Abstract
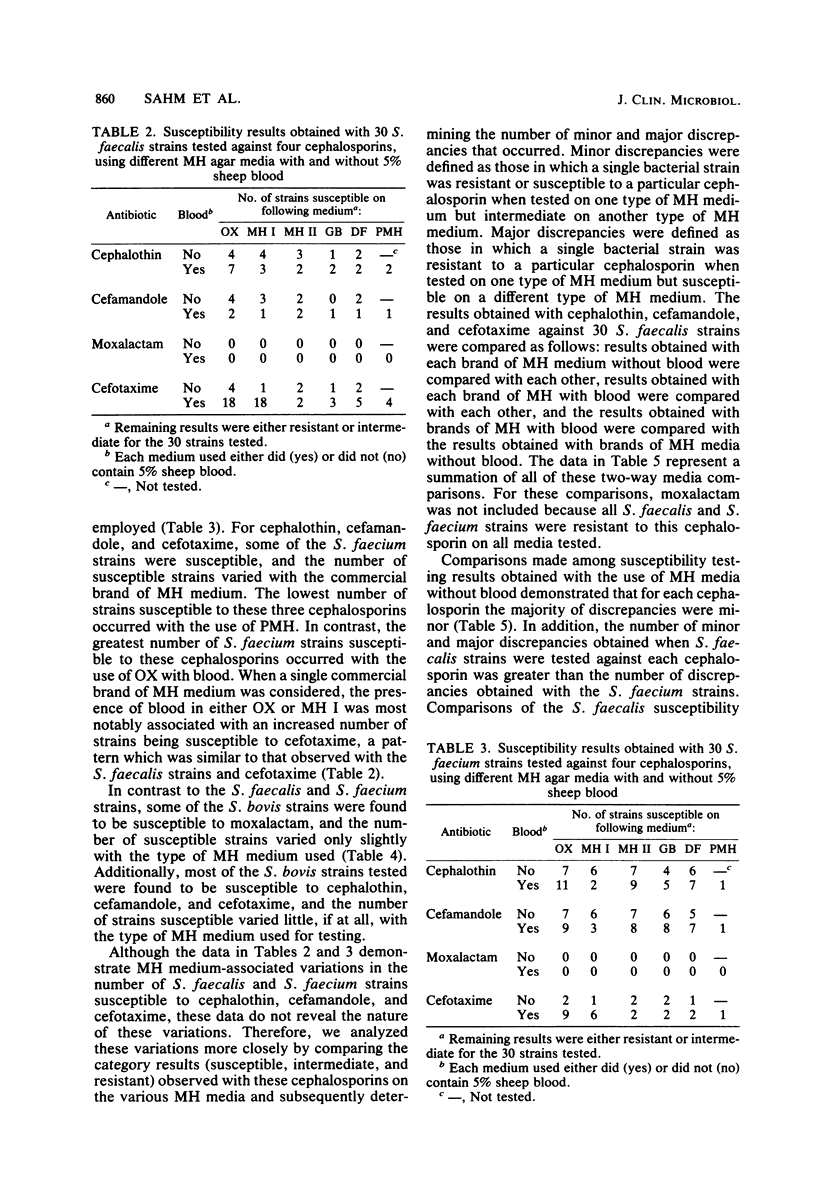
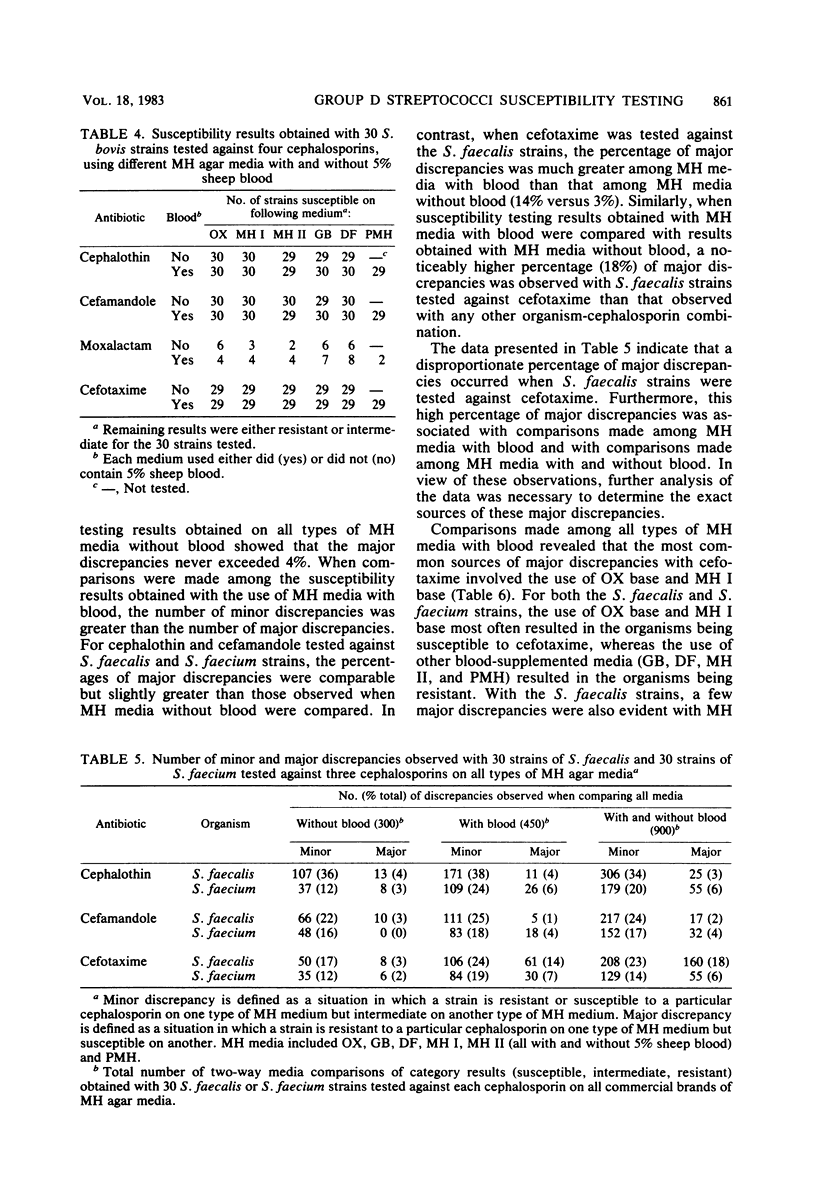
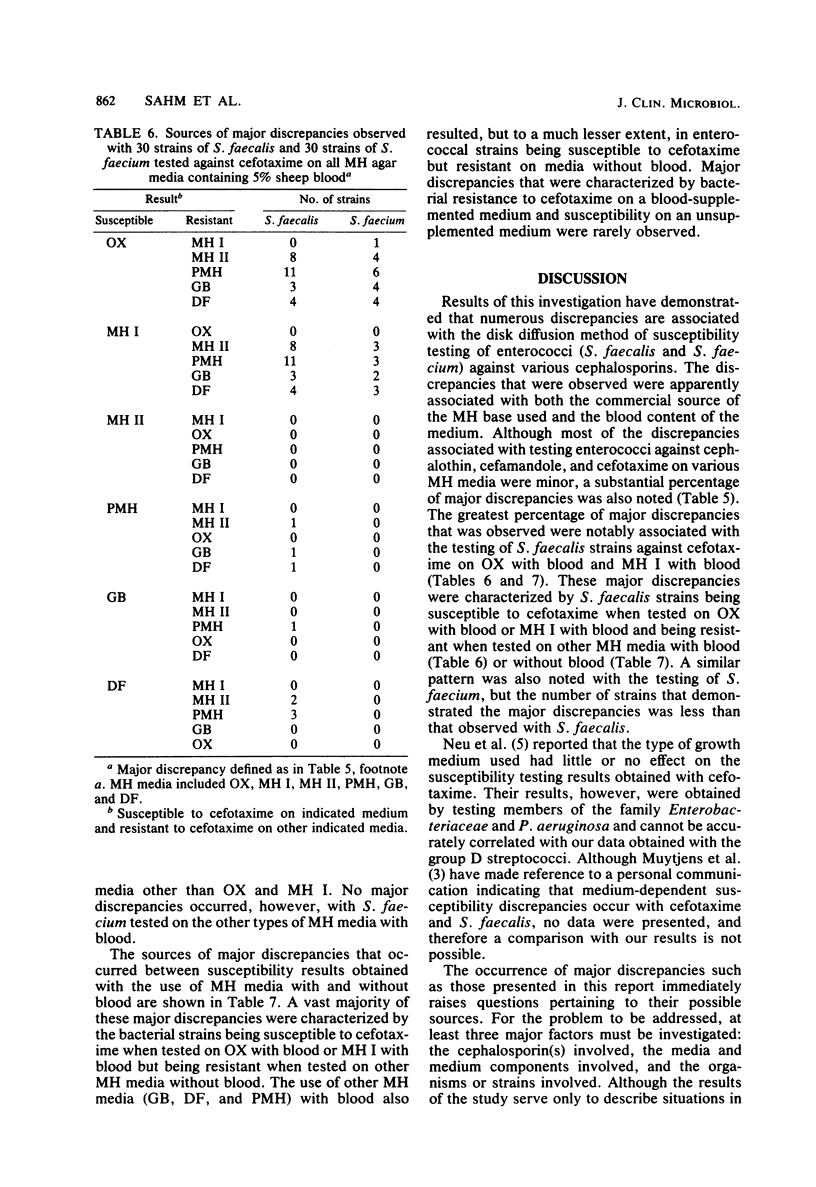
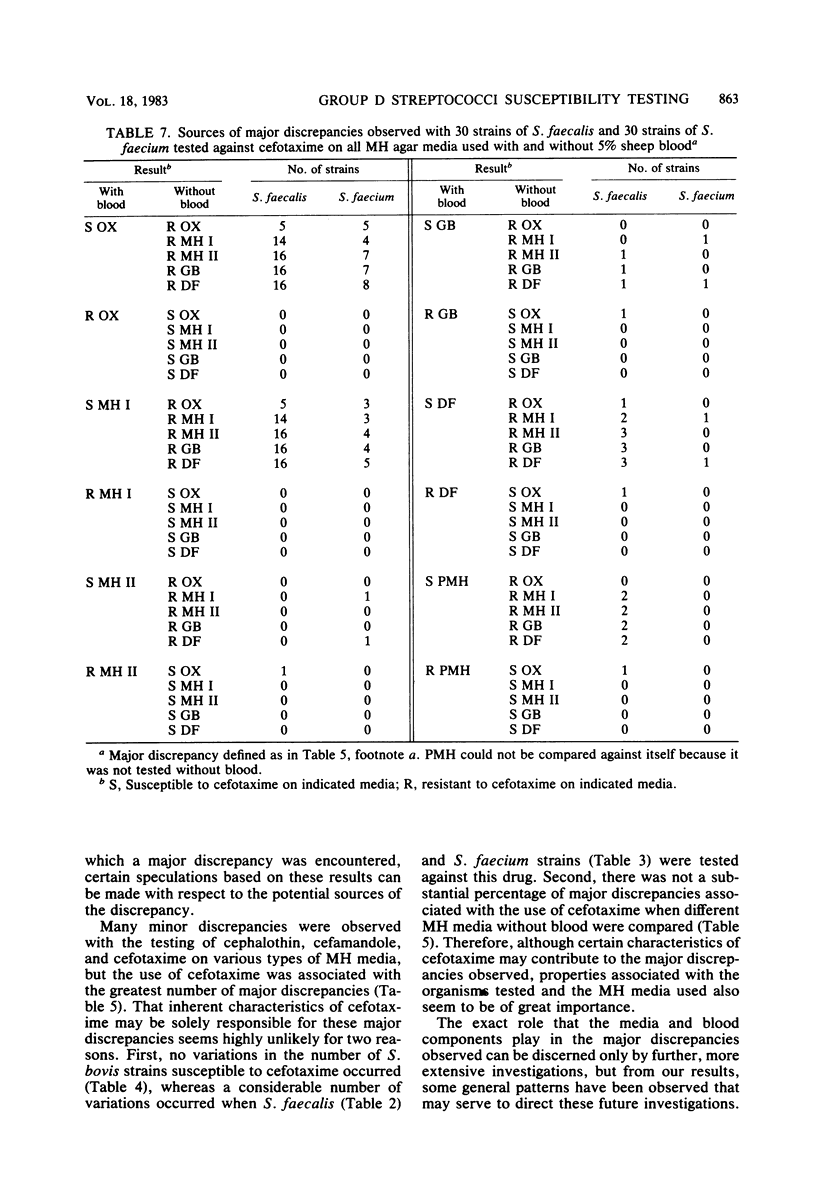
Mueller-Hinton (MH) agar media from various commercial sources, either supplemented or not supplemented with 5% sheep blood, were studied to determine their effect on disk diffusion susceptibility testing results obtained with 90 strains of group D streptococci and four cephalosporins. The cephalosporins investigated included cephalothin, cefamandole, moxalactam, and cefotaxime. Results showed that a number of Streptococcus faecalis and Streptococcus faecium strains were susceptible to cephalothin, cefamandole, and cefotaxime, but the number varied with both the commercial source and blood content of the MH medium used. Regardless of the MH medium used, none of the S. faecalis or S. faecium strains were found to be susceptible to moxalactam. The apparently medium-associated variations in the number of strains susceptible to cephalothin, cefamandole, and cefotaxime were largely due to minor discrepancies (one result being intermediate) among the various types of MH media used. However, major discrepancies (one result being resistant and the other susceptible or vice versa) were observed when S. faecalis strains were tested against cefotaxime. These major discrepancies were associated with both the commercial source of the MH medium and the blood content of the medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuchs P. C., Barry A. L., Thornsberry C., Jones R. N., Gavan T. L., Gerlach E. H., Sommers H. M. Cefotaxime: in vitro activity and tentative interpretive standards for disk susceptibility testing. Antimicrob Agents Chemother. 1980 Jul;18(1):88–93. doi: 10.1128/aac.18.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Matsumoto Y., Okada N., Mine Y., Nishida M., Goto S., Kuwahara S. Ceftizoxime (FK 749), a new parenteral cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1979 Nov;16(5):540–548. doi: 10.1128/aac.16.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Aswapokee P., Fu K. P. HR 756, a new cephalosporin active against gram-positive and gram-negative aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1979 Feb;15(2):273–281. doi: 10.1128/aac.15.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Cefuroxime, a beta-lactamase-resistant cephalosporin with a broad spectrum of gram-positive and -negative activity. Antimicrob Agents Chemother. 1978 Apr;13(4):657–664. doi: 10.1128/aac.13.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Griffiths A., Thornton J. E. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976 Mar;9(3):511–519. doi: 10.1128/aac.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosna J. P., Murray P. R., Medoff G. Comparison of the in vitro activities of HR756 with cephalothin, cefoxitin, and cefamandole. Antimicrob Agents Chemother. 1978 Dec;14(6):876–879. doi: 10.1128/aac.14.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]


