and theories of the conscious state are discussed to demonstrate how measurable brain properties can allow a physical understanding of the state without psychological assumptions. The role of consciousness in the different stages/types of sleep or seizure (14) are beyond the scope of the current report.
Results
Behavioral Index and Energetic Basis for the Loss of Consciousness.
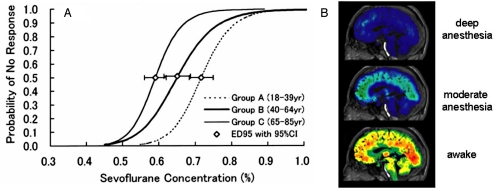
Studying loss of consciousness with anesthesia is an active neurophysiological research area (15, 16), with the majority of studies trying to identify specific brain regions or signaling patterns that change significantly when consciousness is lost. We define the loss of the conscious state, similar to anesthesiologists, as occurring when a person or animal can no longer respond to stimulus (Fig. 1A). PET experiments have measured cerebral energies of human subjects at deepening levels of anesthesia (17–19). At deep levels, subjects were not conscious based on their inability to respond to sensory stimuli and/or questions from the anesthesiologist (11). Although there are many circumstances where humans do not have the abilities necessary to respond (i.e., sleep, coma, physical impairment, etc.), for the sake of simplicity, we limit the definition of unresponsiveness to individuals who have full expressive faculties. To avoid a proliferating terminology, we note that there are caveats to this definition. In the rat, the conscious state has been distinguished from the deeply anesthetized state by the loss of righting reflex (20). Based on these behavioral criteria of the loss of consciousness, humans and rats have the similar dose-response curves for common anesthetics (16).
Fig. 1.
Loss of consciousness with anesthesia, as assessed by behavioral output (A) and cerebral metabolism measured by PET (B). Hot colors indicate higher energy demand. [Fig. 1A reproduced with permission from Katoh T, Bito H, Sato S (2000) Influence of age on hypnotic requirement, bispectral index, and 95% spectral edge frequency associated with sedation induced by sevoflurane. Anesthesiology 92(1):55–61.] [Fig. 1B reprinted by permission from Macmillan Publishers Ltd: Alkire MT (2008) Probing the mind: Anesthesia and neuroimaging. Clin Pharmacol Ther 84:149–152, copyright 2008.]
PET images of brain metabolism (of glucose and oxygen consumption; CMRglc and CMRO2) in varied depths of anesthesia show, to a first-order, ubiquitous depression of energy demand from the awake state (Fig. 1B; Table S1) (21). These suppressions were not clearly regional or anesthesia specific, but beyond these first-order similarities specified differences abound. We propose that the near-homogeneous reductions in brain energy by anesthetics (Table S1) support the hypothesis that, to a first approximation, global energy reduction is responsible for loss of consciousness (Fig. 1B).
Three target sites have been identified for common anesthetics (16), and all sites directly or indirectly inhibit glutamate neurotransmitter activity. Actions of all known anesthetic agents (with the exception of ketamine, which is anomalous in that it raises metabolic activity at low dosages and could be considered an upper energy bound) are consistent with decreases in the glutamate-GABA synaptic activity, and would cause the reductions observed in cerebral energy consumption. Because glutamatergic neurotransmission is widely spread throughout the brain (22), its responsiveness to anesthetics (small molecules that diffuse readily throughout the brain) supports a global reduced neuronal firing consistent with the uniform energy reduction observed in the PET experiments.
In summary, we propose that the conscious state is supported by a high and relatively uniform state of baseline brain energy consumption and its associated neuronal activity. Consciousness is absent during anesthesia when regional energy levels are uniformly reduced by 40–50% from the awake resting values. However, the brain does not only exist in either of 2 extreme states, one conscious the other not. Rather, existing data suggests that there is a continuous variation of brain activity and the behavioral properties associated with consciousness as the brain energy level gradually falls (23). Heinke and Schwarzbauer (24), and others (25), have shown that secondary processing regions in the human fMRI maps are decreased more at a moderate level of anesthesia than are the primary sensory regions. Although the awake fully conscious state can be distinguished clearly from its loss, future experiments could delineate details of these graded transitions. We propose that energy would be a unifying parameter in such experiments.
Uniformly Distributed High Baseline Energy Supports the Conscious State.
The human brain, which claims ≈2% of our body mass, is responsible for ≈20% of our body oxygen consumption. Continuous energy supply is imperative for brain function, because endogenous energy reserves are minimal (26). Normal function needs blood circulation to efficiently provide nutrients and remove waste (27). The energetic costs for brain work are mainly met by ATP derived from glucose oxidation (22). Because activities at the nerve terminal are in continuous need of energy, demand for it is a fundamental requirement (28).
To determine exactly how high the energy demand for neuronal activity is, rat experiments were performed using 13C MRS. The studies were based on the ability of MRS in combination with 13C labeled substrates such as glucose and acetate to measure the cerebral energy production rates in neurons by the rate of glucose oxidation (CMRglc(ox),N) in the tri-carboxylic acid cycle and the coupled rates of neurotransmitter glutamate and GABA release and recycling (Vcyc) (29). The rates of glucose oxidation and neurotransmitter cycling were measured over a wide range of brain activities, taking the rats from isoelectric pentobarbital anesthesia, under which there is no neuronal signaling, to mildly anesthetized states with higher neuronal signaling activity. The plot of Vcyc vs. CMRglc(ox),N fitted a straight line of a slope of unity and an intercept of ≈0.1 μmol/g/min (Fig. 2A).
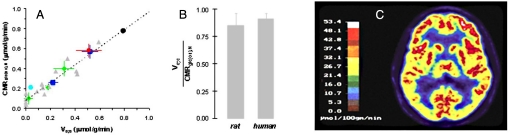
Fig. 2.
13C MRS and PET results of baseline energy. (A) Experimental results of Vcyc and CMRglc(ox),N. Values of Vcyc and CMRglc(ox),N for the rat brain reported in studies published between 1998 and 2006. The dark blue squares are from Patel et al. (58), the red circle is from Oz et al. (59), the light blue circle is from Choi et al. (60), the green diamonds are from de Graaf et al. (61), and the gray triangles are from Sibson et al. (29). For details of these papers, see ref. 30. [Reproduced with permission from Hyder et al. (30) (Copyright 2006).] (B) The ratio of Vcyc/CMRglc(ox),N in the nonanesthetized resting awake state in rat (extrapolated from A; see ·) and human brain (see ref. 30 for details). Similarity of the Vcyc/CMRglc(ox),N ratio in rats and humans suggests that the relationship between Vcyc and CMRglc(ox),N are similar. [Reproduced with permission from Hyder et al. (30) (Copyright 2006).] (C) PET image of CMRglc of the human brain showing uniform and high energy metabolism in the resting, awake state. From ref. 32 with permission. [Reprinted by permission from Wolters Kluwer Health: Alkire MT (2008) Loss of effective connectivity during general anesthesia. Int Anesthesiol Clin 46(3):55–73.]
These results (29), in agreement with reports from other laboratories (30), are significant in several respects. They establish a quantitative molecular relationship between cortical oxidative energy production and the glutamate neurotransmitter flux, which is coupled to the rate of neuronal firing (22). At the intercept where Vcyc falls to zero, CMRglc(ox),N had fallen to ≈0.1 μmol/g/min, which is only 15–20% of the value in the awake rat brain (of ≈0.7 μmol/g/min). Hence, in the awake, resting (basal) state 80–85% of the neuronal energy consumption is devoted to supporting neuronal signaling. A similar high ratio of Vcyc to CMRglc(ox),N was found in 13C MRS studies of the awake human brain (Fig. 2B) (30, 31). This high level of neuronal signaling in the absence of specific stimulations required fundamental reevaluations of results from higher level brain studies, including those using functional imaging (1).
PET images of CMRglc in the awake, resting (basal) state show regional distribution of the high baseline energy demand (Fig. 2C; Table S2) (17–19, 32). Because PET and 13C MRS metabolic measurements are averaged over minutes, temporal fluctuations cannot be accurately assessed. However, it is unlikely that spontaneous variations are larger than the ≈15% evoked changes observed during strong sensory stimulation in awake humans (33).
These results suggest that the high and widely distributed level of brain energy exists in the conscious human brain to support a high level of neuronal firing, which is globally reduced during the loss of consciousness at deep anesthesia.
Effect of Baseline Energy on fMRI Activation Patterns.
The total energy of neuronal activity in a brain volume and the incremental energy during a stimulus can be measured noninvasively by 13C MRS and calibrated fMRI methods, respectively (34). When there is an increase in cortical neuronal activity, there is a concomitant increase in cerebral blood flow (CBF) and volume CBV) to bring more oxygen to the brain to provide fuel for the neurons and glia (26). The blood-oxygenation-level-dependent (BOLD) fMRI signal is sensitive to these changes, as well as to changes in CMRO2 (35). Recent work combining BOLD measurements with measurements of CBF (and CBV), using calibrated fMRI, have allowed CMRO2 increments (ΔCMRO2) to be derived from the BOLD signal (36). Because fMRI has good spatiotemporal resolution and allows ample brain coverage, it has become attractive to calculate ΔCMRO2 with calibrated fMRI even for event-related paradigms (37), which is desired in cognitive fMRI studies.
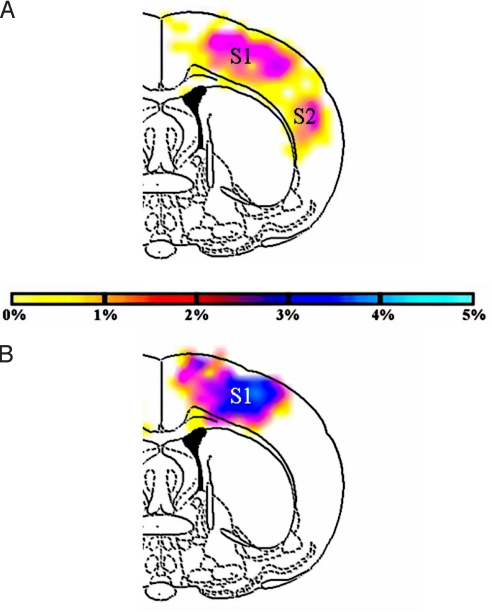
For fMRI experiments, it has been conventional to describe the total energy in the unstimulated state as the “baseline” energy (2, 38). The different meanings attributed to the term baseline are simplified when we simply consider it an operational term. In our usage, baseline is an adjective that describes a property of the brain state that exists before a stimulation of interest. That state has values of baseline CMRO2, baseline BOLD signal, and baseline CBF etc., and on stimulation, we detect their increments. The imaging consequences of baseline energies were explored by experiments in rats at 2 anesthetic states, characterized by very different baseline energies (39), where energy in the lower state (α-chloralose) was 40–60% less than in the higher energy state (halothane). Forepaw stimulation that was administered in both states excited the contralateral primary somatosensory cortex. However, there were major differences between fMRI activations in the 2 states. At high baseline, there were additional weak activations in anterior brain regions (Fig. 3A), which included primary and secondary somatosensory cortices in the contralateral side along with other distal regions. At low baseline, strong activations were found mainly in the contralateral primary somatosensory cortex (Fig. 3B). Several studies have suggested that activity patterns become more localized in deep anesthetized states (40, 41). Functional MRI studies in primates and humans are supportive of these findings (42, 43).
Fig. 3.
Averaged fMRI maps (from 2 subjects, 2 single runs, 30-s block design, forepaw stimulation) of anterior coronal slices under halothane showed weak widespread activities beyond contralateral primary (S1) and secondary (S2) somatosensory cortices (A), whereas under α-chloralose demonstrated strong localized activation in contralateral S1 (B). Darker colors represent greater overlap across experiments (i.e., reproducibility). All activation maps were thresholded at the same value (P < 0.02). [Copyright 2007 by The National Academy of Sciences of the USA.]
In summary, these results indicate that at higher baseline energy fMRI activations are spread across larger cortical areas. At present, due to experimental limitations, fMRI studies of awake rodents have been limited. However, the few that have been performed are consistent with delocalized fMRI activation patterns (44).
Effect of Baseline Energy on Distribution of Neuronal Firing.
The chemical relationship between the rates of glutamate neurotransmitter release and neuronal oxidative energy production/consumption were experimentally related to electrophysiological measurements of neuronal firing rates. Studies of high resolution calibrated fMRI and multiunit recordings (in rats) showed a similar relationship between changes in neuronal oxygen consumption and the average rate of neuronal firing during sensory stimulation (45). Changes in CMRO2 in an fMRI voxel were proportional to changes in the neuronal firing frequencies of a representative ensemble of neurons in that voxel. When the same neuronal population was measured across different states, relative changes in CMRO2 were quantitatively related to shifts in ensemble firing rates.
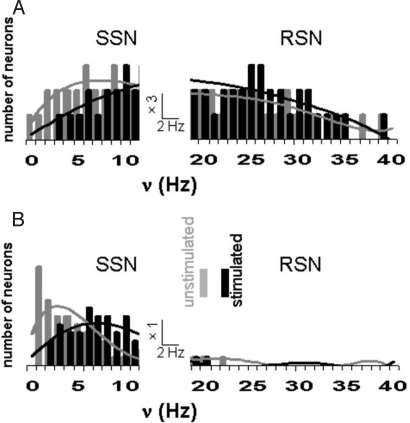
Baseline energy level affects the distribution of neuronal firing rates in the activated volume (39). Neuronal histograms in the two states (α-chloralose and halothane) showed that the population can be divided into subgroups of slow and rapid signaling neurons (SSN and RSN, respectively). Greater RSN and SSN subpopulations were observed in the higher (Fig. 4A) and lower (Fig. 4B) energy baseline states, respectively. The most conspicuous difference between the 2 anesthetized states of different energy was the dominance of RSN activity under halothane (i.e., RSN/SSN ratio is high) in contrast to α-chloralose (i.e., RSN/SSN ratio is low). Similar anesthesia-dependent shifts in high vs. low frequency neuronal signaling have been reported (46). High frequency signaling, including γ-band electrical activity, has been extensively studied with EEG (15), and has been shown to be attenuated by anesthesia (47). The γ-band activity tends to be synchronized between homologous sensorimotor regions of both hemispheres (48), and can be modified by variations in global activity (49). These results gave rise to the hypothesis that the RSN activity supports intracortical signaling and synchronization, in accord with previous assignments of similar frequencies observed electrically as the γ-band type signaling in EEG (39). However, it has been suggested that the reduction in γ-band is not a unitary correlate of anesthetic-induced unconsciousness (50).
Fig. 4.
Total activity represented by distribution of firing rates (ν; 10-s bins) in the primary somatosensory ensemble of ≈200 neurons for halothane (A) and α-chloralose states (B). Activity under halothane is dominated by the RSN subgroup, which seems unaffected by stimulation. The SSN subgroup, shifting to higher frequencies on stimulation, is similar in both states but more significant under α-chloralose. [Copyright 2007 by The National Academy of Sciences of the USA.]
In summary, these results indicate that at higher baseline energy consumption the elevated RSN population supports interactions across distant brain regions. We hypothesize that in the high energy baseline state rich intracortical interactions represented by high frequency γ-band type signaling support evoked changes in fMRI activation in areas beyond the primary area.
Discussion
Our understanding of the conscious state grows from measurements of its properties (Fig. S1). The high global neuronal activity/energy is a necessary property of the conscious state, which if reduced by anesthesia, causes loss of consciousness. High brain activity is not consciousness; rather, it is a brain property that provides necessary, but not sufficient support of the conscious state. Objective, reliable understandings of the conscious state are expected to gradually emerge from measurable brain properties. This growing understanding of the “state” of consciousness, although impoverished compared with philosophical and quotidian formulations of the “contents” of consciousness, benefits from scientific reliability supplied by the bottom-up physically based neurophysiological measurements.
The relative energetics of the contents of consciousness and the state of consciousness offer clarification to ongoing debates about the role of brain activities in mental processes. The brain/mind question is a generalization of experimental hopes of understanding the relation between subjective mental processes and objective brain function. Christof Koch overcomes this divide by biting the bullet: “I take subjective experience as given and assume that brain activity is both necessary and sufficient for biological creatures to experience something” (ref. 51, p. 19). He assumes that there must be neuronal correlates of consciousness (NCC) that contain explicit correspondence between a mental event and its neuronal correlates (ref. 51, p. 17). His research starts with visual processes with hopes of proceeding from their NCC to correlates of subjective mental processes, which form the contents of consciousness. However, we and others have repeatedly found it necessary to distinguish between the neuronal responses to sensory stimuli and the prospects for identifying NCC of subjective concepts (52–54). Koch recognizes that even if NCC of visual processes are found, the Cartesian divide has not yet been crossed. He postulates that even with NCC in hand, additional activities would be needed to link them with subjectivity, and therefore, introduces an enabling factor (NCCe) that supports the contents of consciousness (ref. 51, p. 88).
We propose that a range of high global energy production and consumption is a brain property necessary to support the conscious state; thus, fulfilling the role envisaged for the NCCe. Without a high level of brain energy, a person does not display the brain properties of the conscious state we have measured (the delocalized fMRI activities and the high frequency neuronal firing). Although suggesting that the conscious state agrees with their perceived role for an enabling condition, we are not in agreement with the view, as expressed by Koch, that “brain activity is both necessary and sufficient for biological sentience” (ref. 51, p. 9). Rather, we propose that an understanding of the conscious state should build on neurophysiological properties that are necessary for, but not sufficient for, the person's ability to have complex behavioral functions.
Our approach does not start with a priori assumptions about the nature of mental processes, including the concept of consciousness. Our proposed approach does not question the value of using mental concepts like intention, memory, attention or love in everyday life. However, using mentalistic-, philosophical-, and folk-psychological concepts in neurophysiological studies of brain properties assumes that the Cartesian dualism is resolved.
The different magnitudes of baseline and incremental energies provide a physical basis for Llinas's views that “sensory cues earn representation via their impact on the preexisting functional disposition of the brain” (ref. 54, p. 8). Llinas, from his electrophysiological studies that followed many reports, especially by Singer and coworkers (55), proposed that the 40-Hz electrical signals recorded on the scalp measure consciousness, and further suggested that their synchronicity (56) binds together separate brain activities into a “substrate of self” (ref. 54, p. 126). Crick and Koch (57) extended this idea to propose that the 40-Hz oscillations bind together the different inputs to thereby create awareness.
The increase in the ≈40-Hz signal in the conscious awake state is a consequence of the general increase in energy in the state. Histograms of neuronal firing frequencies in the rat (Fig. 4), and similar results in other species (42), show an increase in activity across a broad band of RSN frequencies including 40 Hz. Although its coherence may or may not have a role in binding together brain activities (57), its increased magnitude in the conscious state (15, 54) is most likely a consequence of the high baseline energy.
Conclusions
Our experimental protocols are describing brain properties of the person in the state of consciousness. This approach does not promise descriptions of the contents of consciousness, which would be defining subjective mental processes in objective terms. However, it has identified brain properties for the human in a conscious state that change with loss of consciousness; properties, at the neuronal level which in their interconnections display the characteristics of consciousness.
Why do we identify the conscious state in so limited a behavioral way, by the human's response to stimuli, which is simplistic compared with psychological efforts to formulate its meaning? Certainly, the property of responding to a stimulus does not do justice to the richness of mental processes usually intended for the word. However, the very jarring nature of this limited test for the existence of consciousness illustrates an important point that we have made above and elsewhere (52, 53). Namely, to study mental processes, we do not assume them to be already described by cognitive psychology. Generalizations of psychological concepts like fear, memory, attention, or cognition are of great utility in everyday life, but assuming them introduces subjectivity into brain research. In gaining scientific objectivity by avoiding the study of subjective concepts, we have confined our studies to bottom-up neuronal properties that are sketching a neuronal basis for a behaviorally defined state of consciousness.
Materials and Methods
All experiments in humans (for PET, see refs. 17–19; for 13C MRS, see details in refs. 30, 31) and rats (for fMRI-electrical, see refs. 39, 45; for 13C MRS, see details in ref. 30) were conducted with approved protocols as described in the original papers. CMRglc and CMRO2 data from PET studies were (17–19) analyzed for different regions (Tables S1 and S2). The fMRI activation patterns and the neuronal activity patterns were obtained from the primary somatosensory cortex of the rat (39).
Supplementary Material
Acknowledgments.
We thank Robert Turner, Barbara Herrnstein Smith, and Jennifer Roth for comments. This work was supported by National Institutes of Health Grants R01 MH-067528, R01 NS-037527, R01 NS-051854, and P30 NS-52519.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903941106/DCSupplemental.
References
- 1.Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. NeuroImage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posner MI, Raichle ME. Images of Mind. New York: Scientific American Library; 1994. [Google Scholar]
- 5.Fodor J. The Mind Doesn't Work That Way: The Scope and Limits of Computational Psychology. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 6.Shulman RG. Interview with Robert G. Shulman. J Cogn Neurosci. 1996;8:474–480. doi: 10.1162/jocn.1996.8.5.474. [DOI] [PubMed] [Google Scholar]
- 7.Friston KJ, et al. The trouble with cognitive subtraction. NeuroImage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- 8.Buchel C, Wise RJ, Mummery CJ, Poline JB, Friston KJ. Nonlinear regression in parametric activation studies. NeuroImage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- 9.Gazzaniga MS. The Ethical Brain. New York: Dana Press; 2005. p. p 88. [Google Scholar]
- 10.Zeman A. Consciousness: A User's Guide. New Haven, CT: Yale Univ Press; 2002. [Google Scholar]
- 11.Katoh T, Bito H, Sato S. Influence of age on hypnotic requirement, bispectral index, and 95% spectral edge frequency associated with sedation induced by sevoflurane. Anesthesiology. 2000;92:55–61. doi: 10.1097/00000542-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Putnam Publishing; 1994. p. xvii. [Google Scholar]
- 13.Bennett MR, Hacker PMS. History of Cognitive Neuroscience. Chichester, UK: John Wiley and Sons; 2008. [Google Scholar]
- 14.Tononi G, Koch C. The neural correlates of consciousness: An update. Ann NY Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 15.Kulli J, Koch C. Does anesthesia cause loss of consciousness? Trends Neurosci. 1991;14:6–10. doi: 10.1016/0166-2236(91)90172-q. [DOI] [PubMed] [Google Scholar]
- 16.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 17.Alkire MT, et al. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82:393–403. doi: 10.1097/00000542-199502000-00010. discussion 327A. [DOI] [PubMed] [Google Scholar]
- 18.Alkire MT, et al. Functional brain imaging during anesthesia in humans: Effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. doi: 10.1097/00000542-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kaisti KK, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–213. doi: 10.1007/s001140100230. [DOI] [PubMed] [Google Scholar]
- 21.Alkire MT. Probing the mind: Anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- 22.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinke W, Schwarzbauer C. In vivo imaging of anaesthetic action in humans: Approaches with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) Br J Anaesth. 2002;89:112–122. doi: 10.1093/bja/aef155. [DOI] [PubMed] [Google Scholar]
- 25.Qiu M, Ramani R, Swetye M, Rajeevan N, Constable RT. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: An fMRI study in normal human subjects. Magn Reson Med. 2008;60:987–996. doi: 10.1002/mrm.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyder F. Dynamic imaging of brain function. Methods Mol Biol. 2009;489:3–22. doi: 10.1007/978-1-59745-543-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26:68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- 28.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 29.Sibson NR, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyder F, et al. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 31.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Alkire MT. Loss of effective connectivity during general anesthesia. Int Anesthesiol Clin. 2008;46:55–73. doi: 10.1097/AIA.0b013e3181755dc6. [DOI] [PubMed] [Google Scholar]
- 33.Hoge RD, Pike GB. Oxidative metabolism and the detection of neuronal activation via imaging. J Chem Neuroanat. 2001;22:43–52. doi: 10.1016/s0891-0618(01)00114-4. [DOI] [PubMed] [Google Scholar]
- 34.Hyder F, et al. Quantitative functional imaging of the brain: Towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa S, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F. High-resolution CMR(O2) mapping in rat cortex: A multiparametric approach to calibration of BOLD image contrast at 7 Tesla. J Cereb Blood Flow Metab. 2000;20:847–860. doi: 10.1097/00004647-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Sanganahalli BG, Herman P, Blumenfeld H, Hyder F. Oxidative neuroenergetics in event-related paradigms. J Neurosci. 2009;29:1707–1718. doi: 10.1523/JNEUROSCI.5549-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maandag NJ, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erchova IA, Lebedev MA, Diamond ME. Somatosensory cortical neuronal population activity across states of anaesthesia. Eur J Neurosci. 2002;15:744–752. doi: 10.1046/j.0953-816x.2002.01898.x. [DOI] [PubMed] [Google Scholar]
- 41.Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–221. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Disbrow EA, Slutsky DA, Roberts TP, Krubitzer LA. Functional MRI at 1.5 tesla: A comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci USA. 2000;97:9718–9723. doi: 10.1073/pnas.170205497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinke W, et al. Sequential effects of propofol on functional brain activation induced by auditory language processing: An event-related functional magnetic resonance imaging study. Br J Anaesth. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 44.Sachdev RN, et al. Experimental model for functional magnetic resonance imaging of somatic sensory cortex in the unanesthetized rat. NeuroImage. 2003;19:742–750. doi: 10.1016/s1053-8119(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 45.Smith AJ, et al. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkire MT. Quantitative EEG correlations with brain glucose metabolic rate during anesthesia in volunteers. Anesthesiology. 1998;89:323–333. doi: 10.1097/00000542-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald KD, Brett B, Barth DS. Inter- and intra-hemispheric spatiotemporal organization of spontaneous electrocortical oscillations. J Neurophysiol. 1996;76:423–437. doi: 10.1152/jn.1996.76.1.423. [DOI] [PubMed] [Google Scholar]
- 49.Engel A, Konig P, Kreiter A, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- 50.van Eijsden P, Hyder F, Rothman DL, Shulman RG. Neurophysiology of functional imaging. NeuroImage. 2009;45:1047–1054. doi: 10.1016/j.neuroimage.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch C. The Quest for Consciousness: A Neurobiological Approach. Englewood, CO: Roberts and Co.; 2004. [Google Scholar]
- 52.Shulman RG. Functional imaging studies: Linking mind and basic neuroscience. Am J Psychiatry. 2001;158:11–20. doi: 10.1176/appi.ajp.158.1.11. [DOI] [PubMed] [Google Scholar]
- 53.Shulman RG, Hyder F, Rothman DL. Cerebral metabolism and consciousness. C R Biol. 2003;326:253–273. doi: 10.1016/s1631-0691(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 54.Llinas R. I of the Vortex: From Neurons to Self. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 55.Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 56.Singer W. Neurobiology. Striving for coherence. Nature. 1999;397:391–393. doi: 10.1038/17021. [DOI] [PubMed] [Google Scholar]
- 57.Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- 58.Patel AB, et al. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- 59.Oz G, et al. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi IY, et al. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: On the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- 61.de Graaf RA, et al. Regional glucose metabolism and glutamatergic neurotransmission in rat brain in vivo. Proc Natl Acad Sci USA. 2004;101:12700–12705. doi: 10.1073/pnas.0405065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.