Abstract
Antimicrobial proteins comprise a significant component of the acute innate immune response to infection. They are induced by pattern recognition receptors as well as by cytokines of the innate and adaptive immune pathways and play important roles in infection control and immunomodulatory homeostasis. Lipocalin 2 (siderocalin, NGAL, 24p3), a siderophore-binding antimicrobial protein, is critical for control of systemic infection with Escherichia coli; however, its role in mucosal immunity in the respiratory tract is unknown. In this study, we found that lipocalin 2 is rapidly and robustly induced by Klebsiella pneumoniae infection and is TLR4 dependent. IL-1β and IL-17 also individually induce lipocalin 2. Mucosal administration of IL-1β alone could reconstitute the lipocalin 2 deficiency in TLR4 knockout animals and rescue them from infection. Lipocalin 2-deficient animals have impaired lung bacterial clearance in this model and mucosal reconstitution of lipocalin 2 protein in these animals resulted in rescue of this phenotype. We conclude that lipocalin 2 is a crucial component of mucosal immune defense against pulmonary infection with K. pneumoniae.
Gram-negative bacteria are a significant contributor to disease in many healthcare settings. For example, Gram-negative organisms surpassed Staphylococcus aureus as a major cause of bacteremia at a university medical center and prevalence of Gram-negative isolates continued to rise at that institution through 2003 (1). Moreover, the community is becoming a significant reservoir harboring these organisms (2, 3). Internationally, Klebsiella species constitute a large proportion of Gram-negative isolates and many strains demonstrate a disturbing trend toward extended spectrum β-lactamase expression and multidrug resistance (4).
Klebsiella pneumoniae (KP)3 is an insidious organism, causing both pulmonary and extrapulmonary invasive, suppurative infections. It has a predilection for individuals already immunodeficient from other conditions such as diabetes, chemotherapy-induced neutropenia, alcohol abuse, or organ transplant (5–7). The population of the susceptible and the number of virulent strains loom against a shrinking armamentarium of existing antibiotics. A better understanding of the innate and adaptive immune response to infection could present opportunities to enhance this response for improved antibacterial response.
Previously, our group has shown that IL-17, a T cell-derived cytokine, is induced in a mouse model of KP infection. Its over-expression improved bacterial clearance in the lung (8). Conversely, loss of IL-17 function correlated with increased susceptibility to KP, which is reflected by the early mortality of IL-17R knockout (KO) mice in this model (9). Subsequent studies have shown that IL-23 expression with downstream induction of IL-17 in KP infection was TLR4 dependent, making IL-17 an attractive prospect as a nexus in the innate and adaptive immune cytokine cross-talk.
The protective antimicrobial response elicited by IL-17 is multifactorial and remains to be fully characterized. Although it is known that IL-17 elicits expression of antimicrobial peptides such as β-defensins and S100 class proteins (10), gene expression profiling additionally showed up-regulation of Lcn2 from IL-17 stimulation or Klebsiella infection (11, 12). Lcn2 encodes for lipocalin 2 (also known as siderocalin, NGAL, 24p3, or uterocalin in the literature), a unique protein with bacteriostatic properties mediated through a mechanism divergent from that of classical antimicrobial peptides.
The insolubility of ferric (Fe(III)) ion and the toxicity of free ferrous (Fe(II)) ion result in vanishingly small amounts of free iron in the environment. Yet, it is an essential nutrient and hence efficient uptake mechanisms have evolved in many organisms. For their own metabolic processes, bacteria produce and uptake siderophores, small molecules that bind Fe(III) with exceedingly high affinity. Siderophores, such as enterobactin, have become important survival and virulence factors for bacteria, allowing them to survive in their host’s iron-poor environment. Lipocalin 2 arrests bacterial growth by sequestering enterobactin, depriving bacteria of their ability to scavenge iron, and consequently “starving” bacteria of growth-essential iron (13). This presents an elegant host antimicrobial response, centered around competition for a scarce resource. It differs from classical antimicrobial peptide killing mechanisms and, in contrast to prior antimicrobial peptide gene KO, the Lcn2 KO mouse is more susceptible to infection in an E. coli bacterial peritonitis model (14).
In the current study, we examine the role of lipocalin 2 in pulmonary defense against bacterial infection and the mechanism of its regulation in a mouse model of KP infection. In this study, we show that recombinant lipocalin 2 has in vitro activity against KP. In vivo, we find that this protein is robustly up-regulated by TLR4- and IL-1 β-dependent pathways. Moreover, lipocalin 2 is a crucial component of the early host defense against bacterial infection in the lung as demonstrated by the rescue of animals from infection after its reconstitution in lipocalin 2-deficient mouse models.
Materials and Methods
Cell lines and cytokine stimulation
HBE1, a papillomavirus-transformed human bronchial epithelial cell line (15), was a gift from Dr. K. Adler (North Carolina State University, Raleigh, NC). Primary normal human bronchial epithelial cells (NHBE) were obtained from the Human Lung Tissue Core Laboratory at the University of Pittsburgh (Pittsburgh, PA), which operates with an approved protocol under the University of Pittsburgh Institutional Review Board, or were purchased from Lonza. Cells were cultured and passaged as previously described (16) or per the manufacturer’s specifications and grown as polarized air-liquid interface cultures for stimulation. Before stimulation, cultures were serum-starved for 24 h and stimulation medium containing rIL-17A, IL-17F, and/or TNF-α (R&D Systems) was added basolaterally. IL-17 cytokines were added at 50 ng/ml (alone or cumulatively in the case of combined IL-17A and IL-17F stimulation). TNF-α was added at 10 ng/ml. Cells were cultured in stimulation medium 24 h before harvest. The results were reproduced for three different patients.
Mouse lung epithelial cells (MLE) were purchased from American Type Culture Collection (www.atcc.org; CRL-2110) and cultured per their web-posted instructions. For stimulation experiments, they were grown to confluence and serum starved 24 h before cytokine addition. Stimulation medium containing rIL-1β at 20 ng/ml (R&D Systems) and/or TNF-α at 10 ng/ml was added and cultures were incubated for 24 h before harvest.
Mice
Specific pathogen-free mice were used in all experiments and housed in specific pathogen-free conditions within animal care facilities at Children’s Hospital of Pittsburgh. All mouse experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. TLR4-deficient (JAX strain C57BL/10ScNJ, denoted TLR4 KO in the text), IL-12 p35−/− (p35 KO), IL-12/23 p40−/− (p40 KO), TIR domain-containing adaptor protein-inducing IFN-β-deficient mice (Trif KO), IL-1R-deficient (IL-1R KO), and their appropriate age-matched strain controls were purchased from The Jackson Laboratory. Strain controls were either C57BL/10 (TLR4 KO background strain) or C57BL/6 (all other KO backgrounds listed above). IL-23 p19−/− (p19 KO) were a gift from Dr. N. Ghilardi (Genentech, San Francisco, CA). MyD88 KO mice were a gift from Dr. T. Billiar (University of Pittsburgh). IL-17R KO were bred at the University of Pittsburgh (9). IL-17A KO were provided by Dr. Y. Iwakura (University of Tokyo, Tokyo, Japan) (17). IL-22 neutralization was conducted as previously described (18). Lipocalin 2 KO mice (Lcn2 KO) (19) were a gift from Dr. T. Mak (University of Toronto, Toronto, Canada). All KO mice that were bred in-house were backcrossed 8–10 generations onto a C57BL/6 background and homozygous animals from F1 or F2 intercrosses were used. Control animals were age-matched C57BL/6 animals purchased from The Jackson Laboratory.
KP strain analysis and antimicrobial assays
For siderophore analysis, overnight cultures of KP (ATCC 43816) and two other KP clinical isolates were centrifuged and the pellet was resuspended in 0.1 M NaOH to lyse. This was neutralized with 1 M Tris (pH 8.0). Aerobactin iucA promoter region was detected using the following primers: 5′-CCCAAGCAGAGTAAAGCTTGC-3′ and 5′-CGTCATGATAAT GAGAATTTTGTCG-3′. PCR controls for aerobactin gene amplification included known aerobactin-positive and -negative KP clinical isolates and a known aerobactin-negative E. coli clinical isolate (all courtesy of Dr. D. Paterson, University of Pittsburgh). For growth inhibition assays, ATCC 43816 was grown to mid-log phase and added at 103 CFU/well in Nutrient Broth (BD Difco) on a 96-well plate. Recombinant lipocalin 2 was produced as previously described (13, 20) and added to the culture in increasing dilutions. Each dilution was tested in triplicate. Serial OD600 readings were taken at specified time points. For iron-repletion assays, FeCl3 was added at the specified concentrations to the growth medium and the above assay was repeated as previously reported (13). Data shown are for ATCC 43816 only.
Infection and rescue
All infections were done with KP ATCC 43816. KP was grown and prepared as previously described (8). For intratracheal (i.t.) induction of experimental pneumonia, mice were anesthetized with isoflurane inhalation and 1 × 104 CFU delivered by retropharyngeal instillation. The results from each cohort represent the findings from four to six mice per cohort. For IL-1β reconstitution experiments, rIL-1β (3 µg/mouse) or PBS control was delivered i.t. before bacterial challenge. For lipocalin 2 reconstitution experiments, a dose titration was first done to determine the amount of lipocalin 2 required to reconstitute Lcn2 KO mice to strain control levels (data not shown). Animals were anesthetized as above and several doses of lipocalin 2 were delivered. The animals were then sacrificed 4 h later and the lungs were aseptically removed and homogenized for ELISA analysis of lipocalin 2. The optimum dose determined to reconstitute Lcn2 KO to strain control levels at 4 h of infection was 200 µg. This dose was delivered to mice before KP challenge for subsequent reconstitution experiments. Recombinant lipocalin 2 protein was tested for endotoxin contamination and found to have a low level (12 ng/ml total protein preparation or 1.2 ng/mg protein). Controls and Lcn2 KO mice received a similar dose of BSA in PBS with endotoxin added to match the level of trace contamination in the recombinant protein. After 12 h of infection, lungs were removed aseptically, each placed in 1 ml of sterile PBS and weighed to obtain a final volume of lung and PBS. Dilutions of lung homogenates were plated to determine CFU/ml and CFU/mouse was calculated from this value using the volume obtained above. Wet:dry ratios were performed on the left lungs of the mice in the cohorts indicated. Lungs were taken after 12 h of infection and weighed. Subsequently, they were dried overnight in a vacuum oven and their dry weight was obtained to perform the ratio calculation. A paired t test was performed to determine statistical significance where indicated.
Lipocalin 2 localization and quantitation
C57BL/6 mice were subjected to KP challenge as above and sacrificed at specified time points. For immunolocalization, lungs were inflated at 10 cm of H2O pressure with i.t. 10% neutral-buffered formalin, tied off, and fixed in 10% neutral-buffered formalin overnight followed by paraffin embedding. Lung sections were stained per standard protocols (21) using affinity-purified goat anti-mouse lipocalin 2 (R&D Systems) followed by HRP-conjugated rabbit anti-goat IgG (Pierce) and development using a HRP chromogen kit (Abcam) for immunohistochemical sections. For immunofluorescence, sections were additionally stained with rabbit anti-Clara cell secretory protein (CCSP, a gift from Dr. B. Stripp, Duke University, Durham, NC) and secondary Abs were Alexa Fluor 488-conjugated anti-goat IgG and Alexa Fluor 594-conjugated anti-rabbit IgG (Invitrogen).
For ELISA and Western blot analysis, cell cultures or lung tissue were homogenized in PBS, 1% Triton X-100, and Complete Mini Protease Inhibitor Cocktail (Roche). Protein concentrations were determined by bicinchoninic acid assay (Pierce) and then lysates were diluted to 1 µg/ml before application to wells for ELISA analysis. Ab sandwich ELISA was performed per standard protocol (22) by coating 96-well plates with affinity-purified anti-mouse lipocalin 2, application of 100 µl of diluted protein lysate, detection with monoclonal rat anti-mouse lipocalin 2 (R&D Systems), and HRP-conjugated goat anti-rat IgG (Invitrogen), followed by colorimetric development using a 3,3′,5,5′-tetramethylbenzidine substrate reagent set (BD Biosciences). For Western blots, 10 µg of protein lysate per well was run on NuPAGE 10% Bis-Tris gels (Invitrogen), transferred to polyvinylidene difluoride membrane, and probed with monoclonal rat anti-mouse lipocalin 2 or monoclonal rat anti-human lipocalin 2 (R&D Systems). Detection Ab was HRP-conjugated goat anti-rat IgG and blots were developed using SuperSignal West Pico ECL substrate (Pierce). Loading controls were subsequently assessed on the same blot using anti-GAPDH followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates) and development with a 5-bromo-4-chloro-3-indolyl-phosphate/NBT kit (Bio-Rad) to reveal a colorimetric result.
Results
Lipocalin 2 is produced by human bronchial epithelium (HBE) and kills KP
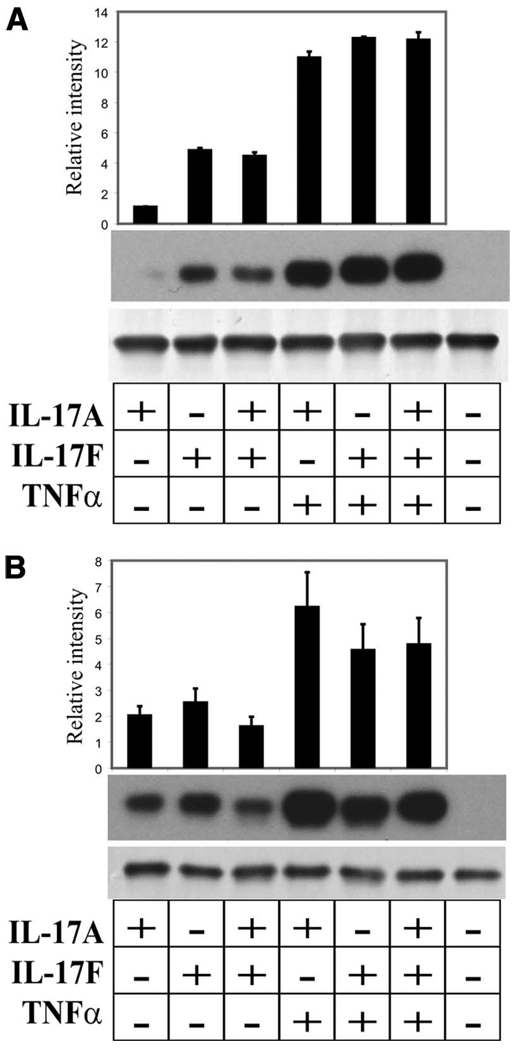
We have previously demonstrated that IL-17 can induce Lcn2 expression in mouse tracheal epithelial cells (18). To investigate whether lipocalin 2 was inducible and present at the protein level in HBE, we examined protein levels in both immortalized (HBE1) and primary NHBE. HBE1 and NHBE were grown as described and stimulated on the basolateral surface with combinations of IL-17A and IL-17F with or without synergistic activation by TNF-α. Previously, we have shown that IL-17 cytokines and TNF-α have synergistic cytokine stimulatory effects on HBE (16). In this study, we found that IL-17A or IL-17F alone induced lipocalin 2 in HBE1 and this effect was augmented with the addition of TNF-α (Fig. 1A). In primary cells, this effect was again observed from three patients tested (Fig. 1B). TNF-α stimulation alone produced similar results to the unstimulated samples (data not shown).
FIGURE 1.
Lipocalin 2 is inducible in HBE. Air-liquid interface cultures of HBE1 (A) or NHBE (B) were stimulated on the basolateral surface with IL-17A, IL-17F, or both, with and without synergistic TNF-α. In each panel, Western blot analysis of cell lysates using monoclonal anti-lipocalin 2 detects a 25-kDa protein shown in the top blot. Bottom blot in each section shows the results of probing the same blot with anti-GAPDH. Densitometry results from Western blots run on three replicates (A) or three patients (B) are above each gel in both panels. In each set, relative intensities were determined by taking absolute intensities quantified from the Western blot and dividing by the absolute intensity of the unstimulated lane on that blot.
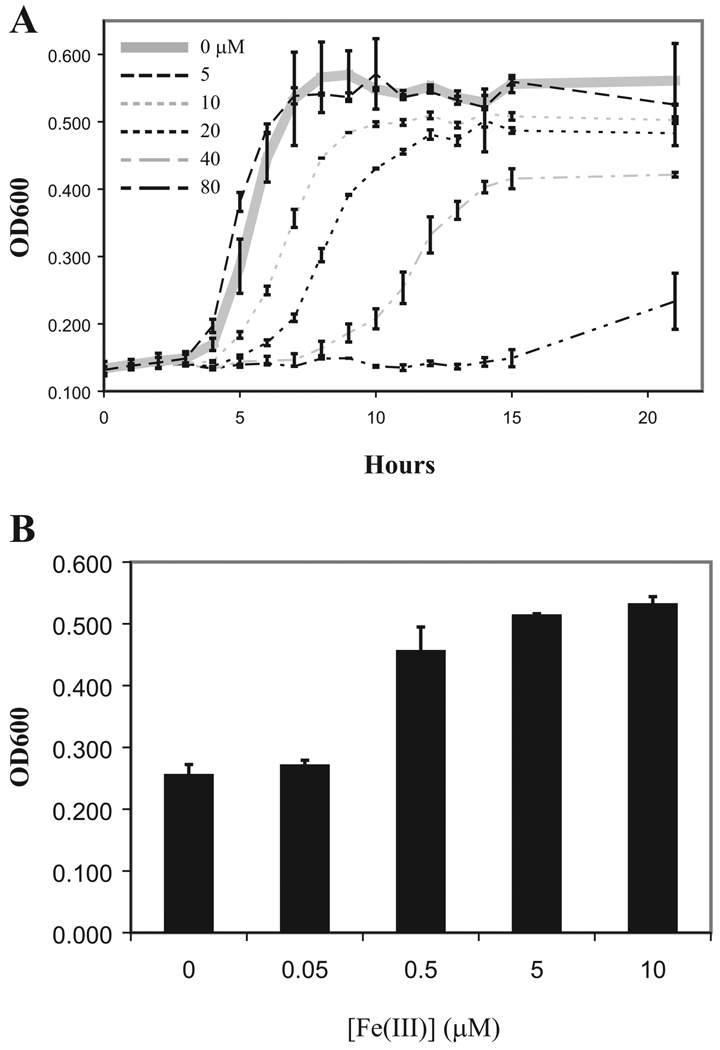
It has been described that the antimicrobial activity of lipocalin 2 is due to its ability to bind enterobactin, a phenolate class of bacterial siderophores, and discriminates among chemically distinct siderophores (14). Bacterial strains producing the siderophore aerobactin are resistant to lipocalin 2-mediated killing. We tested our strain of KP for aerobactin by PCR analysis and found that it was negative for this siderophore (data not shown). We found that recombinant lipocalin 2 exhibited dose-dependent inhibition of growth in vitro (Fig. 2A) and that this effect was reversed by iron supplementation in the medium (Fig. 2B).
FIGURE 2.
Non-aerobactin producing KP strain is inhibited by lipocalin 2. A, KP ATCC strain 43816 was seeded into wells of a 96-well plate and cultured in the presence of increasing concentrations of recombinant lipocalin 2. OD600 readings from triplicate wells of each concentration were taken as shown. Growth inhibition is seen starting at 10 µM. B, Lipocalin 2 effect is reversed with iron repletion. Assay above was repeated with 40 µM recombinant lipocalin 2 and increasing concentrations of FeCl3 in the medium. Final OD600 was taken after 15 h of culture. Results reflect averages of triplicate wells.
Lipocalin 2 protein is up-regulated in the lung following KP infection
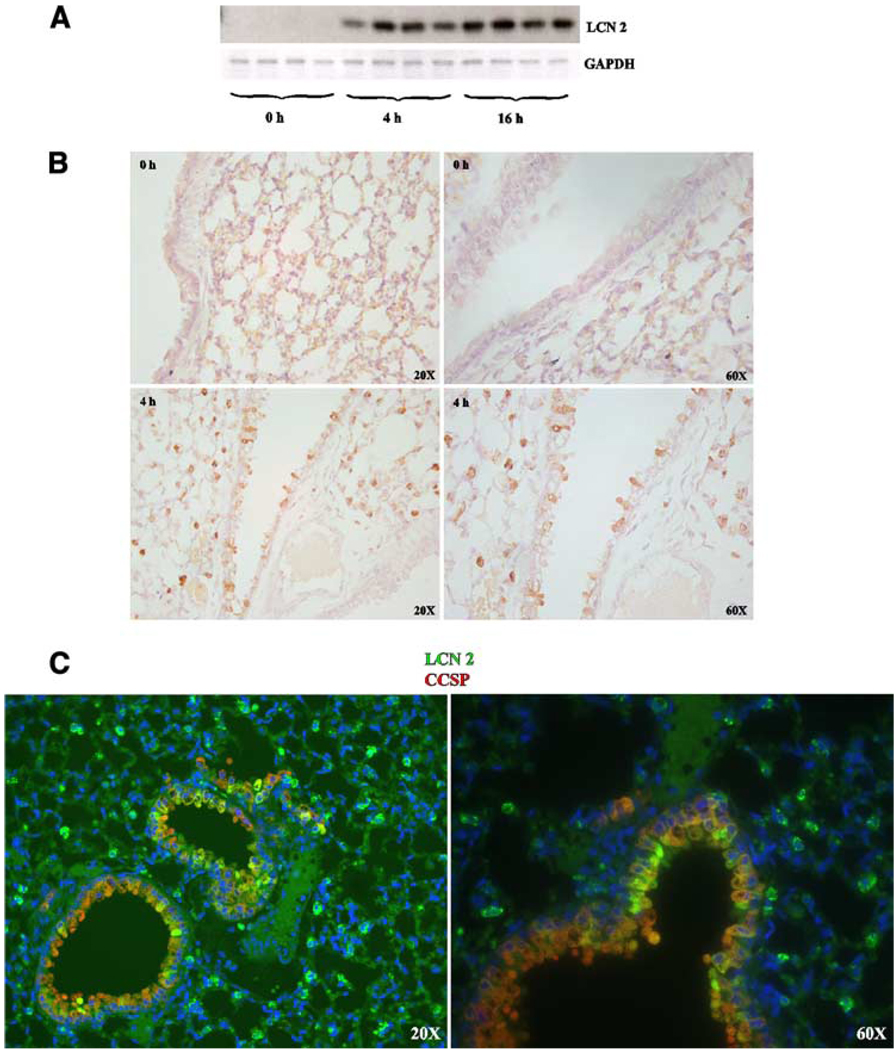
Male C57BL/6 mice were challenged with KP strain 43816 and sacrificed at three time points after infection. Lipocalin 2 protein was assayed in lung homogenates by Western blot analysis. Lipocalin 2 protein levels increased in the lung following infection as early as 4 h after infection and persisted at 16 h after infection (Fig. 3A). Lipocalin 2 elevation in the lung following infection was confirmed by immunohistochemical analysis in paraffin-embedded lungs (Fig. 3B). Immunofluorescence costaining with anti-CCSP and anti-lipocalin 2 reveal that lipocalin 2-positive cells actually consist of two distinct CCSP+ and CSSP− populations (Fig. 3C), indicating that lipocalin 2 protein is present in both epithelial and nonepithelial populations.
FIGURE 3.
Lipocalin 2 is up-regulated in the lung during KP infection in CCSP+ and CCSP− cells. A, Twelve C57BL/6 (WT) mice were infected with KP and four mice per cohort were sacrificed at 0, 4, and 16 h after infection. Top panel, Lung homogenates were analyzed by Western blot using anti-lipocalin 2. Bottom panel, Western blot was reprobed with Ab against GAPDH. Increased lipocalin 2 levels are seen as early as 4 h after infection and persist to 16 h. B, Immunohistochemical analysis of lung sections using anti-lipocalin 2 shows lipocalin 2-positive cells as early as 4 h after infection. This finding was reproduced for three animals. C, Dual immunofluorescence analysis using anti-lipocalin 2 and anti-CCSP Abs shows that lipocalin 2 is present in both CCSP+ and CCSP− cells.
Lipocalin 2 up-regulation in the lung is TLR4 dependent but IL-12 and IL-23 independent
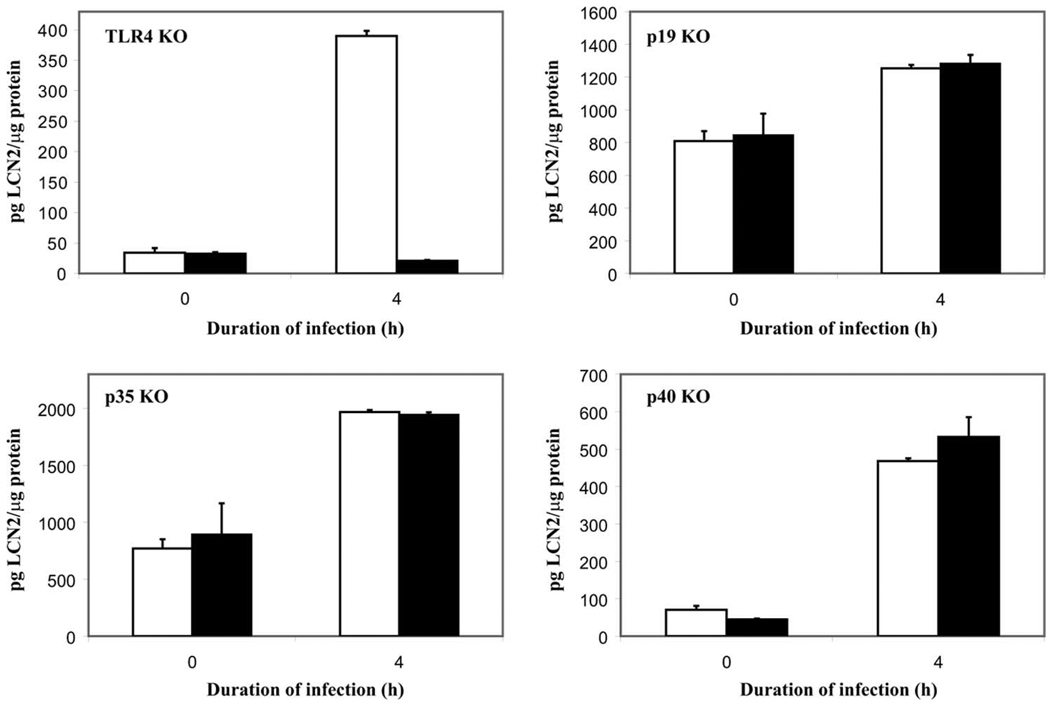
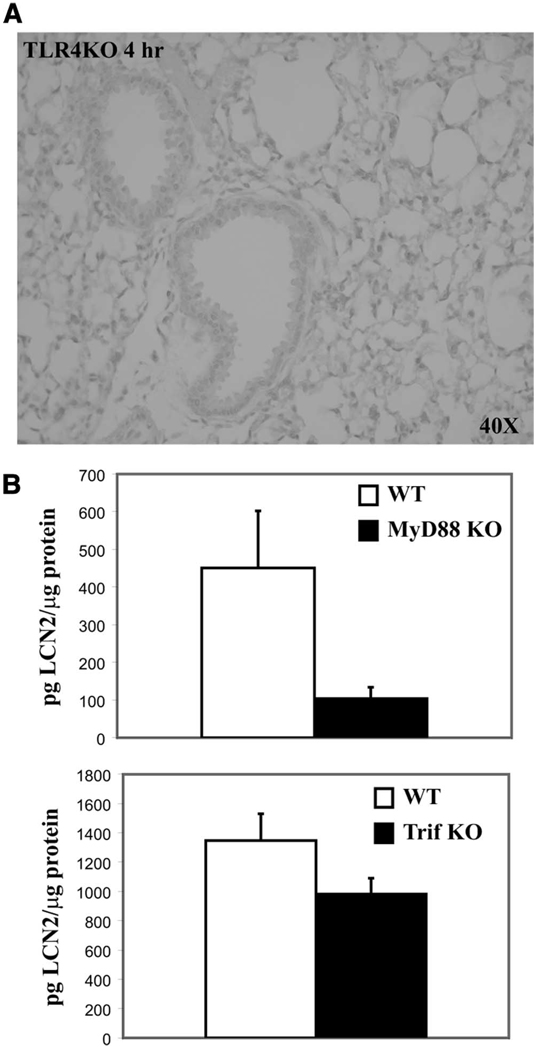
Since IL-17 induces lipocalin 2, we examined the IL-17 upstream signaling pathway to characterize pathways up-regulating lipocalin 2. It was previously shown that TLR4 activation leads to up-regulation of IL-17 via production of IL-23 (23). IL-23 is a heterodimeric cytokine in the IL-12 family that consists of a p19 subunit and a p40 subunit that IL-12 also shares. IL-12 is distinguished from IL-23 in that it has a p35 subunit bound to p40 instead (24, 25). We examined the effect of KP infection in various KO mouse models to evaluate the role of IL-12 and IL-23 in lipocalin 2 induction. TLR4-deficient (TLR4 KO), IL-23p19-deficient(p19 KO), IL-12p35-deficient (p35 KO), and IL-12/23p40-deficient (p40 KO) mice were challenged with KP as above and sacrificed at 0 and 4 h after infection. Lipocalin 2 levels after infection were nearly identical in strain control mice in comparison to p19 KO, p35 KO, and p40 KO mice, indicating that IL-12 and IL-23 are not necessary for lipocalin 2 induction in vivo (Fig. 4). However, lipocalin 2 levels were significantly reduced in TLR4 KO lung homogenates at 4 h, indicating TLR4 dependence for lipocalin 2 up-regulation in the setting of acute KP infection. Similarly, no lipocalin 2 protein could be seen in paraffin-embedded lung sections 4 h after infection (Fig. 5A). Further dissection of the TLR4 signaling pathway by examining the response of MyD88 KO and Trif KO mice to KP infection reveals a strong dependence on the MyD88-dependent pathway for protein up-regulation (Fig. 5B).
FIGURE 4.
Lipocalin 2 up-regulation is TLR4 dependent. Littermate controls (□) and KO mice (■) were infected and sacrificed after 4 h, n = 4–6 animals/cohort. Lung homogenates were analyzed by ELISA for lipocalin 2 levels. TLR4 KO animals are unable to up-regulate lipocalin 2 protein levels after 4 h of infection, while p19 KO, p35 KO, and p40 KO animals retained this ability.
FIGURE 5.
TLR4 regulation of lipocalin 2 is largely MyD88 dependent. TLR4 KO, MyD88 KO, Trif KO mice, and their strain controls (WT) were infected and sacrificed after 4 h, n = 4–6 animals/cohort. Immunohistochemistry results were reproduced for three animals. Lungs were fixed in formalin and paraffin embedded for immunohistochemical analysis or obtained for homogenization for ELISA analysis. A, Results of staining for lipocalin 2 in the TLR4 KO at 4-h infection showing no discernible lipocalin 2 signal. B, Lipocalin 2 ELISA results from lung homogenates obtained after 4 h of infection. Lipocalin 2 is diminished in both the MyD88 KO and Trif KO mice but the levels are more profoundly affected by MyD88 deficiency.
IL-17 is sufficient but not necessary for lipocalin 2 induction
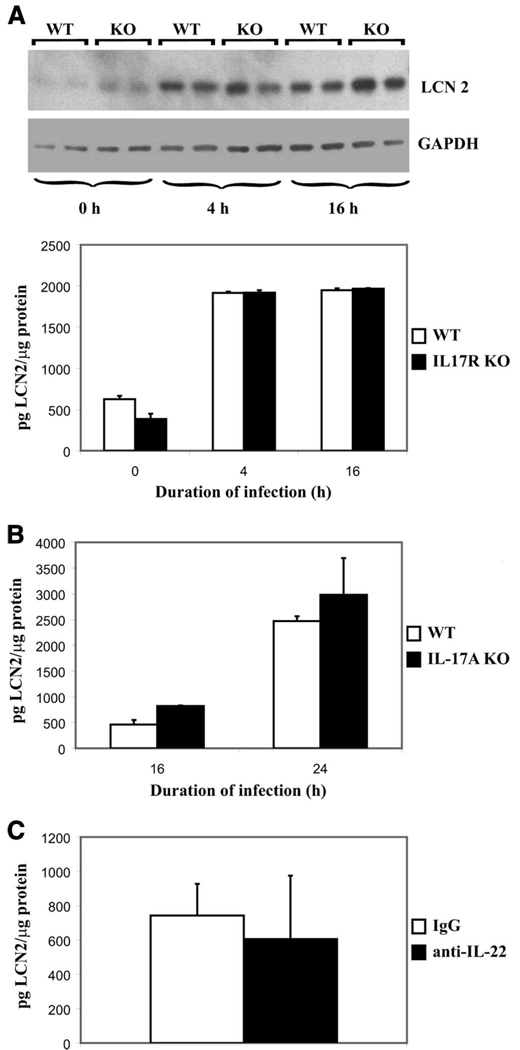
Because both IL-17 and IL-22 can up-regulate Lcn2 in both human and murine respiratory epithelial cells (18), we next examined whether these cytokines are required for lipocalin 2 induction in vivo. We studied lipocalin 2 levels in the setting of IL-17 or IL-22 deficiency and found that although each cytokine can induce lipocalin 2, neither is absolutely necessary in vivo. IL-17R KO up-regulated lipocalin 2 at both 4 and 16 h after KP infection similar to wild- type (WT) mice (Fig. 6A). Because peak IL-17 cytokine expression occurs within 24 h, later time points were examined to see whether IL-17 played a role in the stabilization of lipocalin 2 levels at later time points. IL-17A KO mice challenged with KP were also able to up-regulate lipocalin 2 at 4 (data not shown), 16, and 24 h after infection, similar to their strain controls (Fig. 6B). Moreover, neutralization of IL-22 in WT mice before infection also minimally affected lipocalin 2 up-regulation at 4 h (Fig. 6C).
FIGURE 6.
Th17 cytokines are not necessary for lipocalin 2 induction. A, Six strain controls (WT) and six IL-17R KO mice were infected and two animals of each genotype were sacrificed at 0, 4, and 16 h of infection. Western blot analysis was performed on lung homogenates. Top panel shows anti-lipocalin 2 probe with anti-GAPDH-loading control. Bottom panel shows ELISA results of the same infection repeated with three animals per cohort. B, Strain controls (WT) and IL-17A KO animals were subjected to infection and sacrificed at 16 and 24 h (four animals per cohort). Lung homogenates were analyzed for lipocalin 2 levels by ELISA. C, Six C57BL/6 (WT) animals were given neutralizing doses of anti-IL-22 (three animals) or IgG control (three animals) and infected and sacrificed after 4 h of infection. Lung homogenates were analyzed for lipocalin 2 levels by ELISA.
IL-1β induces lipocalin 2
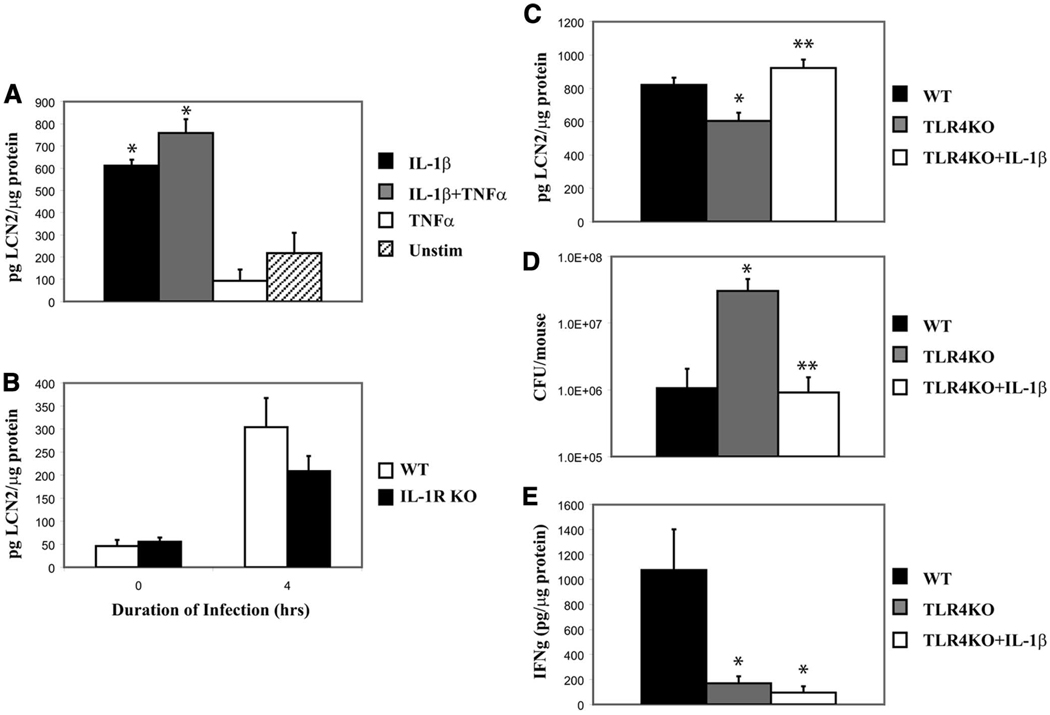
Based on our findings of the MyD88 role in lipocalin 2 up-regulation, we studied the potential contribution of another MyD88-dependent signaling pathway, IL-1. Previous studies have implicated a role for IL-1β in lipocalin 2 induction in vitro (26–28), which we confirmed by stimulating MLE cells with IL-1β. IL-1β induced lipocalin 2 protein in MLE cells with a mild synergistic effect from added TNF-α (Fig. 7A). Next, we examined the role of IL-1R-mediated signaling by infecting IL-1R KO mice and their strain controls and examining lung homogenates for lipocalin 2. The IL-1R KO mice showed a mild defect in lipocalin 2 up-regulation in response to KP infection (Fig. 7B). We next examined whether IL-1β reconstitution in TLR4 KO could restore lipocalin 2 expression in vivo. We delivered rIL-1β to the lung before KP challenge in TLR4 KO mice. IL-1β indeed reconstituted lipocalin 2 levels in the TLR4-deficient mouse challenged with KP (Fig. 7C) and IL-1β treatment resulted in significantly lower bacterial burden in the lung (Fig. 7D). Interestingly, although IFN-γ was up-regulated in the WT infected lung, the mechanism of IL-1β-mediated lipocalin 2 rescue was independent of IFN-γ (Fig. 7E).
FIGURE 7.
IL-1β induces lipocalin 2 and rescues TLR4 KO from KP infection in an IFN-γ-independent manner. A, MLE were stimulated in triplicate with IL-1β with or without TNF-α. Cell lysates were assayed for lipocalin 2 levels by ELISA. B, Three strain controls (WT) and three IL-1R KO mice were infected and lung homogenates were analyzed for lipocalin 2 levels. IL-1R KO mice show decreased levels of lipocalin 2 after infection. C, Six WT and six TLR4 KO mice were infected and another cohort of TLR4 KO mice (six animals) was given IL-1β. Animals were sacrificed at 12 h of infection and lung homogenates were assayed for lipocalin 2. Lipocalin 2 levels in the TLR4 KO again were significantly diminished compared with WT and significantly increased in TLR4 KO mice that received IL-1β. D, TLR4 KO mice receiving IL-1β were rescued from infection as demonstrated by bacterial load after 12 h of infection. E, WT mice up-regulate IFN-γ during infection, but TLR4 KO, including TLR4 KO receiving IL-1β do not; therefore, the mechanism of IL-1β - mediated rescue from infection is not due to IFN-γ up-regulation. *, Statistical significance compared with unstimulated or WT and **, statistical significance compared with untreated KO.
Lipocalin 2 is required for lung defense against KP
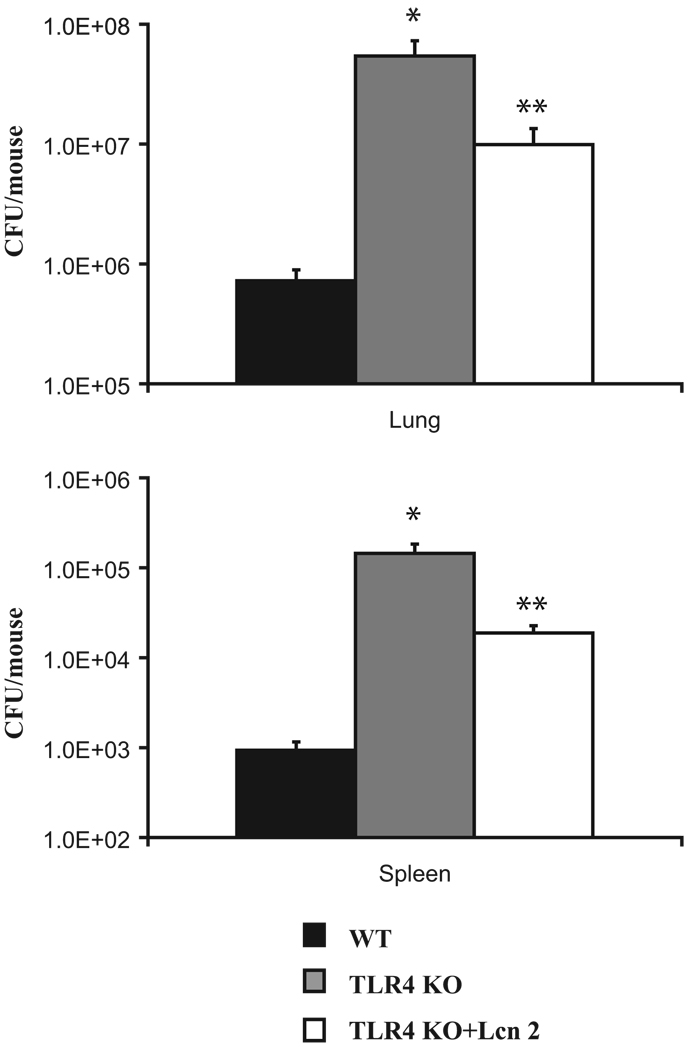
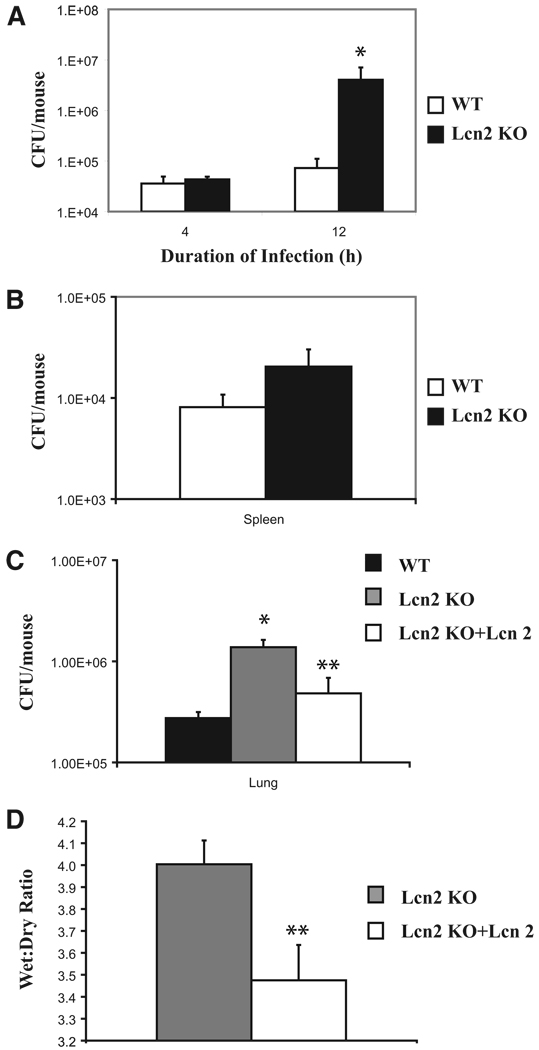
Lipocalin 2 deficiency confers profound susceptibility to bacterial sepsis. This has been shown in the lipocalin 2 KO mouse by i.p. E. coli injection (14, 19). We investigated the role of lipocalin 2 in localized organ defenses by examining its effects in our model of pulmonary KP infection. Lung reconstitution of lipocalin 2 protein in the TLR4 KO led to a significantly reduced bacterial burden in the lungs and dissemination to the spleen (Fig. 8). After KP challenge, TLR4 KO mice had significantly higher lung CFU with more extrapulmonary dissemination compared with their strain control littermates. Lcn2 KO show decreased KP clearance as well. Lung CFU in Lcn2 KO mice are significantly higher than in controls and they also demonstrate a trend toward more extrapulmonary spread of infection (Fig. 9, A and B). Lung reconstitution of lipocalin 2 protein in Lcn2 KO significantly decreased bacterial burden (Fig. 9C). Finally, we found that reconstitution of lipocalin 2 in the Lcn2 KO mice resulted in significantly less lung injury from infection as measured by lung wet:dry ratios (Fig. 9D).
FIGURE 8.
Recombinant lipocalin 2 rescues TLR4 KO from KP infection. Two Lcn2 KO cohorts (six mice per group) and their strain controls (six mice) were infected with KP as before. One Lcn2 KO cohort was given recombinant lipocalin 2 while the other two cohorts received PBS plus BSA. Mice were sacrificed at 12 h of infection and lung homogenates were plated to determine CFU. Reconstitution of the TLR4 KO results in statistically significant reduction in lung bacterial load and spleen dissemination of KP. *, Statistical significance compared with WT and **, statistical significance compared with untreated KO.
FIGURE 9.
Recombinant lipocalin 2 rescues Lcn2 KO from KP infection. A, Lcn2 KO mice were infected and are susceptible as demonstrated by bacterial loads in lung homogenates after 4 and 12 h of infection (four animals per cohort). B, Extrapulmonary dissemination of KP to the spleen is also increased in the same Lcn2 KO animals compared with controls at 12 h of infection. C, Reconstituting Lcn2 KO mice with recombinantly expressed lipocalin 2 protein (Lcn 2) results in statistically significant reduction in lung bacterial load at 12 h of infection in these mice compared with Lcn2 KO mice that did not receive Lcn 2 (six animals per cohort). D, Wet:dry ratios in Lcn2 KO mice receiving Lcn 2 are significantly lower compared with Lcn2 KO mice that did not receive Lcn 2. Data reflect six mice per group. *, Statistical significance compared with WT and **, statistical significance compared with untreated KO.
Discussion
Klebsiella is a virulent Gram-negative organism, causing disseminated infection with a relatively small inoculum, particularly in an immunosuppressed host. Infections with Klebsiella are becoming an increasing concern as escalating antibiotic resistance becomes more widespread. Rarely does this organism stay localized to one organ system, instead causing multiorgan system infection as a result of its ability to subvert innate and humoral host defenses. Understanding bacterial host interactions may lead to new treatment modalities that have been heretofore not considered.
Previously, our group had established a model of this infection as an example of Gram-negative pneumonia to study the host immune response and investigate new avenues of antimicrobial defense. In previous studies, we found that IL-17, a T cell-derived cytokine, played a key role in controlling infection (8, 9, 23, 24). Furthermore, IL-17 induces antimicrobial proteins such as β-de-fensin (29). We postulated that this was a major mechanism of antimicrobial defense elicited by IL-17 and set about to characterize novel antimicrobial proteins induced by IL-17. Microarray data on IL-17-treated cells and Klebsiella-infected lung tissue revealed up-regulation of the gene for lipocalin 2 (11, 30).
Lipocalin 2 is so named because of its analogy to a family of proteins with a conserved tertiary structure. Its label has metamorphosed as independent investigations have assigned various descriptive appellations to the protein. Initially discovered and named 24p3 in the mouse, it was found by differential screening of a cDNA library from kidney cells stimulated out of G0 cell cycle arrest by SV40 large T Ag (31). This finding led to its putative assignment as an oncogene because SV40 large T Ag is known to inactivate p53 (32). However, it appears to be an acute phase response protein up-regulated with a variety of situations and stimuli including parturition (33), renal injury (34), LPS (35), serum, fibroblast growth factor, PG, phorbol ester, dexamethasone, and turpentine injection (36, 37).
Its ligand was discovered when it was found that bacterial-produced recombinant lipocalin 2 purified as a red-colored compound in contrast to the colorless compound made in a baculovirus expression system. This led to the identification of enterobactin, an iron-binding chromophore (known as a siderophore) as its ligand (13, 38). Nearly all living things require iron for metabolism. However, the more soluble Fe(II) form of it is harmful because of its potential to generate oxidizing radicals (39, 40). Mammals protect themselves from this oxidative damage by sequestering a majority of their iron stores in protective heme-containing proteins. This, coupled with the low solubility of the Fe(III) form, leads to only trace amounts being available in the environment. Bacteria have similarly evolved mechanisms to scavenge this rare resource by elaborating low molecular weight carriers called siderophores which bind free iron with remarkably high affinity (41). Lipocalin 2 binds catecholate-type siderophores such as enterobactin made by E. coli. Interestingly, lipocalin 2 was shown to have bacteriostatic effects on E. coli and this effect was abolished when iron or siderophore was replenished in the medium. Thus, lipocalin 2 can arrest E. coli growth by depriving bacteria of their iron- uptake ability, distinguishing it from the conventional pore-forming mechanisms of cationic antimicrobial peptides.
In this work, we examined the role of lipocalin 2 at the protein level in an in vivo model of pulmonary KP infection and study its regulation. Previous studies have shown a role for this protein in other infections such as Salmonella (42, 43), Chlamydia (44), and Mycobacterium tuberculosis (45, 46) and have similarly attributed the induction of lipocalin 2 to TLR-dependent signaling and subsequent T cell activation pathways. Indeed, we have recently confirmed the ability of Th17 cytokines, IL-17 and IL-22 to induce Lcn2 and inhibit Klebsiella in an in vitro model (18). To our knowledge, this current study demonstrates the most comprehensive in vivo study of the lipocalin 2 role and regulation in a pulmonary model of infection. Moreover, we expand the known in vivo antibacterial activity of lipocalin 2 to the opportunistic pathogen used in this study and establish the in vivo relevance of MyD88-dependent signaling in the regulation of lipocalin 2 at the protein level.
Curiously, although IL-17 was sufficient for lipocalin 2 induction in bronchial epithelial cells in vitro, it was not necessary for induction in vivo. This is consistent with other findings implicating various cytokines such as Il-1β, IL-1α (47), and IFN-γ (43) in the induction of lipocalin 2. Like other antimicrobial proteins, it is reasonable to invoke an evolutionarily conserved mechanism of redundant induction pathways. Early innate inflammatory cytokines, as well as adaptive cytokines from the Th1 and Th17 pathways, specialized against bacterial defense may all independently induce lipocalin 2. Using in vitro models, two groups have shown that lipocalin 2 can be induced in a NF-κB- dependent manner by IκB-ζ, a transcription factor induced by TLR and IL-1 pathways (28, 48). Based on the convergence of TLR and IL-1R signaling on inflammasome activation and magnification of the IL-1β response by enzymatic activation in these pathways, as well as our observation of profound TLR4 dependence of lipocalin 2 in early infection, we examined the role of IL-1β in our model. Exogenous IL-1β alone was able to completely reconstitute lipocalin 2 levels equivalent to WT levels and this had a protective effect. The mechanism of this reconstitution was not IFN-γ dependent. Furthermore, we showed that restoration of lipocalin 2 in its protein-deficient models, TLR4 KO and Lcn2 KO, reduced their bacterial burden to a WT phenotype. In addition, it significantly reduced the amount of lung injury. This is shown by the lower wet:dry ratio in the lipocalin 2-reconstituted Lcn2 KO which reflects a lower amount of lung edema and injury.
Early lipocalin 2 induction in this model is dependent on the TLR4 pathway. This is consistent with previous studies from our group that TLR4 regulates nearly 75% of the gene induction in the lung in the first 4 h of this infection (11). Lungs in the i.t. IL-1β experiments were harvested 12 h after infection and it is clear from Fig. 7C that lipocalin 2 levels eventually do increase in the TLR4 KO at later time points, albeit still at significantly reduced levels compared with controls. Despite the later presence of lipocalin 2 in this model, it appears inadequate in the defense against Klebsiella infection in the TLR4 KO. Thus, the early presence of lipocalin 2 in the lungs is crucial for the defense against bacterial pneumonia.
This effect might be due to more than its simple, yet elegant mechanism of antimicrobial activity. In fact, some KP strains have been shown to elaborate alternate siderophore systems, including yersiniabactin and salmochelin, each, respectively, important for in vivo virulence and evasion of lipocalin 2 (49, 50). Salmochelin has been shown to increase E. coli virulence in an i.p. infection model and it does so in a lipocalin 2-independent manner (49). Although it is possible that KP 43816 may express salmochelin, it does not appear to impact lipocalin 2 function in our i.t. model of pulmonary infection which may represent unique innate immune mechanisms compared with an i.p. peritonitis model.
Lipocalin 2 might possibly act in an autocrine-paracrine manner, itself capable of eliciting a robust, early inflammatory cytokine burst of IL-1β, and other chemokines. In multiplex cytokine analysis of Lcn2 KO reconstituted with recombinant lipocalin 2, we observed magnified induction of IL-1α, IL-1β, IFN-γ, and IL-17 compared with the Lcn2 KO animals that did not receive recombinant protein (data not shown). All of these cytokines have been shown either in the current study or by others to independently up-regulate and/or amplify the antimicrobial effect of lipocalin 2 (43, 47). Indeed, our IL-1β rescue experiments show that the TLR4 defect could be overcome by robust stimulation via alternate MyD88-dependent pathways. Also, lipocalin 2 administration to Lcn2 KO animals increased GM-CSF, IL-12, and IL-9 levels, which were reduced in infected Lcn2 KO animals relative to controls (data not shown). The early, acute presence of lipocalin 2 may give the host advanced warning of infection, launch a more specialized adaptive response further magnifying antimicrobial protein expression, and ultimately clearing infection through a combined cellular and humoral response.
An aspect that warrants further investigation is the role of lipocalin 2 in its different iron-bound states as well as its status of binding to matrix metalloproteinase 9 (MMP-9). Apo-lipocalin 2 (ligand and iron-free) was produced for the experiments done in the current study. It would be reasonable to infer that mammalian-derived lipocalin 2 would also be in an apo- form as there should be no endogenous siderophore production in the host. Studying the role of apo- and holo-lipocalin 2 (ligand or iron-bound) and their next protein targets and molecular fates could reveal interesting insights on innate immune signaling and homeostasis. However, the current status of technology does not easily allow determination of lipocalin 2 iron-binding states in vivo.
Lipocalin 2 has been associated with confusing and contradictory roles in human disease. Although it is clear that up-regulation of lipocalin 2 improves host defense against infections such as E. coli (14), M. tuberculosis (46), and Salmonella typhimurium (43) through its augmentation of neutrophil and macrophage function in both human and mouse models, its role in other disease states is not so clear. It is up-regulated and associated with the worse prognosis in various neoplastic states (51–53) yet is linked to prodifferentiation and antiangiogenic roles (54). Lipocalin 2 was originally copurified with MMP-9 from human neutrophils (55–57) and the state of the lipocalin 2/MMP-9 complex and its iron-bound state may determine the variable role lipocalin 2 seems to have in infection vs neoplasia and differentiation. The binding of MMP-9 by lipocalin 2 is thought to have a stabilizing influence on MMP-9 activity (58). This might be difficult to study in a mouse model as the domain responsible for heterodimerization with MMP-9 appears to be absent in mouse lipocalin 2 (59). However, lipocalin 2 and MMP-9 have been colocalized in mouse models of inflammation (60). An interesting future possibility would be to create a lipocalin 2 knock-in mouse containing heterodimerization domains for MMP-9 so as to better approximate human models of inflammatory disease.
Supplementary Material
Acknowledgments
Drs. Thorsten Berger and Tak Mak at the University of Toronto generated and generously provided us with the lipocalin 2 KO mice that were used in our studies. Dr. Matthew Clifton (Strong Lab, Seattle, WA) is appreciated for his mentorship and support in generating the recombinant proteins used. Many thanks to Dr. David Paterson for providing clinical Klebsiella isolates and Dr. Barry Stripp for providing the anti-CCSP Ab used in the immunofluorescence experiments.
Footnotes
This work is supported by grants from the National Institutes of Health, K08 HL089189-01 (to Y.R.C.) and R01HL079142, P30DK072506, and 5P50HL084932 (J.K.K.).
Abbreviations used in this paper: KP, Klebsiella pneumoniae; KO, knockout; CCSP, Clara cell secretory protein; HBE, human bronchial epithelium; lcn2/Lcn2, lipocalin 2; NHBE, normal human bronchial epithelial cell; MLE, mouse lung epithelial cell; i.t., intratracheal; MMP-9, matrix metalloproteinase 9.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Albrecht SJ, Fishman NO, Kitchen J, Nachamkin I, Bilker WB, Hoegg C, Samel C, Barbagallo S, Arentzen J, Lautenbach E. Reemergence of Gram-negative health care-associated bloodstream infections. Arch. Intern. Med. 2006;166:1289–1294. doi: 10.1001/archinte.166.12.1289. [DOI] [PubMed] [Google Scholar]
- 2.Wiener J, Quinn JP, Bradford PA, Goering RV, Nathan C, Bush K, Weinstein RA. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–523. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 3.Yinnon AM, Butnaru A, Raveh D, Jerassy Z, Rudensky B. Klebsiella bacteraemia community versus nosocomial infection. Q. J. Med. 1996;89:933–941. doi: 10.1093/qjmed/89.12.933. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Ko W-C, Gottberg AV, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial infections. Ann. Intern. Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chen KY, Hsueh PR, Liaw YS, Yang PC, Luh KT. A 10-year experience with bacteriology of acute thoracic empyema: emphasis on Klebsiella pneumoniae in patients with diabetes mellitus. Chest. 2000;117:1685–1689. doi: 10.1378/chest.117.6.1685. [DOI] [PubMed] [Google Scholar]
- 6.Kofteridis DP, Papadakis JA, Bouros D, Nikolaides P, Kioumis G, Levidiotou S, Maltezos E, Kastanakis S, Kartali S, Gikas A. Nosocomial lower respiratory tract infections: prevalence and risk factors in 14 Greek hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:888–891. doi: 10.1007/s10096-004-1245-y. [DOI] [PubMed] [Google Scholar]
- 7.Velasco E, Byington R, Martins CA, Schirmer M, Dias LM, Goncalves VM. Comparative study of clinical characteristics of neutropenic and non-neutropenic adult cancer patients with bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:1–7. doi: 10.1007/s10096-005-0077-8. [DOI] [PubMed] [Google Scholar]
- 8.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 9.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of Toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect. Immun. 2005;73:532–545. doi: 10.1128/IAI.73.1.532-545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 13.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 14.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 15.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA, Jr., Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am. J. Physiol. 1993;264:C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 16.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 18.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HEH, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abergel RJ, Clifton MC, Pizarro JC, Warner JA, Shuh DK, Strong RK, Raymond KN. The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport 1. J. Am. Chem. Soc. 2008;130:11524–11534. doi: 10.1021/ja803524w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofman F. Current Protocols in Immunology. 2002 Ed. New York: Wiley; 2003. Immunohistochemistry. Chapter 21, Unit 21.4. [DOI] [PubMed] [Google Scholar]
- 22.Hornbeck P. Current Protocols in Immunology. New York: Wiley; 2003. Enzyme-linked immunosorbent assays. Chapter 2, Unit 2.1. [DOI] [PubMed] [Google Scholar]
- 23.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 26.Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kκB. Am. J. Pathol. 2006;169:2245–2253. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraman A, Roberts KA, Yoon J, Yarmush DM, Duan X, Lee K, Yarmush ML. Identification of neutrophil gelatinase-associated lipocalin (NGAL) as a discriminatory marker of the hepatocyte-secreted protein response to IL-1β: a proteomic analysis. Biotechnol. Bioeng. 2005;91:502–515. doi: 10.1002/bit.20535. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo S, Yamazaki S, Takeshige K, Muta T. Crucial roles of binding sites for NF-κB and C/EBPs in IκB-ζ-mediated transcriptional activation. Biochem. J. 2007;405:605–615. doi: 10.1042/BJ20061797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao C-Y, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 30.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17-and TNF-α-induced genes in bone cells. J. Leukocyte Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 31.Hraba-Renevey S, Turler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989;4:601–608. [PubMed] [Google Scholar]
- 32.Flower DR, North ACT, Attwood TK. Mouse oncogene protein 24p3 is a member of the lipocalin protein family. Biochem. Biophys. Res. Commun. 1991;180:69–74. doi: 10.1016/s0006-291x(05)81256-2. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Ryon J, Nilsen-Hamilton M. Uterocalin: a mouse acute phase protein expressed in the uterus around birth. Mol. Reprod. Dev. 1997;46:507–514. doi: 10.1002/(SICI)1098-2795(199704)46:4<507::AID-MRD9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2008;17:127–132. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 35.Meheus LA, Fransen LM, Raymackers JG, Blockx HA, Van Beeumen JJ, Van Bun SM, Van de Voorde A. Identification by microsequencing of lipopolysaccharide-induced proteins secreted by mouse macrophages. J. Immunol. 1993;151:1535–1547. [PubMed] [Google Scholar]
- 36.Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J. Biol. Chem. 1995;270:22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- 37.Owen HC, Roberts SJ, Ahmed SF, Farquharson C. Dexamethasone-induced expression of the glucocorticoid response gene lipocalin 2 in chondrocytes. Am. J. Physiol. 2008;294:E1023–E1034. doi: 10.1152/ajpendo.00586.2007. [DOI] [PubMed] [Google Scholar]
- 38.Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 39.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 40.Gutteridge JM, Rowley DA, Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts: detection of “free” iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem. J. 1981;199:263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagg A, Neilands JB. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Baumler AJ. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 2008;76:2008–2017. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-γ limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez N, Mages J, Dietrich H, Wantia N, Wagner H, Lang R, Miethke T. MyD88-dependent changes in the pulmonary transcriptome after infection with Chlamydia pneumoniae. Physiol. Genomics. 2007;30:134–145. doi: 10.1152/physiolgenomics.00011.2007. [DOI] [PubMed] [Google Scholar]
- 45.Landro L, Damas JK, Flo TH, Heggelund L, Ueland T, Tjonnfjord GE, Espevik T, Aukrust P, Froland SS. Decreased serum lipocalin-2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin. Exp. Immunol. 2008;152:57–63. doi: 10.1111/j.1365-2249.2008.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bando M, Hiroshima Y, Kataoka M, Shinohara Y, Herzberg MC, Ross KF, Nagata T, Kido J-i. Interleukin-1α regulates antimicrobial peptide expression in human keratinocytes. Immunol. Cell Biol. 2007;85:532–537. doi: 10.1038/sj.icb.7100078. [DOI] [PubMed] [Google Scholar]
- 48.Cowland JB, Muta T, Borregaard N. IL-1β-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IκB-ζ. J. Immunol. 2006;176:5559–5566. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- 49.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. USA. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawlor MS, O’Connor C, Miller VL. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 2007;75:1463–1472. doi: 10.1128/IAI.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arlinghaus R, Leng X. Requirement of lipocalin 2 for chronic myeloid leukemia. Leuk. Lymphoma. 2008;49:600–603. doi: 10.1080/10428190701859664. [DOI] [PubMed] [Google Scholar]
- 52.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur. J. Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Villalva C, Sorel N, Bonnet ML, Guilhot J, Mayeur-Rousse C, Guilhot F, Chomel JC, Turhan AG. Neutrophil gelatinase-associated lipocalin expression in chronic myeloid leukemia. Leuk. Lymphoma. 2008;49:984–988. doi: 10.1080/10428190801942360. [DOI] [PubMed] [Google Scholar]
- 54.Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty A, Bresalier RS, et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100–6108. doi: 10.1158/0008-5472.CAN-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- 56.Kjeldsen L, Johnsen A, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 57.Triebel S, Blaser J, Reinke H, Tschesche H. A 25 kDa α2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. doi: 10.1016/0014-5793(92)81511-j. [DOI] [PubMed] [Google Scholar]
- 58.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–3335. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 59.Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 60.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.