Abstract
It has been known for at least 20 years that growth factors induce the internalization of cognate receptor tyrosine kinases (RTKs). The internalized receptors are then sorted to lysosomes or recycled to the cell surface. More recently, data have been published to indicate other intracellular destinations for the internalized RTKs. These include the nucleus, mitochondria, and cytoplasm. Also, it is recognized that trafficking to these novel destinations involves new biochemical mechanisms, such as proteolytic processing or interaction with translocons, and that these trafficking events have a function in signal transduction, implicating the receptor itself as a signaling element between the cell surface and the nucleus.
INTRODUCTION
Growth factor binding to a cognate receptor tyrosine kinase (RTK) initiates receptor activation of several well-described signal transduction pathways that relay biochemical signals to points of signal reception, such as promoter elements in the nucleus, to effect cellular responses [1]. While receptor activation of these pathways occurs predominantly at the cell surface, there are data indicating that signal transduction also occurs from intracellular RTKs [2,3]. Coincident with the initiation of cell surface signaling, growth factor: receptor complexes translocate to clathrin-coated pits and are rapidly internalized as endosomal complexes. Subsequently, the intracellular receptors, which remain active for several minutes, are trafficked to the lysosome where both ligand and receptor are degraded. While the lysosome is the predominant destination and the trafficking pathway to it is reasonably well understood, it is also clear, depending on cell content, that internalized receptors can be recycled to the cell surface [3].
More recently, evidence has accumulated to support the trafficking of the RTKs from the cell surface to other intracellular destinations: cytoplasm, nucleus, and mitochondria. There is, in some instances, mechanistic information regarding the trafficking route, as well as data pertaining to biologic significance. It is the focus of this review to summarize these results. Mechanisms that involve secretase-mediated RTK cleavage are addressed first followed other less extensively understood mechanisms. As ErbB-1 and ErbB-4 are the best understood examples, they will be described in more detail.
γ-Secretase-Dependent Trafficking
The role of secretase-dependent processing of cell surface molecules is most clear in the case of Notch [4]. In this case, ligand-binding initiates sequential proteolytic processing by α-secretase, which removes the ectodomain, and by γ-secretase, which cleaves within the transmembrane domain of the cell-associated receptor fragment to release an intracellular domain (ICD) fragment into the cytosol. The ICD subsequently escorts a transcription activation factor into the nucleus to initiate a cellular response to the ligand.
The Notch scenario is recapitulated to different extents by several RTKs, as indicated in Table I. In the case of ErbB-4, all essential steps are repeated and the ErbB-4 data are reviewed below and illustrated in Figure 1. Secretase processing is reported for several other RTKs (ephrin, CSF-1R, VEGFR1, Tie1, plus preliminarily for the insulin and IGF-1 receptors) and these data are also discussed. It should be mentioned that the list can be expected to lengthen as additional RTKs are known to be subject to ectodomain cleavage and this is a necessary precursor step for intramembranous cleavage by γ-secretase. Since biochemical detection of ICD fragments is known to be problematic, as these fragments are produced in substoichiometric amounts and are metabolically labile, more effective antibodies or protocols may be required.
Table 1.
Receptor Tyrosine Kinases Subject to Intramembrane Proteolysis*
| Receptor Tyrosine Kinase | Stimulating Ligand | ICD |
|
|---|---|---|---|
| Location | Functional Evidence | ||
| ErbB-4 | neuregulin, TPA | cytoplasm, nucleus, mitochondria | yes |
| Ephrin | ephrin, ionomycin | cytoplasm, nucleus | no |
| CSF-1R | CSF-1, LPS, TPA | cytoplasm, nucleus | no |
| Tie 1 | VEGF, TPA | cytoplasm | yes |
| VEGFR1 | PEDGF | cytoplasm | yes |
| Insulin, IGF-1 | TPA | cytoplasm | no |
References are in the text.
Figure 1.
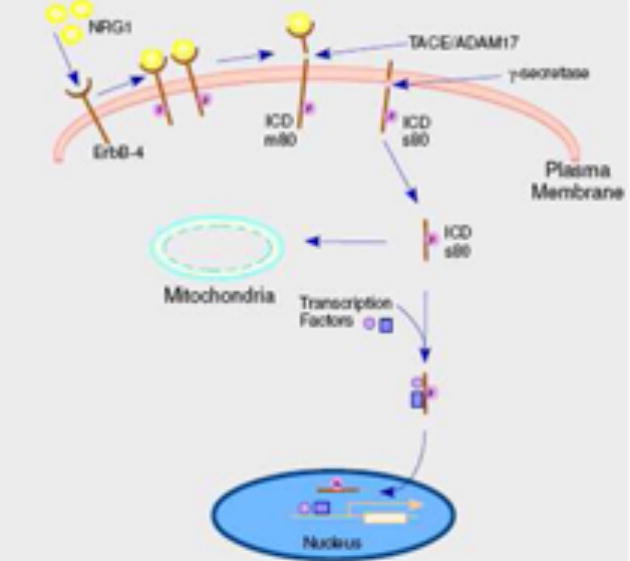
Secretase-mediated trafficking of ErbB-4 from the cell surface to intracellular compartments. Following ligand binding and receptor activation and dimerization, ErbB-4 is subject to ectodomain cleavage by ADAM17(TACE) with release of the ectodomain fragment into the media. The cell-associated fragment m80 is then cleaved by γ-secretase to produce an ICD fragment also termed s80. The ICD fragment is translocated to intracellular organelles. In the case of nuclear translocation, transcription factors are reported to be chaperoned into the nucleus with the ICD.
In addressing these examples of RTK intramembranous cleavage two points are emphasized. First, is the cleavage process stimulatable by a ligand? Second, what is the evidence that the released ICD fragment produces a relevant biologic activity? These issues are important as it has been hypothesized that secretase processing of transmembrane proteins may be a cellular housekeeping mechanism to degrade these molecules, as the presence of a transmembrane domain(s) would seem to present a barrier to other proteolytic systems [5,6]. These are not, however, necessary mutually exclusive interpretations. For example, α- or β-secretase release of an ectodomain fragment may be biologically important, while the γ-secretase degradation of the remaining cell-associated fragment may proceed as a housekeeping function. However, when the cleavage is stimulated by a ligand, especially the cognate ligand, and there is a biologic function to the ICD fragment, then it seems very likely that these trafficking events also represent a signal transduction mechanism.
Also, it is instructive to note that an increasing number of non-RTK cell surface molecules are subject to secretase cleavage and these are tabulated in Table 2. Within the RTK field of research, the processing of receptor phosphotyrosine phosphatases and growth factor precursors are especially relevant. Also, within the RTK and ligand categories are two ligand:receptor pairs: nueregulin1 Type III and ErbB-4 plus ephrin and the ephrin receptor. Available evidence indicates that these are similar to the Notch system in that formation of the ligand:receptor complex in a juxtacrine manner initiates forward and backward signaling between two adjacent cells in a secretase-dependent manner.
Table 2.
Cell Surface Transmembrane Protein Subject to Intramembrane Proteolysis
| Category | Protein | Stimulating Ligand | ICD |
||
|---|---|---|---|---|---|
| Localization | Functional Evidence | References | |||
| Ligands | TNFa | none | cytoplasm | yes | [64,65] |
| NRG1 Type III | ErbB-4 | cytoplasm, nucleus | yes | [66,67] | |
| Delta, Jagged | Notch | cytoplasm, nucleus | yes | [68–70] | |
| Ephrin | Eph, TPA | cytoplasm, nucleus | yes | [71,72] | |
| PTPases | RPTPκ,μ | High cell density, antibody | cytoplasm, nucleus | yes | [73] |
| LAR | TPA | cytoplasm, nucleus | yes | [74] | |
| Ligand Receptor | Notch | Delta, Serrate | cytoplasm, nucleus | yes | [4](ref therein) |
| p75 | BDNF, TPA | cytoplasm, nucleus | no | [75–81] | |
| IL-1R2 | TPA | cytoplasm | no | [82] | |
| Growth Hormone Receptor | TPA | cytoplasm, nucleus | no | [83] | |
| GluR3 | none | ICD not produced | yes | [84] | |
| LDLRPs | TPA | cytoplasm, nucleus | yes | [85–90] | |
| CXCL1&16 | none | cytoplasm | none | [91] | |
| IFNaR2 | IFN-α, TPA | cytoplasm, nucleus | yes | [92–94] | |
| Channels Adhesion | Na channel-β2 | none | cytoplasm | no | [95–97] |
| CD44 | Ionomycin, TPA | cytoplasm, nucleus | yes | [98–101] | |
| Cadherins | Apoptosis, Ca influx, toxin | cytoplasm | yes | [102–108] | |
| DCC | none | cytoplasm | yes | [109] | |
| Syndecan 3 | TPA, bFGF | cytoplasm | yes | [110] | |
| L1 | none | cytoplasm | no | [111] | |
| Miscellaneous | APP | TPA | cytoplasm, nucleus | yes | [112](ref therein) |
| TMEFF2 | TPA | cytoplasm | no | [113] | |
| CD43 | none | cytoplasm, nucleus | yes | [114,115] | |
| CD74 | none | cytoplasm, nucleus | yes | [116] | |
| SorLA | none | cytoplasm, nucleus | yes | [117] | |
| Fibrocystin | Ca+2 mobilization | cytoplasm, nucleus | no | [118] | |
| RAGE receptor | ionomycin | cytoplasm, nucleus | no | [119] | |
| HLA-A2 | TPA | cytoplasm | no | [120] | |
| Bri 2 | none | cytoplasm | no | [121] | |
| Nectin-1α | TPA | cytoplasm | no | [122] | |
| Neogenin | none | cytoplasm, nucleus | yes | [123] | |
| NRADD | none | cytoplasm | no | [124] | |
| Tyrosinase | none | cytoplasm | no | [125] | |
| GnT-V | none | cytoplasm | no | [126] | |
ErbB-4
Ectodomain proteolytic processing of ErbB-4 includes a basal level, which can be increased by TPA in all cells or by the addition of neuregulin (heregulin) to certain cells [7,8]. As depicted in Figure 1, this cleavage results in the formation of two receptor fragments: a 120 kDa ectodomain fragment that is released into the media and an 80 kDa membrane-bound fragment, termed m80. Cleavage requires ADAM 17 (TACE) and it is likely this is the enzyme that executes cleavage of ErbB-4 between His651 and Ser652 within the extracellular stalk or ecto-juxtamembrane region [9,10]. Hence, the m80 fragment includes eight ectodomain residues, the transmembrane domain and entire ICD.
Sensitivity to ectodomain shedding is likely determined, at least in part, by the length of the stalk region in various transmembrane proteins, as demonstrated for the selectins [11]. There are two ErbB-4 isoforms termed Jm-a, in which the ectodomain is sensitive to cleavage, and Jm-b, which is not cleavable [12]. Since ADAM-mediated cleavage events do not involve a defined sequence or cleavage site in the substrate, it seems that longer stalk regions in substrates may simply permit accessibility of the protease. Interestingly, the stalk region in Jm-b is much shorter (6 residues) than the corresponding region of Jm-a (16 residues) and ErbB-1, -2 and -3 also have relatively short stalk regions (6–9 resides) and are not subject to a significant level of metalloprotease mediated ectodomain cleavage [7]. Hence the unique sensitivity of the Jm-a ErbB-4 isoform to secretase-dependent processing and signaling seem likely due to the length of its stalk region.
It seems probable, though not formally demonstrated, that the shed ErbB-4 ectodomain may function to block receptor activation by binding neuregulin. The function of the m80 fragment, however, is known. The capacity of γ-secretase to cleave substrates requires that the substrate have a short ectodomain region of 50 or fewer residues [13]. Hence, the ADAM-mediated removal of a large portion of the ErbB-4 ectodomain is a prerequisite step for subsequent γ-secretase cleavage of the m80 fragment [14].
γ-Secretase is a complex of at least four distinct transmembrane proteins of which presenilin is the catalytic protease [15]. It has been shown that the nicastrin subunit of the γ-secretase complex recognizes transmembrane proteins with shortened or nub-like ectodomains and thereby acts as a targeting subunit for intramembrane cleavage by presenilin [16]. This would predict that nicastrin recognition of the ErbB-4 m80 fragment initiates intramembranous cleavage. As shown in Figure 1, presenilin activity converts the ErbB-4 m80 fragment to a soluble s80 or ICD fragment that is found in the cytoplasm, nucleus and, in one report, mitochondria [17,14].
The C-terminus of ErbB-4 encodes a PDZ domain recognition motif, which is required for presenilin cleavage of the m80 fragment [18]. Deletion of this motif (TVV) does not influence ectodomain cleavage, but does abrogate presenilin association with the m80 fragment and production of the ICD fragment. Presenilin also contains a PDZ domain recognition motif, and, it is possible that a scaffold of PDZ domain containing proteins may be required for γ-secretase cleavage. Proteins that recognize the PDZ domain recognition motifs in ErbB-4 [149,150] and presenilin [151,152] have been reported.
Presenilin cleavage of substrates occurs within the transmembrane domain and, based on APP and Notch processing, this may occur at multiple sites, producing several species of ICD fragments that may have differing levels of metabolic stability based on the N-end rule [19]. Hence, mutation within the transmembrane domain can diminish cleavage and/or alter the metabolic stability of the ICD fragments. This is shown in the case of Notch where a transmembrane mutation appears to prevent cleavage, but actually results in a new ICD fragment that is very rapidly degraded due to the presence of a metabolically destabilizing N-terminal residue [20].
It has been reported that the Val675Ala [21] or Val673Ile [22] mutations within the ErbB-4 transmembrane domain abrogate γ-secretase cleavage, as judged by the inability to detect the ICD fragment. In view of the Notch mutagenesis data, it is not clear whether these mutations actually prevent cleavage or result in a less stable ICD fragment. Given the low level of ICD fragment normally detectable, a modest change in stability may render the fragment undetectable by the same methodology.
In terms of the physiological relevance of the ErbB-4 ICD, it is now clear that endogenous generation of the ICD by γ-secretase is required for control of astrogenesis in the developing mouse [23]. In this system the ICD fragment interacts with TAB2, an adaptor protein, and thereby with N- CoR, a co-repressor, and chaperones this complex into the nucleus. A similar chaperone mechanism between the ErbB-4 ICD and STAT5 has been proposed to be operative in mammary differentiation in vitro [24] and it is clear that ErbB-4 is functionally involved in mammary development in the animal [25]. While ErbB-4 nuclear localization has been observed in normal and tumor mammary tissue and exogenous ICD expression provokes differentiation events, it has not yet been demonstrated that ErbB-4 cleavage is physiologically relevant in this tissue. Also, in line with a role of the ErbB-4 ICD fragment in various cell differentiation systems, is the report that γ-secretase inhibition prevents neuregulin generation of the nuclear ErbB-4 ICD in oligodendrocytes and maturation of this cell type [26].
In addition to STAT 5 and the TAB 2:N-CoR complex mentioned above, several other proteins (Eto-2 [27], YAP [28,29], WWOX [30], ER [31], Mdm 2 [32], AIP4/Itch [33]) have been reported to associate with the ErbB-4 ICD. Eto-2, YAP and ER are transcription factors/co-activators and the ICD may regulate their nuclear localization similar to STAT 5 and N-CoR. WWOX is a cytoplasmic protein and its interaction with the ICD attenuates nuclear translocation of the ICD, while AIP4/Itch is a cytoplasmic ubiquitin ligase that modulates the levels of intact ErbB-4 and the ICD. The ICD is an active tyrosine kinase [34] that phosphorylates Mdm2, a regulator of p53, which is predominantly localized in the nucleus [32].
As mentioned above, the ErbB-4 ICD has also been localized in mitochondria and in that location may function as a proapoptotic protein. This is based on the capacity of the ICD to induce cell death, the presence of a BH3 domain in the ICD, the loss of apoptotic capacity following mutagenesis of this domain, and detection of an interaction with the antiapoptotic protein BCL-2, which, when over-expressed, abrogated ICD-induced cell death [17]. This and other aspects of the ErbB-4 ICD are reviewed elsewhere [25].
Ephrin Receptor
Addition of the ephrin receptor ligand ephrin provokes secretase cleavages of the receptor releasing the ectodomain fragment and an ICD fragment [35]. The cleavage events are also stimulatable by ionomycin or activation of the NMDA receptor, agents that mediate Ca2+ influx into cells. In this system, ephrin-mediated cleavage events require endocytosis, while cleavage mediated by ionomycin in NMDA receptor activation occurs on the cell surface. A similar endocytosis relationship between neuregulin and TPA mediated cleavage of ErbB-4 was noted [8]. To date it is unclear whether the receptor ICD fragment is translocated from the cytoplasm to another organelle and there is no data related to a physiological function in mediating ligand cell responsiveness.
CSF-1 Receptor
Secretase cleavage of the CSF-1R can be stimulated by CSF-1, LPS and TPA [36–38]. LPS is a ligand for the Toll4 receptor and agonists for other Toll-like receptors also stimulate cleavage of CSF-1R. This heterologous stimulation of CSF-1R cleavage may be related to the fact that in macrophages both receptor systems are thought to be involved in producing innate immune responses. While the CSF-1 ICD fragment does appear in both cytoplasm and nucleus, a physiologic function has not been identified.
VEGF Receptor 1
Pigment epithelium-derived factor (PEDF) binds to an unknown receptor and promotes an anti-angiogenic response and can oppose the capacity of VEGF to promote endothelial cell proliferation. The addition of PEDF to endothelial cells promotes the γ-secretase mediated cleavage of VEGFR1 with release of its ICD fragment [39]. This ICD fragment was only present when cells were treated simultaneously with PEDF and VEGF and the fragment was detected in the cytoplasm, but not in the nucleus. In this system, the intact VEGFR1 molecule is found in the nucleus following the addition of VEGF (see below) and PEDF reduces VEGF-induced angiogenesis and the nuclear level of intact VEGFR in a manner dependent on γ-secretase activity. This implies that the PEDF stimulated production of the VEGFR1 ICD fragment negatively regulates intact VEGFR1 levels in the nucleus and VEGF-induced angiogenesis, based on the action of γ-secretase inhibitors. However, other signaling systems (Notch, etc.) will also be blocked by pharmacologic γ-secretase inhibitors.
Tie 1
Tie 1 is an orphan receptor that forms a hetero-oligomeric complex with Tie 2, the receptor for angiopoietin 1 (Ang 1). Addition of Ang 1 activates Tie 2 and provokes tyrosine phosphorylation of Tie 1. In this system ectodomain cleavage of Tie 1 is stimulated by a variety of agents (TPA, VEGF, TNFα, sheer stress) and increases Ang 1 activation of Tie 2, apparently by allowing greater access of the ligand to its Tie 2 binding site. Following ectodomain release, the Tie1 cell-associated cleavage fragment (45 kDa) is processed by γ-secretase to produce a 42 kDa cytoplasmic ICD fragment [40]. In this receptor system, the ectodomain secretase action is physiologically important: however, the significance of γ-secretase activity may be simply to remove the highly tyrosine phosphorylated 45 kDa fragment. While the addition of Ang 1 promotes rapid endocytosis and degradation of Tie 2, Tie1 is not cleared from the cell surface by this same route. Therefore, a secretase mechanism may provide the means by which Ang1- phosphorylated Tie 1 is inactivated.
IGF-1 and Insulin Receptors
In preliminary reports, it has been demonstrated that the insulin and IGF-1 receptors can be cleaved by secretase action to produce ICD fragments [41,42]. However, while TPA stimulates formation of the ICD fragments neither cognate ligand was demonstrated to do so.
Non-Secretase Formation of RTK ICD Fragments
In the case of several RTKs (ErbB-2 [43–45], Ret [46], ALK [47], TrkC [48], Met [49–51]) there is evidence that caspases cleave the cytoplasmic domain to produce an ICD fragment. Since the fragment is often produced by two cleavage events within the cytoplasmic domain, the fragment is often considerably smaller than that produced by intramembrane proteolysis. In no reported case are these caspase cleavages stimulated by ligand binding or by TPA and in some studies the presence of the cognate ligand prevents cleavage. In the above RTKs the formation of caspase ICD fragments is functionally associated with the induction of apoptosis and in one instance [44] the fragment has been localized to the mitochondria. Thus, this group of RTKs can be added to the list of dependence receptors in which the receptor mediates opposing cellular responses (apoptosis, cell proliferation) depending on the absence or presence of ligand.
Trafficking of Intact Receptors to the Nucleus
An accounting of recently published reports demonstrating the appearance of intact RTKs and other cell surface receptors in the nucleus is presented in Table 3. In a few instances the data relies on immuno-histochemistry alone and it is not clear that intact receptor is distinguishable from an ICD fragment. In nearly all cases, however, it does appear that the receptor is present in the nucleoplasm and not the nuclear envelope. The presence of a transmembrane protein in a non-membranous environment requires a mechanism to extract the intact receptor from the surrounding lipid bilayer. Such a mechanism has recently been reported and is described below and illustrated in Figure 2.. Whether this trafficking route can be applied to other receptors listed in Table 3 remains to be seen.
Table 3.
Intact Receptors Trafficked to Nucleus or Mitochondria
| Receptor Tyrosine Kinase | Stimulating Ligand | Organelle | Functional Evidence | References |
|---|---|---|---|---|
| ErbB-1 | EGF | nucleus | yes | [53,57,127,60,52,56,54,55,58] |
| ErbB-1 | Src | mitochondria | no | [128] |
| ErbB-1 vIII | none | nucleus | yes | [129] |
| ErbB-2 | over-expression | nucleus | yes | [130,131] |
| ErbB-3 | none | nucleus | no | [132] |
| FGF-R1, R2 | FGF | nucleus | yes | [133,134](ref therein) |
| IFN-γR1 | IFN-γ | nucleus | yes | [135–137] |
| TrkA | NGF, LPS | nucleus | no | [138,139] |
| TGF-β | TGF-β | nucleus | no | [140] |
| GH | Growth hormone | nucleus | yes | [141–143] |
| IL-15R2 | IL-15 | nucleus | no | [144] |
| VEGFR1/FLT1 | VEGF | nucleus | no | [145–147] |
| Met | HGF | nucleus | no | [148] |
Figure 2.
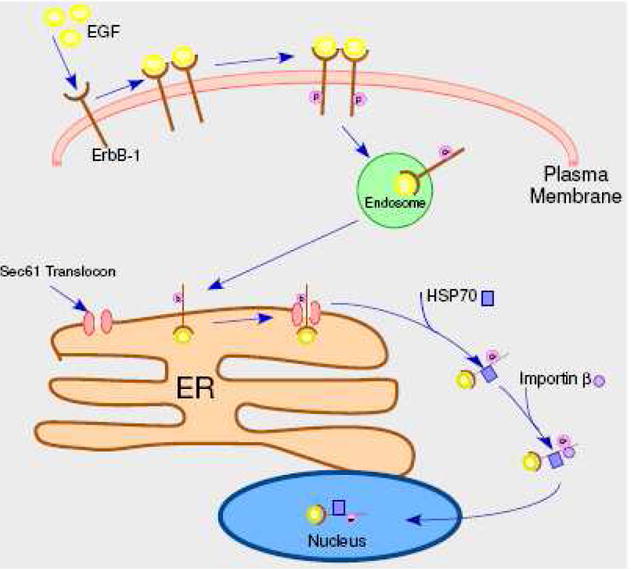
Translocon-mediated trafficking of ErbB-1 (EGF receptor) from the cell surface to the nucleus. Following ligand binding and receptor activation and dimerization, the receptor is internalized and trafficked to the endoplasmic reticulum (ER). Interaction with the Sec 61 translocon mediates extraction from the lipid bilayer, interaction with chaperones, such as Hsp 70, and extrusion into the cytoplasm. Subsequent association with importin-β leads to nuclear import.
ErbB-1 and Translocon –Mediated Trafficking
The capacity of EGF to induce trafficking of the intact EGF receptor (ErbB-1) to the nucleus was first reported in 2001 and a nuclear function was also identified [52]. The nuclear receptor was reported to recognize the promoter of cyclin D1 and to transactivate this promoter in a reporter system. Subsequently, the same group established the following points: 1) other promoters are also recognized by the ErbB-1 (though direct binding to any promoter remains to be shown [53–55]), 2) importin β is required for ErbB-1 nuclear localization [56], 3) a nuclear localization sequence is present in the ErbB-1 sequence [57], and 4) the nuclear receptor associates with PCNA in the nucleus and modifies its stability [58]. The nuclear receptor was identified by both biochemical fractionation and morphological methods and shown to be in a non-membranous environment. Furthermore, the ligand (EGF) was reported to also be present in the nucleus [52].
While no RTK trafficking system was known to extract the transmembrane receptor from its lipid bilayer, another group suggested that a protein translocon could provide such a step [59]. Specifically, the Sec 61 translocon located in the endoplasmic reticulum was known to mediate the trafficking of certain extracellular toxins from the cell surface to the cytoplasm and also, as part of the ERAD pathway, to retrotranslocate malfolded transmembrane proteins from the endoplasmic reticulum to the cytoplasm. Subsequent testing of this possibility showed that EGF induced trafficking of ErbB-1 to the endoplasmic reticulum where it interacted with the Sec61 translocon that then mediated receptor retrotranslocation to the cytoplasm and import into the nucleus [60]. The data showed that knock-down of a Sec61 subunit abrogates both EGF nuclear localization of ErbB-1 and EGF induction of cyclin D1. This trafficking pathway is depicted in Figure 2. HSP 70 has been shown to be essential in the in vitro retrotranslocation process [60] and likely functions by interacting with the receptor transmembrane domain and thereby maintaining the receptor in a soluble state following extraction from the ER membrane. The route by which the receptor is trafficked from the cell surface to the endoplasmic reticulum was not determined, but precedent exists with both toxins [153,154] and the SV40 virus [155]. In the former case, the Golgi serves as an intermediate, while in the latter caveosomes translocate virus to the endoplasmic reticulum.
The translocon pathway described above for ErbB-1 is distinguished form the endocytic pathways leading to lysosomal degradation or recycling to the cell surface on the basis of time and quantity of receptors involved. The translocon pathway is relatively slow and involves a smaller fraction of the receptor population [60]. Therefore it is not clear whether the translocon pathway represents a third destination for receptors internalized through clathrin-coated pits or whether receptors destined for the translocon and nucleus are internalized by a separate cell surface mechanism.
There are several examples of ligand/receptor pairs that translocate to the nucleus, perhaps as a complex. However, this has not been convincingly demonstrated. The interested reader is referred to recent reviews that address the issue of ligand trafficking to the nucleus [61 – 63].
Acknowledgments
The author appreciates the efforts of Sue Carpenter in manuscript preparation and acknowledges support of NIH grant CA125649.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–6. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–14. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 4.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 5.Kopan R, Ilagan MX. γ-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 6.Small DH. Is γ-secretase a multienzyme complex for membrane protein degradation? Models and speculations, Peptides. 2002;23:1317–21. doi: 10.1016/s0196-9781(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 7.Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J Biol Chem. 1996;271:18989–95. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Carpenter G. Heregulin-dependent trafficking and cleavage of ErbB-4. J Biol Chem. 2000;275:34737–43. doi: 10.1074/jbc.M003756200. [DOI] [PubMed] [Google Scholar]
- 9.Cheng QC, Tikhomirov O, Zhou W, Carpenter G. Ectodomain cleavage of ErbB-4: characterization of the cleavage site and m80 fragment. J Biol Chem. 2003;278:38421–7. doi: 10.1074/jbc.M302111200. [DOI] [PubMed] [Google Scholar]
- 10.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor- α-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–87. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 11.Migaki GI, Kahn J, Kishimoto TK. Mutational analysis of the membrane-proximal cleavage site of L-selectin: relaxed sequence specificity surrounding the cleavage site. J Exp Med. 1995;182:549–57. doi: 10.1084/jem.182.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–8. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 13.Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–36. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 14.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–21. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a γ-secretase-substrate receptor. Cell. 2005;122:435–47. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–20. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 18.Ni CY, Yuan H, Carpenter G. Role of the ErbB-4 carboxyl terminus in γ-secretase cleavage. J Biol Chem. 2003;278:4561–5. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]
- 19.Varshavsky A. The N-end rule pathway of protein degradation. Genes Cells. 1997;2:13–28. doi: 10.1046/j.1365-2443.1997.1020301.x. [DOI] [PubMed] [Google Scholar]
- 20.Tagami S, et al. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol. 2008;28:165–76. doi: 10.1128/MCB.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muraoka-Cook RS, Sandahl M, Husted C, Hunter D, Miraglia L, Feng SM, Elenius K, Earp HS., 3rd The intracellular domain of ErbB4 induces differentiation of mammary epithelial cells. Mol Biol Cell. 2006;17:4118–29. doi: 10.1091/mbc.E06-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent γ-secretase processing regulates multiple ERBB4/HER4 activities. J Biol Chem. 2005;280:19777–83. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- 23.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–97. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones FE. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:247–58. doi: 10.1007/s10911-008-9076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai C, Feng L. Implication of γ-secretase in neuregulin-induced maturation of oligodendrocytes. Biochem Biophys Res Commun. 2004;314:535–42. doi: 10.1016/j.bbrc.2003.12.131. [DOI] [PubMed] [Google Scholar]
- 27.Linggi B, Carpenter G. ErbB-4 s80 intracellular domain abrogates ETO2-dependent transcriptional repression. J Biol Chem. 2006;281:25373–80. doi: 10.1074/jbc.M603998200. [DOI] [PubMed] [Google Scholar]
- 28.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–41. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 29.Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L, Gulino A, Alimandi M. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res. 2004;294:469–79. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65:6764–72. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, Vadlamudi RK, Jones FE. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res. 2006;66:7991–8. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 32.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. J Biol Chem. 2005;280:30783–7. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 33.Omerovic J, et al. The E3 ligase Aip4/Itch ubiquitinates and targets ErbB-4 for degradation. FASEB J. 2007;21:2849–62. doi: 10.1096/fj.06-7925com. [DOI] [PubMed] [Google Scholar]
- 34.Linggi B, Cheng QC, Rao AR, Carpenter G. The ErbB-4 s80 intracellular domain is a constitutively active tyrosine kinase. Oncogene. 2006;25:160–3. doi: 10.1038/sj.onc.1209003. [DOI] [PubMed] [Google Scholar]
- 35.Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007;282:16155–63. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenn G, van der Geer P. CSF-1 and TPA stimulate independent pathways leading to lysosomal degradation or regulated intramembrane proteolysis of the CSF-1 receptor. FEBS Lett. 2007;581:5377–81. doi: 10.1016/j.febslet.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glenn G, van der Geer P. Toll-like receptors stimulate regulated intramembrane proteolysis of the CSF-1 receptor through Erk activation. FEBS Lett. 2008;582:911–5. doi: 10.1016/j.febslet.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelmsen K, van der Geer P. Phorbol 12-myristate 13-acetate-induced release of the colony-stimulating factor 1 receptor cytoplasmic domain into the cytosol involves two separate cleavage events. Mol Cell Biol. 2004;24:454–64. doi: 10.1128/MCB.24.1.454-464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–13. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 40.Marron MB, Singh H, Tahir TA, Kavumkal J, Kim HZ, Koh GY, Brindle NP. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J Biol Chem. 2007;282:30509–17. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasuga K, Kaneko H, Nishizawa M, Onodera O, Ikeuchi T. Generation of intracellular domain of insulin receptor tyrosine kinase by γ-secretase. Biochem Biophys Res Commun. 2007;360:90–6. doi: 10.1016/j.bbrc.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 42.McElroy B, Powell JC, McCarthy JV. The insulin-like growth factor 1 (IGF-1) receptor is a substrate for γ-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun. 2007;358:1136–41. doi: 10.1016/j.bbrc.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 43.Benoit V, Chariot A, Delacroix L, Deregowski V, Jacobs N, Merville MP, Bours V. Caspase-8-dependent HER-2 cleavage in response to tumor necrosis factor α stimulation is counteracted by nuclear factor κB through c-FLIP-L expression. Cancer Res. 2004;64:2684–91. doi: 10.1158/0008-5472.can-03-2914. [DOI] [PubMed] [Google Scholar]
- 44.Strohecker AM, Yehiely F, Chen F, Cryns VL. Caspase cleavage of HER-2 releases a bad-like cell death effector. J Biol Chem. 2008;283:18269–82. doi: 10.1074/jbc.M802156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tikhomirov O, Carpenter G. Caspase-dependent cleavage of ErbB-2 by geldanamycin and staurosporin. J Biol Chem. 2001;276:33675–80. doi: 10.1074/jbc.M101394200. [DOI] [PubMed] [Google Scholar]
- 46.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 2000;19:4056–63. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mourali J, et al. Anaplastic lymphoma kinase is a dependence receptor whose proapoptotic functions are activated by caspase cleavage. Mol Cell Biol. 2006;26:6209–22. doi: 10.1128/MCB.01515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tauszig-Delamasure S, Yu LY, Cabrera JR, Bouzas-Rodriguez J, Mermet-Bouvier C, Guix C, Bordeaux MC, Arumae U, Mehlen P. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci U S A. 2007;104:13361–6. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foveau B, Leroy C, Ancot F, Deheuninck J, Ji Z, Fafeur V, Tulasne D. Amplification of apoptosis through sequential caspase cleavage of the MET tyrosine kinase receptor. Cell Death Differ. 2007;14:752–64. doi: 10.1038/sj.cdd.4402080. [DOI] [PubMed] [Google Scholar]
- 50.Pozner-Moulis S, Pappas DJ, Rimm DL. Met, the hepatocyte growth factor receptor, localizes to the nucleus in cells at low density. Cancer Res. 2006;66:7976–82. doi: 10.1158/0008-5472.CAN-05-4335. [DOI] [PubMed] [Google Scholar]
- 51.Tulasne D, et al. Proapoptotic function of the MET tyrosine kinase receptor through caspase cleavage. Mol Cell Biol. 2004;24:10328–39. doi: 10.1128/MCB.24.23.10328-10339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 53.Hanada N, Lo HW, Day CP, Pan Y, Nakajima Y, Hung MC. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol Carcinog. 2006;45:10–7. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- 54.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–89. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Tao Y, et al. Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein-Barr-encoded oncoprotein latent membrane protein 1. Exp Cell Res. 2005;303:240–51. doi: 10.1016/j.yexcr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 56.Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin β1 and CRM1. J Cell Biochem. 2006;98:1570–83. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- 57.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282:10432–40. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 58.Wang SC, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8:1359–68. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 59.Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15:143–8. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 60.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–72. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. Bioessays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- 62.Olsnes S, Klingenberg O, Wiedlocha A. Transport of exogenous growth factors and cytokines to the cytosol and to the nucleus. Physiol Rev. 2003;83:163–82. doi: 10.1152/physrev.00021.2002. [DOI] [PubMed] [Google Scholar]
- 63.Planque N. Nuclear trafficking of secreted factors and cell-surface receptors: new pathways to regulate cell proliferation and differentiation, and involvement in cancers. Cell Commun Signal. 2006;4:7. doi: 10.1186/1478-811X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fluhrer R, et al. A γ-secretase-like intramembrane cleavage of TNFα by the GxGD aspartyl protease SPPL2b. Nat Cell Biol. 2006;8:894–6. doi: 10.1038/ncb1450. [DOI] [PubMed] [Google Scholar]
- 65.Friedmann E, Hauben E, Maylandt K, Schleeger S, Vreugde S, Lichtenthaler SF, Kuhn PH, Stauffer D, Rovelli G, Martoglio B. SPPL2a and SPPL2b promote intramembrane proteolysis of TNFα in activated dendritic cells to trigger IL-12 production. Nat Cell Biol. 2006;8:843–8. doi: 10.1038/ncb1440. [DOI] [PubMed] [Google Scholar]
- 66.Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–8. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 67.Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–41. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikeuchi T, Sisodia SS. The Notch ligands, Delta1 and Jagged2, are substrates for presenilin-dependent “ γ-secretase” cleavage. J Biol Chem. 2003;278:7751–4. doi: 10.1074/jbc.C200711200. [DOI] [PubMed] [Google Scholar]
- 69.LaVoie MJ, Selkoe DJ. The Notch ligands, Jagged and Delta, are sequentially processed by α-secretase and presenilin/γ-secretase and release signaling fragments. J Biol Chem. 2003;278:34427–37. doi: 10.1074/jbc.M302659200. [DOI] [PubMed] [Google Scholar]
- 70.Six E, Ndiaye D, Laabi Y, Brou C, Gupta-Rossi N, Israel A, Logeat F. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and γ-secretase. Proc Natl Acad Sci U S A. 2003;100:7638–43. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–52. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anders L, Mertins P, Lammich S, Murgia M, Hartmann D, Saftig P, Haass C, Ullrich A. Furin-, ADAM 10-, and γ-secretase-mediated cleavage of a receptor tyrosine phosphatase and regulation of β-catenin’s transcriptional activity. Mol Cell Biol. 2006;26:3917–34. doi: 10.1128/MCB.26.10.3917-3934.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haapasalo A, Kim DY, Carey BW, Turunen MK, Pettingell WH, Kovacs DM. Presenilin/γ-secretase-mediated cleavage regulates association of leukocyte-common antigen-related (LAR) receptor tyrosine phosphatase with β-catenin. J Biol Chem. 2007;282:9063–72. doi: 10.1074/jbc.M611324200. [DOI] [PubMed] [Google Scholar]
- 75.Frade JM. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. J Neurosci. 2005;25:1407–11. doi: 10.1523/JNEUROSCI.3798-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung KM, et al. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–9. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- 77.Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–36. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–32. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Underwood CK, Reid K, May LM, Bartlett PF, Coulson EJ. Palmitoylation of the C-terminal fragment of p75(NTR) regulates death signaling and is required for subsequent cleavage by γ-secretase. Mol Cell Neurosci. 2008;37:346–58. doi: 10.1016/j.mcn.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Urra S, et al. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal γ--mediated release of the p75 intracellular domain. J Biol Chem. 2007;282:7606–15. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 81.Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by α-secretase and γ-secretase requires specific receptor domains. J Biol Chem. 2005;280:14563–71. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
- 82.Kuhn PH, Marjaux E, Imhof A, De Strooper B, Haass C, Lichtenthaler SF. Regulated intramembrane proteolysis of the interleukin-1 receptor II by α-, β-, and γ-secretase. J Biol Chem. 2007;282:11982–95. doi: 10.1074/jbc.M700356200. [DOI] [PubMed] [Google Scholar]
- 83.Cowan JW, Wang X, Guan R, He K, Jiang J, Baumann G, Black RA, Wolfe MS, Frank SJ. Growth hormone receptor is a target for presenilin-dependent γ-secretase cleavage. J Biol Chem. 2005;280:19331–42. doi: 10.1074/jbc.M500621200. [DOI] [PubMed] [Google Scholar]
- 84.Meyer EL, Strutz N, Gahring LC, Rogers SW. Glutamate receptor subunit 3 is modified by site-specific limited proteolysis including cleavage by γ-secretase. J Biol Chem. 2003;278:23786–96. doi: 10.1074/jbc.M301360200. [DOI] [PubMed] [Google Scholar]
- 85.Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003;278:41182–8. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- 86.Liu CX, Ranganathan S, Robinson S, Strickland DK. γ-Secretase-mediated release of the low density lipoprotein receptor-related protein 1B intracellular domain suppresses anchorage-independent growth of neuroglioma cells. J Biol Chem. 2007;282:7504–11. doi: 10.1074/jbc.M608088200. [DOI] [PubMed] [Google Scholar]
- 87.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by γ-secretase. J Biol Chem. 2003;278:37386–92. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 88.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 89.Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem. 2007;101:517–29. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- 90.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem. 2004;279:34302–10. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]
- 91.Schulte A, et al. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by α- and γ-secretases. Biochem Biophys Res Commun. 2007;358:233–40. doi: 10.1016/j.bbrc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 92.El Fiky A, Arch AE, Krolewski JJ. Intracellular domain of the IFNaR2 interferon receptor subunit mediates transcription via Stat2. J Cell Physiol. 2005;204:567–73. doi: 10.1002/jcp.20305. [DOI] [PubMed] [Google Scholar]
- 93.El Fiky A, Pioli P, Azam A, Yoo K, Nastiuk KL, Krolewski JJ. Nuclear transit of the intracellular domain of the interferon receptor subunit IFNaR2 requires Stat2 and Irf9. Cell Signal. 2008;20:1400–8. doi: 10.1016/j.cellsig.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saleh AZ, Fang AT, Arch AE, Neupane D, El Fiky A, Krolewski JJ. Regulated proteolysis of the IFNaR2 subunit of the interferon-α receptor. Oncogene. 2004;23:7076–86. doi: 10.1038/sj.onc.1207955. [DOI] [PubMed] [Google Scholar]
- 95.Kim DY, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–64. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. Presenilin/γ-secretase-mediated cleavage of the voltage-gated sodium channel β 2-subunit regulates cell adhesion and migration. J Biol Chem. 2005;280:23251–61. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 97.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J Biol Chem. 2005;280:23009–17. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 98.Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an A β-like peptide. J Biol Chem. 2002;277:44754–9. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 99.Murakami D, Okamoto I, Nagano O, Kawano Y, Tomita T, Iwatsubo T, De Strooper B, Yumoto E, Saya H. Presenilin-dependent γ-secretase activity mediates the intramembranous cleavage of CD44. Oncogene. 2003;22:1511–6. doi: 10.1038/sj.onc.1206298. [DOI] [PubMed] [Google Scholar]
- 100.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, Wong AJ, Saya H. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–62. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelletier L, Guillaumot P, Freche B, Luquain C, Christiansen D, Brugiere S, Garin J, Manie SN. γ-secretase-dependent proteolysis of CD44 promotes neoplastic transformation of rat fibroblastic cells. Cancer Res. 2006;66:3681–7. doi: 10.1158/0008-5472.CAN-05-3870. [DOI] [PubMed] [Google Scholar]
- 102.Bonn S, Seeburg PH, Schwarz MK. Combinatorial expression of α- and γ-protocadherins alters their presenilin-dependent processing. Mol Cell Biol. 2007;27:4121–32. doi: 10.1128/MCB.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, Daniel J, Fujita Y. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem. 2008;283:12691–700. doi: 10.1074/jbc.M708887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haas IG, Frank M, Veron N, Kemler R. Presenilin-dependent processing and nuclear function of γ-protocadherins. J Biol Chem. 2005;280:9313–9. doi: 10.1074/jbc.M412909200. [DOI] [PubMed] [Google Scholar]
- 105.Hambsch B, Grinevich V, Seeburg PH, Schwarz MK. γ-Protocadherins, presenilin-mediated release of C-terminal fragment promotes locus expression. J Biol Chem. 2005;280:15888–97. doi: 10.1074/jbc.M414359200. [DOI] [PubMed] [Google Scholar]
- 106.Marambaud P, et al. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–56. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–45. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 108.Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944–52. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “ γ-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–8. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 110.Schulz JG, Annaert W, Vandekerckhove J, Zimmermann P, De Strooper B, David G. Syndecan 3 intramembrane proteolysis is presenilin/γ-secretase-dependent and modulates cytosolic signaling. J Biol Chem. 2003;278:48651–7. doi: 10.1074/jbc.M308424200. [DOI] [PubMed] [Google Scholar]
- 111.Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, Altevogt P, Saftig P, Reiss K. L1 is sequentially processed by two differently activated metalloproteases and presenilin/γ-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005;25:9040–53. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ali N, Knauper V. Phorbol ester-induced shedding of the prostate cancer marker transmembrane protein with epidermal growth factor and two follistatin motifs 2 is mediated by the disintegrin and metalloproteinase-17. J Biol Chem. 2007;282:37378–88. doi: 10.1074/jbc.M702170200. [DOI] [PubMed] [Google Scholar]
- 114.Andersson CX, Fernandez-Rodriguez J, Laos S, Baeckstrom D, Haass C, Hansson GC. Shedding and γ-secretase-mediated intramembrane proteolysis of the mucin-type molecule CD43. Biochem J. 2005;387:377–84. doi: 10.1042/BJ20041387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andersson CX, Fernandez-Rodriguez J, Laos S, Sikut R, Sikut A, Baeckstrom D, Hansson GC. CD43 has a functional NLS, interacts with β-catenin, and affects gene expression. Biochem Biophys Res Commun. 2004;316:12–7. doi: 10.1016/j.bbrc.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 116.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell. 2005;16:5061–9. doi: 10.1091/mbc.E05-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bohm C, Seibel NM, Henkel B, Steiner H, Haass C, Hampe W. SorLA signaling by regulated intramembrane proteolysis. J Biol Chem. 2006;281:14547–53. doi: 10.1074/jbc.M601660200. [DOI] [PubMed] [Google Scholar]
- 118.Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P. Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem. 2006;281:34357–64. doi: 10.1074/jbc.M606740200. [DOI] [PubMed] [Google Scholar]
- 119.Galichet A, Weibel M, Heizmann CW. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem Biophys Res Commun. 2008;370:1–5. doi: 10.1016/j.bbrc.2008.02.163. [DOI] [PubMed] [Google Scholar]
- 120.Carey BW, Kim DY, Kovacs DM. Presenilin/γ-secretase and α-secretase-like peptidases cleave human MHC Class I proteins. Biochem J. 2007;401:121–7. doi: 10.1042/BJ20060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin L, Fluhrer R, Reiss K, Kremmer E, Saftig P, Haass C. Regulated intramembrane proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/SPPL2b. J Biol Chem. 2008;283:1644–52. doi: 10.1074/jbc.M706661200. [DOI] [PubMed] [Google Scholar]
- 122.Kim DY, Ingano LA, Kovacs DM. Nectin-1 α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. J Biol Chem. 2002;277:49976–81. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 123.Goldschneider D, Rama N, Guix C, Mehlen P. The neogenin intracellular domain regulates gene transcription via nuclear translocation. Mol Cell Biol. 2008;28:4068–79. doi: 10.1128/MCB.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gowrishankar K, Zeidler MG, Vincenz C. Release of a membrane-bound death domain by γ-secretase processing of the p75NTR homolog NRADD. J Cell Sci. 2004;117:4099–111. doi: 10.1242/jcs.01263. [DOI] [PubMed] [Google Scholar]
- 125.Wang R, Tang P, Wang P, Boissy RE, Zheng H. Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer’s disease mutation. Proc Natl Acad Sci U S A. 2006;103:353–8. doi: 10.1073/pnas.0509822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakahara S, et al. A secreted type of β 1,6 N-acetylglucosaminyltransferase V (GnT-V), a novel angiogenesis inducer, is regulated by γ-secretase. FASEB J. 2006;20:2451–9. doi: 10.1096/fj.05-5066com. [DOI] [PubMed] [Google Scholar]
- 127.Kim J, Jahng WJ, Di Vizio D, Lee JS, Jhaveri R, Rubin MA, Shisheva A, Freeman MR. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer Res. 2007;67:9229–37. doi: 10.1158/0008-5472.CAN-07-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boerner JL, Demory ML, Silva C, Parsons SJ. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol. 2004;24:7059–71. doi: 10.1128/MCB.24.16.7059-7071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ, Levy DE, Depinho RA, Bonni A. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 2008;22:449–62. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–18. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang SC, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 132.Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929–39. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bryant DM, Stow JL. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic. 2005;6:947–54. doi: 10.1111/j.1600-0854.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 134.Larkin J, 3rd, Johnson HM, Subramaniam PS. Differential nuclear localization of the IFNGR-1 and IFNGR-2 subunits of the IFN-γ receptor complex following activation by IFN-γ. J Interferon Cytokine Res. 2000;20:565–76. doi: 10.1089/10799900050044769. [DOI] [PubMed] [Google Scholar]
- 135.Subramaniam PS, Flowers LO, Haider SM, Johnson HM. Signal transduction mechanism of a peptide mimetic of interferon-γ. Biochemistry. 2004;43:5445–54. doi: 10.1021/bi036213t. [DOI] [PubMed] [Google Scholar]
- 136.Subramaniam PS, Johnson HM. Lipid microdomains are required sites for the selective endocytosis and nuclear translocation of IFN-γ, its receptor chain IFN-γ receptor-1, and the phosphorylation and nuclear translocation of STAT1 α. J Immunol. 2002;169:1959–69. doi: 10.4049/jimmunol.169.4.1959. [DOI] [PubMed] [Google Scholar]
- 137.Subramaniam PS, Johnson HM. The IFNAR1 subunit of the type I IFN receptor complex contains a functional nuclear localization sequence. FEBS Lett. 2004;578:207–10. doi: 10.1016/j.febslet.2004.10.085. [DOI] [PubMed] [Google Scholar]
- 138.Bonacchi A, et al. Nuclear localization of TRK-A in liver cells. Histol Histopathol. 2008;23:327–40. doi: 10.14670/HH-23.327. [DOI] [PubMed] [Google Scholar]
- 139.Moughal NA, Waters C, Sambi B, Pyne S, Pyne NJ. Nerve growth factor signaling involves interaction between the Trk A receptor and lysophosphatidate receptor 1 systems: nuclear translocation of the lysophosphatidate receptor 1 and Trk A receptors in pheochromocytoma 12 cells. Cell Signal. 2004;16:127–36. doi: 10.1016/j.cellsig.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 140.Zwaagstra JC, Guimond A, O’Connor-McCourt MD. Predominant intracellular localization of the type I transforming growth factor-β receptor and increased nuclear accumulation after growth arrest. Exp Cell Res. 2000;258:121–34. doi: 10.1006/excr.2000.4905. [DOI] [PubMed] [Google Scholar]
- 141.Conway-Campbell BL, et al. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13331–6. doi: 10.1073/pnas.0600181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lobie PE, Mertani H, Morel G, Morales-Bustos O, Norstedt G, Waters MJ. Receptor-mediated nuclear translocation of growth hormone. J Biol Chem. 1994;269:21330–9. [PubMed] [Google Scholar]
- 143.Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G. Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem. 1994;269:31735–46. [PubMed] [Google Scholar]
- 144.Pereno R, et al. IL-15/IL-15Rα intracellular trafficking in human melanoma cells and signal transduction through the IL-15Rα. Oncogene. 2000;19:5153–62. doi: 10.1038/sj.onc.1203873. [DOI] [PubMed] [Google Scholar]
- 145.Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999;256:192–7. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- 146.Ilan N, Tucker A, Madri JA. Vascular endothelial growth factor expression, β-catenin tyrosine phosphorylation, and endothelial proliferative behavior: a pathway for transformation? Lab Invest. 2003;83:1105–15. doi: 10.1097/01.lab.0000083531.84403.8b. [DOI] [PubMed] [Google Scholar]
- 147.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–51. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garcia RAG, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–01. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB-4 at synapses. Neuron. 2002;26:443–55. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 151.Xu X, Ye S, Gambetti P, Sui D, Cui MZ. Identification of a novel PSD-95/Dlg/ZO-1 (PDZ)-like protein interacting with the C-terminus of presenilin-1. J Biol Chem. 1999;274:32543–6. doi: 10.1074/jbc.274.46.32543. [DOI] [PubMed] [Google Scholar]
- 152.Lau KF, McLoughlin DM, Standen C, Miller CC. X11 alpha and x11 beta interact with presenilin-1 via their PDZ domains. Mol Cell Neurosci. 2000;16:557–65. doi: 10.1006/mcne.2000.0898. [DOI] [PubMed] [Google Scholar]
- 153.Sandvig K, van Deurs B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Letters. 2002;529:49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- 154.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nature Rev Cell Mol Biol. 2006;7:563–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 155.Marsh M, Helenius A. Virus entry: open season. Cell. 2006;124:729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


