Abstract
Probiotic nutrients have shown promise in therapy for the treatment of gastrointestinal inflammation, infection, and atopic disease. Intestinal dendritic cells (DC) play a critical role in shaping the intestinal immune response. In this study, we tested the effect of a probiotic preparation (VSL#3) on DC distribution and phenotypes within the intestinal mucosa using a lineage depletion-based flow cytometric analysis. In naïve C57BL/10J mice, intestinal mucosal DC were composed of plasmacytoid DC (pDC) and myeloid DC (mDC). The pDC were the dominant form in lamina propria and Peyer's patches, whereas mDC were the prevailing type in the mesenteric lymph nodes. Additional characterization of pDC and mDC with flow cytometry revealed that they expressed heterogeneous phenotypes in the intestinal mucosa. In mice gavaged with the probiotic VSL#3 for 7 d, the proportion of pDC within the lamina propria was >60% lower, whereas the pDC subset in the mesenteric lymph nodes was more than 200% greater than in sham-treated controls (P < 0.01). Within pDC, the proportion of functionally unique CX3CR1+ DC was greater than in controls in both the lamina propria and the Peyer's patches (P < 0.01). In contrast to pDC, the mDC number was greater than in controls in all intestinal lymphoid tissue compartments in VSL#3-treated mice (P < 0.01). In conclusion, this study suggests that phenotypically and functionally distinct DC subsets are localized to specific lymphoid tissues within the intestinal mucosa and that the VSL#3 probiotic nutritional supplement alters the distribution of the DC subsets within the intestinal mucosa. These changes may be important in the alteration of mucosal immunity following probiotic VSL#3 therapy.
Introduction
As dietary constituents are processed in the lumen of the gastrointestinal tract, they are exposed to the intestinal immune system (1). Dietary antigens that are captured by dendritic cells (DC)7 in the intestinal mucosa are processed and presented to T-cells. The DC system is a complex and heterogeneous leukocyte population of professional antigen-presenting cells (2). They are involved in orchestrating immune responses to foreign antigens while maintaining tolerance to self-antigens and allergens. DC are classified as myeloid DC (mDC) and plasmacytoid DC (pDC) (3). Resting DC that capture self-antigens in the steady state are able to induce tolerance, whereas in the presence of inflammation, these same cells are able to initiate adaptive immune responses. There are several subsets of DC with functional differences among DC subtypes [reviewed in (4)], including varying cytokine/chemokine secretion, toll-like receptor expression, and T cell-polarizing ability. Luminal contents interact with the various DC subsets in the intestinal mucosa. Evidence exists that nutrients and food antigens are able to alter DC phenotypes and behaviors (5–7), suggesting that intestinal luminal contents are directly involved in modulating mucosal DC function.
It has been shown that specific DC subsets are involved in T cell priming (8–10). Several studies have demonstrated that DC take up residence in the intestinal mucosa, particularly within Peyer's patches and the lamina propria (11). Mucosal DC are major antigen-presenting cells with the capacity to process oral antigens in vivo. They provide a sentinel function, which allows sufficient defense against potential pathogens but restricts the immune response to nonpathogenic resident commensal bacteria and food antigens. Furthermore, a number of independent investigations revealed induction of mucosal tolerance in Peyer's patch deficient animals (12–14), suggesting that DC in lamina propria may play an essential role in mucosal immunity.
Probiotics are live microorganisms, which confer benefits to the host when administrated in sufficient amounts (15). They have been commonly used as nutritional or dietary supplements. A growing body of evidence suggests that administration of probiotics results in modulation of intestinal immunity, improvement of the balance of the gut microflora, enhancement of the recovery of the disturbed gut mucosal barrier, and prevention of microbial translocation [reviewed in (16,17)]. VSL#3, a probiotic preparation combining 8 different probiotic bacteria, has been shown to be effective at attenuating inflammatory bowel disease (18,19) and preventing chemotherapy-induced diarrhea (20). Recent studies suggest that DC functions may be modulated in vitro by VSL#3 probiotic (21), Lactobacillus casei DN-114001 (22), and supernatant from Bifidobacterium (23). However, the effect of VSL#3 probiotic therapy on intestinal DC phenotypes remains largely unknown. The aim of the present study was to characterize the intestinal DC subsets in C57BL/10J mice before and after feedings with probiotics.
Materials and Methods
Mice.
Specific pathogen-free C57BL/6J and C57BL/10J mice (male, 8–12 wk old) were purchased from the Jackson Laboratory. They were housed in a specific pathogen-free animal facility at the Children's Memorial Research Center. All animal experiments were conducted in accordance with NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee of the Children's Memorial Research Center.
Preparation of mononuclear cells from the small intestine.
Five or 6 mice were used for each experiment. They were killed by CO2 asphyxiation. The small intestine was washed extensively with saline and the fatty tissue, mesentery, and Peyer's patches were excised from intestinal segments. The entire intestinal tissue was cut open longitudinally and then cut into 3 pieces, which represented tissue segments of duodenum, jejunum, and ileum. They were processed for isolating mononuclear cells using a protocol modified from the method described by Niess et al. (24). Briefly, each piece was washed with ice-cold calcium- and magnesium-free PBS (CMF-PBS) containing 1 mmol/L dithiothreitol (Sigma Chemicals), treated with ice-cold CMF-PBS containing 30 mmol/L EDTA for 30 s, and finally rinsed with ice-cold CMF-PBS (contain 0.75 mmol/L EDTA). Thereafter, intestines were cut into <1-cm pieces and washed for 20–30 s in CMF-PBS containing 0.75 mmol/L EDTA (vigorously agitation). After rinsing with DMEM, the tissue pieces were treated for 45 min at 37°C in humidified atmosphere containing 5% CO2 with a DMEM-based enzyme cocktail that contains 36 kU/L collagenase IV (Sigma Chemicals), 150 mg/L DNase I (Roche), and 5% fetal bovine serum (FBS). In some experiments, Peyer's patches and mesenteric lymph nodes were digested with the enzyme-cocktail for 20 min. The undigested tissue debris was removed by filtration through a 100-μm–pore size tissue sieve (Bioworld) followed by a second filtration through a 40-μm–pore size cell strainer (BD Falcon). Cells in the digestion buffer were further purified by centrifugation at 700 × g; 8 min and washed twice with CMF-PBS containing 5% FBS, 5 mg/L DNaseI, and 5 mmol/L EDTA. The resulting cells were centrifuged through a 9.5% OptiPrep (ρ = 1055 g/L, Axis Shield) at 1700 × g; 10 min to enrich for mononuclear cells. The mononuclear cell fractions were collected, washed, and immunostained with the antibody cocktails described below. The stained cells were subjected to acquisition and flow cytometric analysis.
Flow cytometry.
Analysis of DC subsets was performed using fluorescein isothiocyanate-, phycoerythrin-, allophycocyanin-, peridinin chlorophyll protein-cyanine 5.5-, or phycoerythrin-cyanine 7-conjugated antibodies against CD11c (HL3), CD4 (RM4–5), CD8α (53–6.7), CD11b (M1/70), B220 (RA3–6B2), Ly6C (AL-21), CX3CR1 (ab8021), CD103 (M290), I-A/I-E (M5/114.15.2), and DEC-205 (NLDC-145). The staining cocktail for lineage-specific cell surface antigens contained biotin-conjugated anti-CD3, anti-CD19, and anti-F4/80 mAbs. Biotinylated antibodies were detected using streptavidin conjugated to APC. Murine cell-specific isotype-matched control Abs were used for the control staining to determine the background immunofluorescence. The above antibodies were purchased from BD Pharmingen, except for DEC205 and CX3CR1, which were obtained from Cedarlane and Abcam, respectively. Before staining, cells were preincubated with FCγIII/IIR antibody (2.4 G2) for 10 min on ice to block non-Fab antibody binding. After washing with PBS, cells (2 × 105 – 5 × 105) were suspended in 100 μL of PBS containing 1% FBS, and appropriate antibodies. All staining was conducted for 40 min on ice in the dark. The resulting cells were analyzed using either a FACScalibur or FACS Aria (Becton Dickinson) cytometer. Data were analyzed with either CellQuest (Becton Dickinson) or FlowJo software (Tree Star).
Characterization of DC subsets with flow cytometry.
The lineage depletion strategy described by Shortman et al. (25,26) was used for identifying of DC. Briefly, the criteria outlined in Table 1 were used for characterizing DC phenotypes in the intestinal mucosa. DC subsets were further defined by staining mononuclear cell preparations with classical DC marker panels (Table 2). To determine the DC functional and gut-associated characteristics, cells were stained with an antibody cocktail that contained antibodies against CX3CR1, CD103, DEC205, B220, CD11c, and lineage markers. Antibodies were conjugated with appropriate fluorochromes or biotin. All immunostained cells were subjected to flow cytometric analysis.
TABLE 1.
Criteria for defining pDC and mDC
| Phenotype | Lineage1 | MHCII | B220 | CD11c |
|---|---|---|---|---|
| pDC | − | −/Low | + | Intermediate |
| mDC | − | + | − | + |
Lineage (lin) markers include CD3, CD19, and F4/80.
TABLE 2.
Classic immunostaining panels for characterization of DC subsets
| DC subsets | Immunostaining panel1 |
|---|---|
| pDC | CD11c, Ly6C, B220, CD8α |
| mDC | CD11c, CD4, CD11b, CD8α |
Lineage cells were depleted from mononuclear cell preparations by the magnetic beads negative selection then stained with cocktails contained monoclonal antibodies stated in the panel. The immunostained cells were subjected to flow cytometric analysis.
Purification of cells by depletion of lineage-specific cells with MACS MicroBeads.
A total of 1 × 107 mononuclear cells from murine intestinal lamina propria were incubated with biotin-conjugated primary mAbs including anti-CD3 (clone KT3), anti-CD19 (clone 6D5), and anti-F4/80 (clone C1:A3–1) (all from Serotec) at a concentration of 1 μg/106 cells in MACS buffer (5 mmol/L EDTA, 1% bovine serum albumin in PBS) at 4°C for 15 min. Cells were washed in MACS buffer, indirectly labeled with streptavidin MACS MicroBeads (Miltenyi Biotec) for 15 min at 4°C, and then negatively selected on a prewashed LD column (Miltenyi Biotec). The columns were washed twice to collect the CD3−CD19−F4/80− unbound mononuclear cells. The latter negatively selected cells were washed with PBS then stained with the cocktail of mAbs against DC subset-specific antigens described above. The stained cells were acquired and analyzed by flow cytometry.
Administration of probiotics VSL#3.
VSL#3 is a medical food probiotic supplement that contains 4 lactobacilli, 3 bifidobacteria, and 1 streptococcal strain. In this study, mice were gavaged with PBS or probiotics VSL#3 (11.25 × 109 bacteria/(100 mg VSL#3 × mouse), suspended in 200 μL PBS) on a daily basis for 7 d. At the end of the treatment, mice were killed by CO2 inhalation. Mononuclear cells in lamina propria, Peyer's patches, and mesenteric lymph nodes were harvested and processed and purified for immunostaining as described above and then examined by flow cytometry.
Statistical analysis.
All the experiments were performed at least twice. Data were tested by 1-way ANOVA and, where appropriate, post hoc Tukey-Kramer multiple comparison tests. Differences were considered significant at P < 0.05. The results were reported as means ± SEM.
Results
Identification of lamina propria DC subsets in naïve C57BL/6J and C57BL/10J mice.
Because murine DC are a CD11cint/+ mononuclear cell population, we measured the level of CD11c expression on the surface of mononuclear cells isolated from lamina propria of C57BL/6J and C57BL/10J mouse strains. In C57BL/6J mice, 0.67 ± 0.06% of the intestinal lamina propria mononuclear cells were positive for CD11c (Supplemental Fig. 1A). In contrast, 5.87 ± 0.12% of the lamina propria mononuclear cells isolated from C57BL/10J mice expressed CD11c (Supplemental Fig. 1B). As the yield of lamina propria CD11c+ cells in C57BL/6J mice was lower (P < 0.05) than the cells in C57BL/10J mice, we decided to use C57BL/10J mice in this study.
Typically, DC were a Lin− mononuclear cell population that acquired a characteristic of CD11cint/+ and I-A/I-E−/+ (Supplemental Fig. 2).
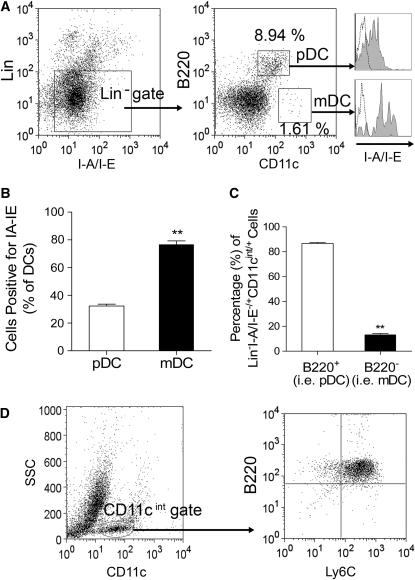
To phenotype DC, we used the criteria stated in Table 1 for defining pDC or mDC. In C57BL/10J mice, the intestinal lamina propria contained 3 populations of Lin−I-A/I-E−/+ cells including B220highCD11cint, B220−CD11c+, and B220−CD11c− (Fig. 1A). Among them, B220highCD11cintI-A/I-E−/lowLin− cells were pDC, whereas B220−CD11chighI-A/I-E+Lin− cells were mDC (Fig. 1A). The mDC expressed a higher level of MHCII molecular (i.e. I-A/I-E) on their surface compared with pDC (Fig. 1B). The majority of lamina propria Lin−I-A/I-E−/+CD11cint/+ cells were positive for B220 (Fig. 1C), indicating that the pDC are the dominant form of DC in the lamina propria. The lamina propria CD11cintB220+Lin− mononuclear cells also expressed Ly6C (Fig. 1D), which is consistent with the pDC characteristic (27).
FIGURE 1 .
Characterization of DC in naïve C57BL/10J mouse intestinal lamina propria. (A) Identification of pDC (i.e. B220highCD11cintI-A/I-E−/lowLin− cells) and mDC (i.e. B220−CD11chighI-A/I-E+Lin−) from intestinal lamina propria with the lineage depletion strategy-based flow cytometric analysis. The histogram indicated by dashed-line represents staining with the isotype-matched negative control mAb, whereas shaded-histogram represents cells stained with an appropriate antibody. (B) Assessment of surface MHCII expression in lamina propria pDC and mDC. Values are means ± SEM, n = 3. **Different from pDC, P < 0.01. (C) Numbers of lamina propria pDC and mDC. Values are means ± SEM, n = 3 pools of samples from 5 or 6 mice. **Different from pDC, P < 0.01. (D) Lamina propria pDC were positive for B220 and Ly6C.
Characterization of DC subsets in lamina propria.
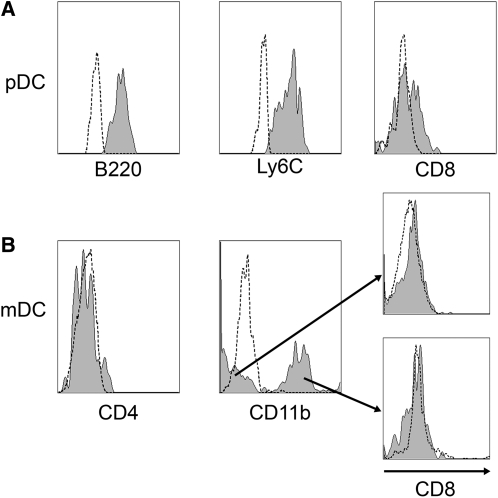
In laminar propria, pDC (Lin−CD11cintB220+Ly6C+) expressed low to intermediate CD8α (Fig. 2A). In contrast, mDC were negative for CD8α, contained CD11b+ and CD11b− subsets, and were negative for CD4 (Fig. 2B). Overall, the data indicated that steady-state lamina propria mDC are a heterogeneous population, including CD11chighCD11b−CD4−CD8− and CD11chighCD11b+CD4−CD8− subsets.
FIGURE 2 .
Phenotypic characteristics of laminar propria pDC (A) and mDC (B) in C57BL/10J mice. Data are representative of 3 separate experiments. Each experiment included pooled tissue from 5 or 6 mice. The histogram indicated by dashed-line represents staining with the isotype-matched negative control mAb, whereas shaded-histogram represents cells stained with an appropriate antibody.
Furthermore, we analyzed lamina propria DC in each intestinal fragment, because it was unclear whether intestinal DC distribution patterns are segment specific. DC were present in duodenum, jejunum, and ileum (Supplemental Fig. 3). The distribution of lamina propria DC subsets within each segment in C57BL/10J mice did not differ (Supplemental Fig. 3).
Relative distribution of lamina propria DC subsets vs. Peyer's patches and mesenteric lymph nodes in C57BL/10J mice.
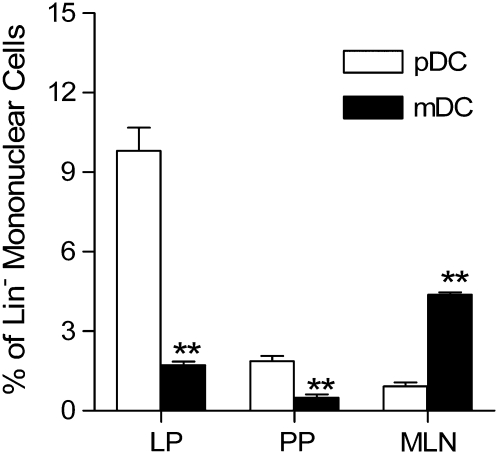
DC are present in various compartments of the intestinal mucosa, including lamina propria, Peyer's patches, and mesenteric lymph nodes. Therefore, we analyzed the relative distribution of DC subsets in the gut mucosa. The pDC subset was dominant in both laminar propria (Figs. 3A and 4) and Peyer's patches (Figs. 3B and 4). The mDC, however, were the predominant subset within the mesenteric lymph node DC populations (Figs. 3C and 4).
FIGURE 3 .
Characterization of DC subsets in lamina propria (A), Peyer's patches (B), and mesenteric lymph nodes (C) in naïve C57BL/10J mice. The histogram indicated by a dashed line represents staining with the isotype-matched negative control mAb, whereas the shaded histogram represents cells stained with an appropriate antibody. Data are representative of 3 separate experiments. Each experiment included pooled tissue from 5 or 6 mice. LP, Lamina propria; PP, Peyer's patches; MLN, mesenteric lymph nodes.
FIGURE 4 .
Phenotypic characteristics of DC in the intestinal mucosa of C57BL/10J mice. Values are means ± SEM, n = 3 pools of samples from 5 or 6 mice. **Different from pDC, P < 0.01. LP, Lamina propria; PP, Peyer's patches; MLN, mesenteric lymph nodes.
Expression of function- and gut-associated surface markers on the DC subsets within the gut mucosa of C57BL/10J mice.
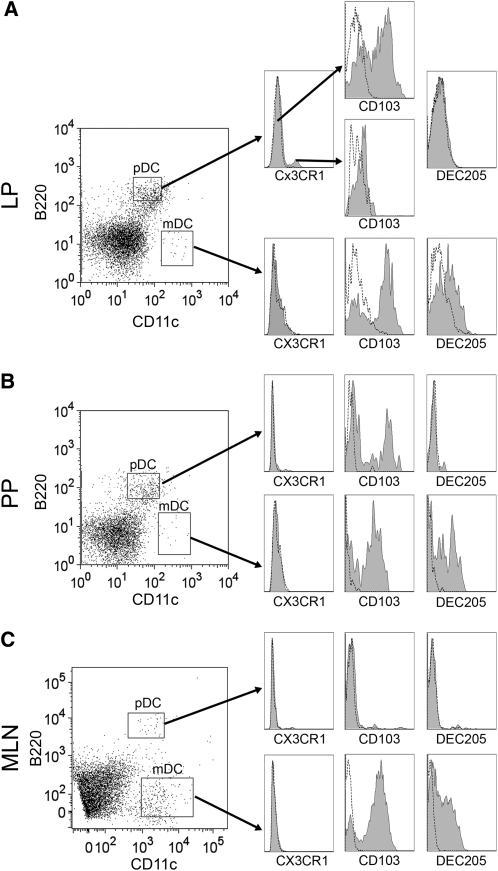
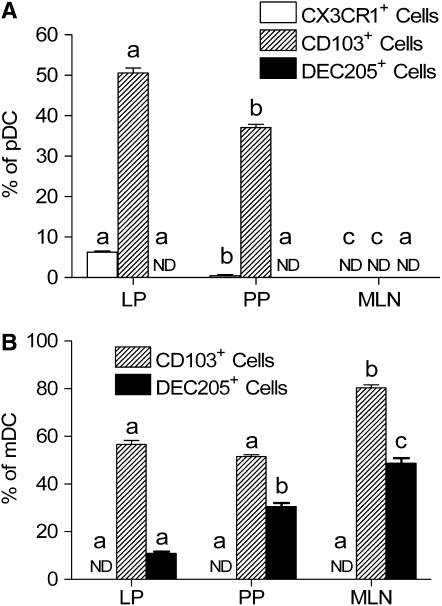
The expression of a DC functional associated marker (i.e. DEC205) and gut-associated markers (i.e. CX3CR1 and CD103) were further measured on the DC isolated from laminar propria, Peyer's patches, and mesenteric lymph nodes. The pDC in all 3 compartments were negative for DEC205 (Figs. 3 and 5A), whereas a large proportion of mucosal mDC expressed DEC205 (Figs. 3 and 5B). Both pDC and mDC were heterogeneous positive for CD103 in lamina propria (Figs. 3A and 5) and Peyer's patches (Figs. 3B and 5). In mesenteric lymph nodes, all pDC were negative for CD103 (Figs. 3C and 5A), whereas ∼80% of mDC were positive for CD103 (Figs. 3C and 5B).
FIGURE 5 .
The distribution of pDC (A) and mDC (B) positive for mucosal-associated DC markers in the intestinal mucosa of C57BL/10J mice. ND, Not detected. Values are means ± SEM, n = 3 pools of samples from 5 or 6 mice. Means within a DC subset without a common letter differ, P < 0.05. LP, Lamina propria; PP, Peyer's patches; MLN, mesenteric lymph nodes.
Previous studies suggested that CX3CR1 is a unique functional marker for intestinal DC (24,28). Therefore, we precisely analyzed CX3CR1 expression on the DC surface in the intestinal mucosa. In the lamina propria, pDC were composed of CX3CR1− and CX3CR1+ subsets (Figs. 3A and 5A). Among them, CX3CR1− cells were the predominant subset within the lamina propria pDC populations in naïve C57BL/10J mice. However, all pDC were negative for CX3CR1 in Peyer's patches (Figs. 3B and 5A) and mesenteric lymph nodes (Figs. 3C and 5A). The mDC resident in all locations of the intestinal mucosa were negative for CX3CR1 (Figs. 3 and 5B).
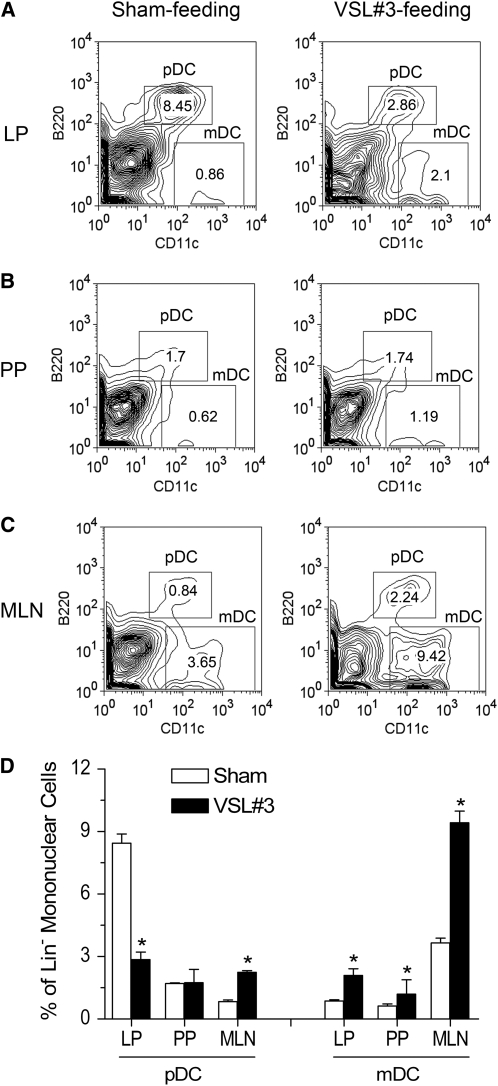
Alterations in the distribution of intestinal mucosal DC subsets after administration of VSL#3 probiotic preparation.
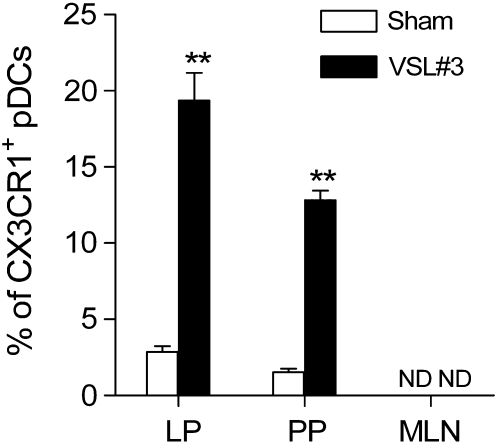
In C57BL/10J mice gavaged with VSL#3 probiotic for 7 d, the proportion of the pDC phenotype within the lamina propria was more than 60% less than in sham-treated controls (P < 0.05) (Fig. 6A,D), whereas the pDC subset within the mesenteric lymph nodes was more than 200% greater (P < 0.05) (Fig. 6C,D). However, administration of probiotic VSL#3 did not alter the number of pDC in Peyer's patches (Fig. 6B,D). Treatment with probiotic VSL#3 significantly increased the percentage of mDC in all intestinal mucosal tissues compared with the sham-treated group (Fig. 6). In addition, the number of CX3CR1+ pDC was also significantly higher in the VSL#3 probiotic-fed mice in lamina propria pDC and Peyer's patches in the controls (Fig. 7 and Supplemental Fig. 4). This change is not associated with alteration of surface expression of CD103 (Supplemental Fig. 4). Administration of VSL#3 did not affect the expression of DEC205 on the pDC surface in the intestinal lamina propria, Peyer's patches, and mesenteric lymph nodes (Supplemental Fig. 4). In addition, feeding mice VSL#3 did not affect the expression of DC functional markers on the mDC surface in the intestinal lamina propria, Peyer's patches, and mesenteric lymph nodes (Supplemental Fig. 5).
FIGURE 6 .
Numerical and phenotypical assessment of DC in lamina propria (A), Peyer's patches (B), and mesenteric lymph nodes (C) in C57BL/10J mice gavaged with PBS (sham) or VSL#3 probiotic preparation for 7 d. Quantitative data are in D. Values are means ± SEM, n = 3 pools of samples from 5 or 6 mice. Each experiment included pooled tissue from 5 or 6 mice. *Different from sham, P < 0.05. LP, Lamina propria; PP, Peyer's patches; MLN, mesenteric lymph nodes.
FIGURE 7 .
Cell populations of intestinal pDC positive for CX3CR1 in C57BL/10J mice gavaged with PBS (sham) or VSL#3 probiotic preparation for 7 d. Values are means ± SEM, n = 3 pools of samples from 5 or 6 mice. Each experiment included pooled tissue from 5 or 6 mice. **Different from sham, P < 0.01. LP, Lamina propria; PP, Peyer's patches; MLN, mesenteric lymph nodes.
Discussion
DC are present in the intestinal microenvironments. They constantly communicate with gut luminal contents such as food components, nutrients, and bacterial flora, which are an important determinant of immunity and immune responses. Previous studies demonstrated that nutrients such as vitamin A and its bioactive metabolite directly modulate the DC development and adhesion (5,29). Furthermore, the VSL#3 probiotic preparation or numerous Lactobacillus species have been shown to alter phenotype, cytokine release, and functions of DC in vitro (21,30,31). Recent investigations revealed that feeding with a probiotic strain Lactobacillus paracasei subsp. paracasei NTU 101 (32) or the probiotic bacteria strain L. casei DN-114001-fermented milk (22,33) resulted in upregulation of the antigen-presenting ability of DC. In the present study, we showed that administration of VSL#3 probiotic nutrient supplement results in a dramatic decrease in the proportion of pDC within the lamina propria, whereas the pDC subset increased significantly within the mesenteric lymph nodes. In contrast to pDC, the proportion of mDC significantly increased in intestinal lamina propria, Peyer's patches, and mesenteric lymph nodes after treatment with VSL#3 probiotic. Together, our results, in conjunction with previous research, suggest that administration of probiotics alters the distribution of DC subsets within the intestinal mucosa. Therefore, we hypothesize that probiotic supplements and microbial nutrients are able to reprogram intestinal DC.
In addition to examining the effect of VSL#3 probiotic treatment on intestinal DC physiology, we also characterized lamina propria DC of C57BL/10J mice here. We found that pDC (Lin−CD11intB220+Ly6C+) is the dominant population of DC within the lamina propria in C57BL/10 mice. Furthermore, we found that lamina propria DC express a range of levels of CD8, CD11b, CD103, DEC205, and CX3CR1. Therefore, DC are highly heterogeneous within lamina propria in steady-state C57BL/10J mice. The function of DC is in a subset-dependent manner (34–38). pDC induce CD4+CD25+ T regulatory cells (39) and provide intrinsic protection against inflammatory responses to harmless antigens (40). Recently, Goubier et al. (41) revealed that pDC mediate oral tolerance. In addition, CD103+ DC mediate induction of Foxp3+ Treg cells (36,37). Mucosal steady-state CD11b+ mDC secrete interleukin-10 and promote Th2 differentiation, whereas the CD11b− mDC subset produces interleukin-12p70 upon bacterial stimulation and induces the Th1 response in naive TCR transgenic CD4+ T cells in vitro (38). Thus, we speculate that lamina propria DC play multiple roles in maintaining immune homeostasis in the intestinal mucosa.
Previous studies suggest that CX3CR1 is an important surface marker associated with the function of mucosal DC. The expression of CX3CR1 leads to DC interacting with intestinal epithelial cells and extending dendrite to the lumen (24). CX3CR1 may play a role in altering the adhesive properties of DC to intestinal epithelium (28). In the present study, we demonstrated that lamina propria pDC are composed of CX3CR1+ and CX3CR1− populations and CX3CR1− DC are dominant at the lamina propria site in C57BL/10J mice. In contrast, DC in Peyer's patches and mesenteric lymph nodes are negative for CX3CR1. Administration of probiotics results in an increase in CX3CR1+ pDC subset in lamina propria and Peyer's patches. Together, our finding suggests that: 1) only a few lamina propria pDC are involved in directly sampling luminal antigens in C57BL/10J mice; 2) all intestinal mDC as well as most pDC in C57BL/10J mice do not directly contact intestinal epithelia or extend their dendrites to the intestinal lumen under the steady state; and 3) treatment with probiotics may promote pDC-forming transepithelial dendrites, which may facilitate the cell contacting luminal antigens.
Besides the major findings stated above, we used the lineage depletion strategy described by Vremec and Shortman (25,26) in the present work. To our knowledge, this is the first time that the lineage depletion strategy has been used to analyze DC phenotypic features in the murine intestinal mucosa. The phenotypic characterization of intestinal DC is involved in preparation of lamina propria mononuclear cells from the mucosal tissue, labeling cells with a range of surface markers followed by analysis of them with the flow cytometry. Intestinal lamina propria mononuclear cells are a mixed population containing T cells, B cells, macrophages, DC, and many other cells. CD11c can be used as a marker to label DC in the lamina propria mononuclear cell prep. However, it is not a unique marker for DC, because several non-DC lineage cells may also express CD11c. Thus, applying the lineage depletion strategy allows us to more precisely gain insight into phenotypic characteristics of DC in the intestinal mucosa.
Finally, Chirdo et al. (42) reported that lamina propria DC in inbred BALB/c mice are mainly composed of the CD11c+CD8α+MHCII+ phenotype. In addition, Niess et al. (24) demonstrated that the CX3CR1-expressing DC population in the intestinal lamina propria express CD11b in inbred BALB/c mice and is thus of myeloid origin in this strain. In contrast, we found that pDC rather than mDC are dominant in lamina propria of inbred C57BL/10J mice. CX3CR1-expressing lamina propria DC are derived from plasmacytoid origin in inbred C57BL/10J mice. In addition, we have shown that lamina propria in C57BL/10J mice have more DC than in C57BL/6J mice. Our result is consistent with a previous report indicating that ∼0.5–1% the lamina propria mononuclear cells in C57BL/6J mice were CD11c+ cells (43). By comparing data obtained from the inbred C57BL mice in the present study to results from BALB/c mice reported by other investigators, we speculate that different inbred mouse strains exhibit differences in the DC distribution pattern and phenotypic characteristics in the intestinal mucosa. The data reported by Asselin-Paturel et al. (27) further support this hypothesis. However, it remains to be determined whether the mouse strain-based differences in intestinal mucosal DC characteristics are physiological meaningful.
In the summary, we characterized the intestinal lamina propria DC subsets in C57BL/10J mice and compared them with DC in Peyer's patches and mesenteric lymph nodes in this study. Furthermore, we investigated the effect of VSL#3 probiotic therapy on DC phenotypes in the intestinal mucosa. We found that lamina propria DC isolated from naïve C57BL/10J mice have unique phenotypic characteristics, which differ from previously reported lamina propria DC phenotype in BALB/c mice (42). In addition, we found that exposure to VSL#3 probiotic causes a shifting DC distribution within intestinal mucosa in both lymphoid tissue compartment and DC subset-dependent mechanisms. We speculate that the action of probiotic VSL#3 on DC physiology influences immune responses in the intestinal mucosa.
Supplementary Material
Supported in part by grant R01DK064240 (to X.-D.T.) from the NIH and Eloise and Warren Batts Investigator Chair (to X.-D.T.)
Author disclosures: X. Wang, M. R. G. O'Gorman, H-F. Bu, V. Koti, X-L. Zuo, and X-D. Tan, no conflicts of interest.
Supplemental Figures 1–5 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: DC, dendritic cell; FBS, fetal bovine serum; mDC, myeloid DC; pDC, plasmacytoid DC.
References
- 1.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–50. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di PT, Connolly J, Fay JW, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–42. [DOI] [PubMed] [Google Scholar]
- 5.Lackey DE, Ashley SL, Davis AL, Hoag KA. Retinoic acid decreases adherence of murine myeloid dendritic cells and increases production of matrix metalloproteinase-9. J Nutr. 2008;138:1512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szeles L, Keresztes G, Torocsik D, Balajthy Z, Krenacs L, Poliska S, Steinmeyer A, Zuegel U, Pruenster M, et al. 1,25-Dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–83. [DOI] [PubMed] [Google Scholar]
- 7.Ren Z, Guo Z, Meydani SN, Wu D. White button mushroom enhances maturation of bone marrow-derived dendritic cells and their antigen presenting function in mice. J Nutr. 2008;138:544–50. [DOI] [PubMed] [Google Scholar]
- 8.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. [DOI] [PubMed] [Google Scholar]
- 9.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132–48. [DOI] [PubMed] [Google Scholar]
- 12.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–87. [DOI] [PubMed] [Google Scholar]
- 14.Spahn TW, Herbst H, Rennert PD, Lugering N, Maaser C, Kraft M, Fontana A, Weiner HL, Domschke W, et al. Induction of colitis in mice deficient of Peyer's patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol. 2002;161:2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. [DOI] [PubMed] [Google Scholar]
- 16.Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50 Suppl 3:III54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong Y, Konrad A, Iqbal N, Elson CO. Probiotics and immune regulation of inflammatory bowel diseases. Curr Drug Targets Inflamm Allergy. 2003;2:145–54. [DOI] [PubMed] [Google Scholar]
- 18.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De SC, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–46. [DOI] [PubMed] [Google Scholar]
- 19.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. [DOI] [PubMed] [Google Scholar]
- 20.Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, De RC, Famularo G. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am J Gastroenterol. 2002;97:2150–2. [DOI] [PubMed] [Google Scholar]
- 21.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Moreno de LA, Dogi CA, Galdeano CM, Carmuega E, Weill R, Perdigon G. Effect of the administration of a fermented milk containing Lactobacillus casei DN-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol. 2008;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, Velge-Roussel F, Lebranchu Y. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS ONE. 2008;3:e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. [DOI] [PubMed] [Google Scholar]
- 25.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–73. [PubMed] [Google Scholar]
- 26.Vremec D, Shortman K. The isolation and identification of murine dendritic cell populations from lymphoid tissues and their production in culture. Methods Mol Biol. 2008;415:163–78. [DOI] [PubMed] [Google Scholar]
- 27.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. [DOI] [PubMed] [Google Scholar]
- 28.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653–9. [DOI] [PubMed] [Google Scholar]
- 30.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci. 2005;102:2880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumann M, Richter JF, Wedell I, Moos V, Zimmermann-Kordmann M, Schneider T, Daum S, Zeitz M, Fromm M, et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut. 2008;57:747–54. [DOI] [PubMed] [Google Scholar]
- 33.Tsai YT, Cheng PC, Fan CK, Pan TM. Time-dependent persistence of enhanced immune response by a potential probiotic strain Lactobacillus paracasei subsp. paracasei NTU 101. Int J Food Microbiol. 2008;128:219–25. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado-Lopez R, De ST, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, et al. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–90. [DOI] [PubMed] [Google Scholar]
- 39.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. [DOI] [PubMed] [Google Scholar]
- 40.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. [DOI] [PubMed] [Google Scholar]
- 43.Anjuere F, Luci C, Lebens M, Rousseau D, Hervouet C, Milon G, Holmgren J, Ardavin C, Czerkinsky C. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J Immunol. 2004;173:5103–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.