Abstract
Purpose of review
Airway mucus plugging has long been recognized as a principal cause of death in asthma. However, molecular mechanisms of mucin overproduction and secretion have not been understood until recently. These mechanisms are reviewed together with ongoing investigations relating them to lung pathophysiology.
Recent findings
Of the five secreted gel-forming mucins in mammals, only MUC5AC and MUC5B are produced in significant quantities in intrapulmonary airways. MUC5B is the principal gel-forming mucin at baseline in small airways of humans and mice, and therefore likely performs most homeostatic clearance functions. MUC5AC is the principal gel-forming mucin upregulated in airway inflammation and is under negative control by forkhead box a2 and positive control by hypoxia inducible factor-1. Mucin secretion is regulated separately from production, principally by extracellular triphosphate nucleotides that bind P2Y2 receptors on the lumenal surface of airway secretory cells, generating intracellular second messengers that activate the exocytic proteins, Munc13-2 and synaptotagmin-2.
Summary
Markedly upregulated production of MUC5AC together with stimulated secretion leads to airflow obstruction in asthma. As MUC5B appears to mediate homeostatic functions, it may be possible to selectively inhibit MUC5AC production without impairing airway function. The precise roles of mucin hypersecretion in asthma symptoms such as dyspnea and cough and in physiologic phenomena such as airway hyperresponsiveness remain to be defined.
Keywords: airway, asthma, mucin, mucous, mucus
Introduction
The central role of mucus hypersecretion in severe asthma has been known since pathologic studies in the late nineteenth and early twentieth centuries. However, exactly what constitutes mucus hypersecretion and how it connects with disease manifestations other than asphyxiation has been poorly understood. This review will present recent data that clarify the relations among mucous metaplasia, mucin production, mucin secretion, and mucus hypersecretion. The connections between these molecular and cellular processes and the symptoms, signs, and pathophysiology of asthma are increasingly studied and will also be reviewed.
Mucus hypersecretion
To begin, a few definitions are warranted. The term ‘mucin’ denotes very large (typically >1000kDa), heavily glycosylated (typically >70% carbohydrate) proteins that contain characteristic domains, including at least one region rich in serines and threonines that are the sites of O-glycosylation [1,2••]. There are approximately 20 mucin and mucin-like genes encoded in mammalian genomes, and these are designated by the letters MUC (all capitals in humans, first letter only capitalized in mice) followed by a number [3,4]. Mucins can be divided into those that are anchored in the plasma membrane and those that are secreted. The secreted mucins can be further divided into those that contain sulfhydryl groups near their termini, which allow cross-linking, and those that do not; the former are primarily responsible for the structure of the mucus gel layer and are often referred to as polymeric or gel-forming mucins. ‘Mucus’ refers to the extracellular mixture of mucins that have been secreted or released by hydrolysis of a membrane anchor, other macromolecules such as proteoglycans and antimicrobial proteins, water, ions, and cellular debris. ‘Mucous’ is an adjective referring to an association with mucin or mucus, as in ‘mucous metaplasia’ or ‘mucous cell’.
The airway epithelium is normally covered by a mucus gel layer approximately 5–50μm thick that overlies a liquid layer approximately 7μm in depth [5–7]. Cilia beat within the liquid layer, propelling the gel layer proximally to the pharynx where it is swallowed together with entrapped particles and pathogens. Under pathological conditions, airway mucins may be produced and secreted in greatly increased quantities (‘mucus hypersecretion’). This can be observed pathologically as an increase in intracellular mucins (‘mucous metaplasia’) or an increase in mucus in the airway lumen. Excessive lumenal mucus may become impacted and lead to airway closure. In this circumstance, cough clearance can complement ciliary clearance. However, the volume of expectorated mucus (sputum) correlates only weakly with distal airway mucus hypersecretion for the following reasons. Mucus produced in submucosal glands in large airways can lead to large amounts of sputum with little distal airway obstruction in diseases such as chronic bronchitis or bronchiectasis. Conversely, distal airways may be impacted with mucus produced by surface epithelium with minimal sputum in diseases such as asthma, bronchiolitis, or emphysema-predominant chronic obstructive pulmonary disease [3].
Mucous metaplasia
In small airways of humans (<2mm lumenal diameter) and all intrapulmonary airways of mice, there are few or no visible ‘mucous’ or ‘goblet’ cells by histochemical staining under baseline conditions. In allergic inflammation [8,9••,10], a rapid and dramatic increase in epithelial staining for carbohydrates (e.g., alcian blue and/or periodic acid Shiff, AB—PAS) occurs that is traditionally termed ‘mucous metaplasia’ (Fig. 1a Fig. 1 [10,11•]). Metaplasia denotes a change in cellular phenotype, and examples include the differentiation of proximal airway epithelium from a mucociliary into a squamous phenotype as a result of cigarette smoke exposure or the differentiation of distal esophageal cells from a squamous to a mucous phenotype as a result of gastric reflux. However, recent work has revealed that airway secretory (Clara) cells of mice produce gel-forming mucins even at baseline but secrete them so rapidly that there is insufficient intracellular mucin to be visible by insensitive histochemical techniques. More sensitive immunohistochemical staining utilizing sandwich amplification techniques reveals intracellular Muc5b at baseline [11•,12••], and mice defective in airway epithelial apical secretion accumulate sufficient Muc5b at baseline to be visible even by simple histochemical staining [12••]. Thus, the amount of intracellular mucin in airway epithelial cells reflects the relative balance between the rates of mucin production and secretion.
Figure 1.
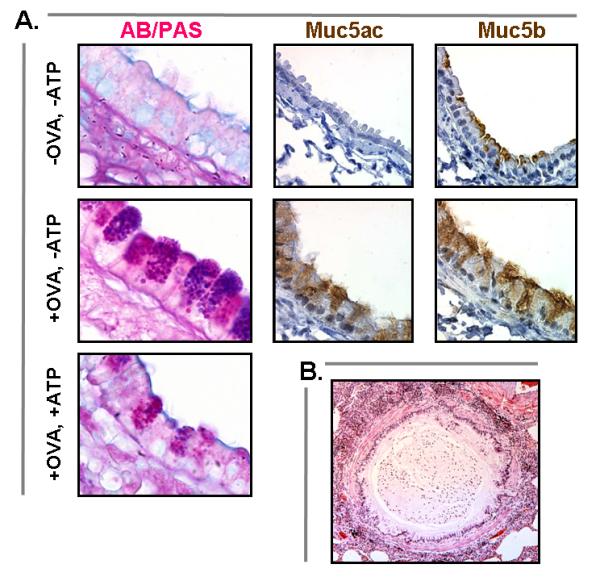
Mucus hypersecretion gr1
(a) In ovalbumin-sensitized and challenged mice (+OVA, -ATP), there is a dramatic increase in the number of AB—PAS-positive mucous cells in the tracheobronchial airways (d). This increase is apparent 24h after challenge and peaks at 3–7 days. Despite the lack of AB—PAS staining at baseline (a), intracellular Muc5b (c) can be demonstrated by sensitive immunohistochemical techniques at the cell apex (-OVA, -ATP). It becomes redistributed throughout the distended cytoplasm (f) during mucous metaplasia (+OVA, -ATP). Muc5ac is not apparent immunohistochemically at baseline (b) but is strongly expressed (f) during mucous metaplasia (+OVA, -ATP). After stimulation of metaplastic mucous cells with aerosolized ATP, there is rapid secretion of most of the accumulated intracellular mucin (+OVA, +ATP) [10,11•]. (b) Airway from a patient who died from asthma showing extensive infiltration of the airway wall and surrounding lung tissue with inflammatory cells and mucus filling the airway lumen. AB—PAS, alcian blue and/or periodic acid Shiff; OVA, ovalbumin. Reproduced with permission from [10,11•] and by courtesy of Martha Warnock, University of California at San Francisco.
The secretory capacity of airway secretory cells does not appear to change substantially during allergic mucous metaplasia, as Clara cells themselves are capable of vigorous regulated secretion, and the expression of molecular components of the secretory machinery does not change during metaplasia [10,13•]. Neither does the rate of activity of the secretory machinery appear to change, as mucous cells in allergic metaplasia are highly responsive to secretagogues present in the inflammatory milieu [10,14•,15]. Rather, the accumulation of intracellular mucin in asthma primarily reflects a greatly increased production of Muc5ac along with a modest increase in Muc5b [9••,16••]. The simple upregulation of secreted products without a fundamental change in underlying secretory function of Clara/mucous cells does not warrant the designation ‘metaplasia’ any more than lymphocytes that upregulate cytokine production or endocrine cells that upregulate hormone production. Nonetheless, we retain the term because of its widespread use. Caution must be used in diagnosing mucous metaplasia histologically, as even cells with greatly increased mucin production can be stimulated to rapidly secrete intracellular mucins and lose their histochemical staining (Fig. 1). Measurement of the expression of Muc5ac and coordinately expressed genes such as calcium-activated chloride channel provides a molecular definition of mucous metaplasia that is not susceptible to the complex kinetics of intracellular mucin accumulation [9••,16••,17,18•,19,20•].
Control of polymeric mucin production
The induction of Muc5ac in allergically inflamed mice is dependent upon two important signaling pathways: the IL-13/IL-4 receptor-α complex [21–23] and the epidermal growth factor receptor (EGFR) [16••,24]. The functional dominance of these signaling pathways, however, does not translate into a simple intracellular pathway for Muc5ac gene activation. The principal signaling molecule activated by IL-13 is signal transducer and activator of transcription 6 (STAT6). STAT6 signaling in mouse airway Clara cells is necessary and sufficient for Muc5ac induction (mucous metaplasia) and airway hyperreactivity in response to IL-13 [22]. STAT6 binds to a canonical motif, 5′-TTCN4GAA-3′, but this motif is not present in the conserved promoter regions of any mammalian MUC5AC orthologs [9••]. One indirect mechanism that may explain IL-13-mediated Muc5ac promoter activation is STAT6-dependent downregulation of forkhead box a2 (Foxa2) (Fig. 2 Fig. 2). Foxa2 is a critical negative regulator of Muc5ac expression, and genetic deletion of Foxa2 in mice leads to constitutive Muc5ac overproduction resembling mucous metaplasia [25].
Figure 2.
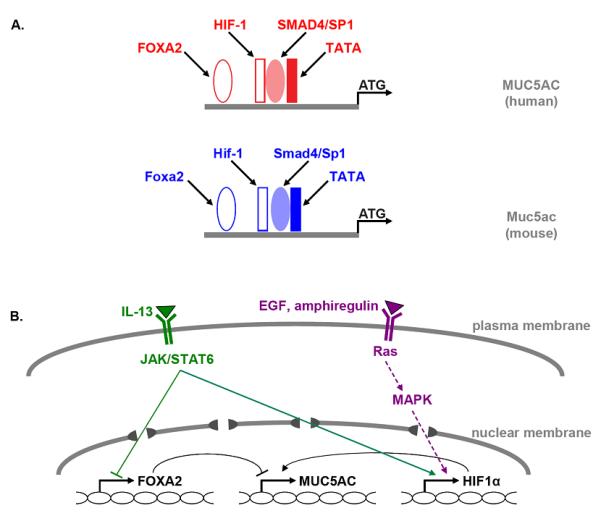
Transcriptional control of Muc5ac production gr2
(a) Conserved consensus-binding sites for transcription factors in the core promoters of the human MUC5AC (red) and the mouse Muc5ac (blue) genes are indicated. (b) Known pathways for activation of MUC5AC/Muc5ac gene transcription by IL-13 (green) and EGFR ligands (violet) are illustrated. Solid lines indicate direct protein interaction with target gene (i.e., STAT6), dotted lines indicate multiple steps of interaction (i.e., EGFR pathway), arrowheads indicate positive interaction, and bars indicate inhibitory interactions (see text for citations). EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; Foxa2, forkhead box a2; HIF, hypoxia inducible factor; JAK, janus-activated kinase; MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
EGFR signaling is also required for mucous metaplasia in a wide variety of animal and in-vitro models [24,26•,16••]. Transcription factors downstream of EGFR include Sp1 [27] and hypoxia inducible factor-1 (HIF-1) [9••]. Sp1 is a ubiquitous transcription factor that binds to a wide variety of promoters where it acts as a transactivator through interactions with other proteins [28], and its activity can be modulated by phosphorylation [29]. A role for HIF-1 was suggested when a consensus-binding motif was found in the core promotors of all presently sequenced mammalian Muc5ac orthologs [9••]. Its mutation dramatically reduces promoter activity, and its binding is induced by IL-13 and EGF stimulation [9••]. A critical role for HIF-1 in the regulation of Muc5ac in allergic mucous metaplasia is further attractive as it contains a conserved STAT6 motif in its promoter. Because HIF-1 is acutely responsive to a wide variety of inflammatory signals [30,31], its activation may be a central regulator of mucous metaplasia in the diverse inflammatory responses to allergens, fungi, viruses, and toxicants. EGFR signaling also downregulates Foxa2 expression [16••]. Nuclear factor-κB (NF-κB) consensus-binding sites have been identified in the human MUC5AC promoter [32], but these are not conserved in other mammalian genomes, and animal models do not support an important role for NF-kB activation in Muc5ac transcription and mucous metaplasia [9••,23,33,34•].
A required permissive role for β2-adrenoceptor signaling in mucous metaplasia has recently been identified. Inverse agonists that reduce β2-adrenoceptor signaling to less than its unliganded level markedly reduce intracellular mucin accumulation and Muc5ac gene expression in a mouse model of allergic mucous metaplasia [11•]. This is due to the loss of physiological β2-adrenoceptor signaling rather than the activation of a novel signaling pathway by these artificial ligands (biased agonism) as the β2-adrenoceptor knockout mouse phenocopies the exposure to inverse agonists (Richard A. Bond, personal communication). Increased β2-adrenoceptor signaling from agonist stimulation did not augment mucin accumulation in this model [11•], although it did (two-fold) in another model [35]. Together, these results suggest that the high density of β2-adrenoceptors in airway epithelium provides sufficient signaling from empty receptors or receptors bound by endogenous ligands to fully support mucous metaplasia induced by strong inflammatory stimuli, but that exogenous β-agonists might augment mucous metaplasia induced by weak stimuli. Whether signaling through a G-protein, β-arrestin, or some other pathway mediates this effect is not yet known.
Control of polymeric mucin secretion
Mucin secretion is controlled separately from mucin production, with triphosphate nucleotides such as ATP and UTP playing central roles in the regulation of secretion [10,13•,36•]. Extracellular nucleotides also play important roles in the regulation of airway surface liquid depth and ciliary beat frequency, suggesting that multiple aspects of mucociliary clearance are coordinately regulated by nucleotides released into the airway surface liquid layer [36•]. Other ligands such as cholinergic agonists can also induce polymeric mucin secretion in vivo [14•,15], but whether this is indirect through the release of extracellular nucleotides induced by smooth muscle contraction is not yet known. Extracellular nucleotides bind to P2Y2 receptors on the apical surface of airway secretory cells to generate intracellular second messengers that activate the exocytic machinery, leading to the rapid release of polymeric mucins into the airway lumen (Fig. 3a Fig. 3 [12•,13•,37•,38,39•,40–42]).
Figure 3.
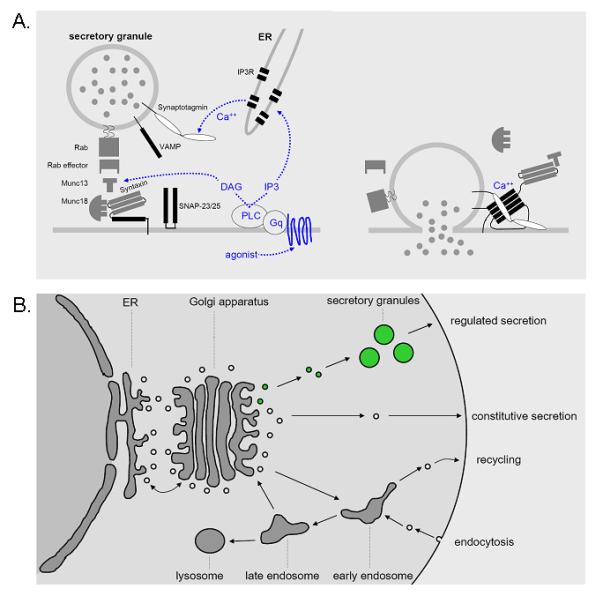
Mechanism of polymeric mucin secretion gr3
(a) Extracellular nucleotides in the airway surface liquid layer bind to apical P2Y2 receptors that activate the trimeric G-protein Gq, which in turn activates phospholipase C β1, generating the intracellular second messengers, diacylglycerol and inositol trisphosphate (IP3) [13•]. Diacylglycerol directly induces mucin granule exocytosis by activating the priming protein, Munc13-2 [12••], and indirectly regulates exocytosis by activating protein kinase Cε [37•]. IP3 induces the release of Ca2+ from intracellular stores [38,39•,40,41], resulting in a rise in cytoplasmic Ca2+ that rapidly triggers mucin granule exocytosis through the activation of synaptotagmin-2. In airway secretory cells, IP3 receptors are localized to endoplasmic reticulum that lies in close apposition to mucin granules at the apical pole (C. William Davis, personal communication). The reliance of airway secretory cells on intracellular Ca2+ stores to activate exocytosis may reflect the instability of Ca2+ concentrations in airway lining fluid that is directly exposed to the external environment [42], in contrast to nonexocrine secretory cells bathed in interstitial fluid or plasma with tightly controlled Ca2+ concentrations. Activation of Munc13 and synaptotagmin allows formation of a four-helix bundle termed the core complex (black rectangles) that draws secretory granule and plasma membranes tightly together and induces their fusion (right). This leads to the release of granule contents, including polymeric mucins, into the airway lumen. The molecular identities of the core complex isoforms in airway secretory cells are not yet known. (b) Data from mouse genetic models suggest that both baseline and stimulated mucin secretion occur through varying rates of activity of a single regulated exocytic machinery acting on a single population of mucin secretory granules (green). DAG, diacylglycerol; ER, endoplasmic reticulum; IP3R, inositol trisphosphate receptor; PLC, phospholipase C; VAMP, vesicle-associated membrane protein.
Analysis of the phenotypes of mice with null mutations in exocytic proteins has been highly informative of the molecular composition of the secretory machinery and its mechanism of function [13•]. Munc13-2 null mice accumulate mucin in the absence of inflammation or increased mucin production, suggesting that baseline mucin secretion is mediated by a low baseline rate of activity of a regulated exocytic machine as Munc13 proteins function exclusively in regulated and not in constitutive exocytosis [12••]. In addition, these mice are defective in stimulated secretion, supporting the notion that a single machine mediates both baseline and stimulated secretion (Fig. 3b). The profound defect in stimulated mucin secretion in Syt-2 null mice but the absence of mucin accumulation under baseline conditions [13•] can be explained by loss of the positive role of Syt-2 at high cytoplasmic Ca2+ concentrations and loss of its inhibitory role at low cytoplasmic Ca2+ concentrations [43]. Together, these results lead to a parsimonious model in which a single population of mucin granules is released at a low tonic rate under baseline conditions and a high phasic rate upon exocytic stimulation (Fig. 3). Additional analyses in mutant mice will further test this model.
Additional mucin secretory proteins have been identified and studied functionally. The myristoylated alanine-rich C-kinase substrate (MARCKS) protein plays roles in positioning secretory granules near the plasma membrane and in remodeling the cortical actin cytoskeleton to allow membrane apposition [13•]. A lipidated peptide that enters cells and interferes with MARCKS function markedly reduced mucin secretion in cultured human airway cells and in mice in a model of allergic mucous metaplasia [15]. This allows pharmacologic manipulation of mucin secretion in pathophysiologic models (see below) and is being developed as a therapeutic [44]. In addition, the cysteine string protein [45] and Munc18b (Kyubo Kim and Michael J. Tuvim, personal communication) regulate mucin secretion.
Mucus hypersecretion in airflow obstruction and airway hyperresponsiveness
Autopsy studies in Germany in the 1880s first identified widespread airway mucus plugging as a central cause of death from asthma [46]. These findings have been repeatedly confirmed [3], and a recent quantitative study of fatal asthma found more than 98% of airways occluded to some extent by mucus [47]. However, the contribution of mucus hypersecretion to airflow obstruction in nonfatal asthma relative to other contributors to airway closure such as extravasated plasma and to airway narrowing from smooth muscle contraction and wall thickening remains to be determined [48,49]. The increase in baseline airway and lung tissue resistance in Foxa2 knockout mice with spontaneous Muc5ac overproduction supports a substantial role for mucus [25,25].
Similarly, the role of mucus hypersecretion in airway hyperresponsiveness relative to closure from other causes and airway narrowing remains to be fully defined. Although it might seem that lumenal occlusion by secreted mucins would be offset by an equal reduction in airway epithelial cell volume with no net change in the aerated lumen cross-sectional area, this is not the case as mucins swell approximately 500-fold after secretion because of hydration [50]. Pharmacological inhibition of mucin secretion with a MARCKS peptide blocked methacholine-induced airway resistance increases by approximately 80% in a mouse model of allergic asthma [14•], suggesting a major contribution by mucus hypersecretion at least under some circumstances. Future studies examining animals deficient in mucin production and secretion and humans treated with inhibitors of mucus hypersecretion will further clarify the role of mucus in baseline airflow obstruction and in airway hyperresponsiveness.
An evolutionary perspective on mucus hypersecretion
The resemblance between allergic asthma and parasitic worm (helminth) infestation of the lungs has been frequently noted. Symptoms of cough and wheezing, airway infiltration by immune cells such as eosinophils and type 2 helper T (Th2) lymphocytes, and elevated Th2 cytokines are prominent in both disorders. This suggests that asthma is an atavistic response to a misperceived pulmonary worm infestation. Insight into how the misperception might occur has been provided by the recent identification of chitin, which is found in helminths and common triggers of asthma such as dust mites and fungi, as a powerful innate stimulus of type 2 inflammation [51,52••]. Similarly, secreted proteases are abundant in helminths as well as in fungi, the feces of dust mites, and the saliva of cats and may serve as another innate stimulus of type 2 inflammation [53••,54••]. Residence of dust mites in homes, domestication of cats, and other factors that increase exposure to type 2 stimuli, in concert with decreased exposure to type 1 and type 17 stimuli (the ‘hygiene hypothesis’), may explain the rise in asthma prevalence in modern societies.
Of what value might mucin hypersecretion be in defense against helminths? Because increased production of MUC5AC can be induced within minutes, and mucin secretion can be induced within seconds, mucin hypersecretion together with airway smooth muscle contraction might trap worms in distal airways and allow their killing before they can ascend to the pharynx to be swallowed to complete their lifecycle in the gastrointestinal tract. Although closure of hundreds of airways from local stimulation by transiting worms does not result in serious pathophysiology, closure of millions of airways from diffuse stimulation by an airborne allergen in asthma can have serious consequences. Such derangement of an adaptive physiologic mechanism is similar to the useful localized vasoconstriction of poorly ventilated segments of lung to maintain ventilation—perfusion matching in pneumonia, but the pathophysiological diffuse vasoconstriction that leads to pulmonary hypertension upon ascent to altitude. Whether airway mucin secretion contributes significantly to defense against worm infestation is a testable hypothesis using mutant mice defective in mucin production and secretion.
Conclusion
The role of mucin hypersecretion in death from asthma has long been recognized, but its contributions to symptoms, signs, and pathophysiology in less severe asthma are subjects of ongoing investigation. MUC5B is the principal gel-forming mucin produced and secreted in small airways under healthy conditions, and MUC5AC is the principal gel-forming mucin upregulated during asthmatic inflammation. Because MUC5AC may serve little or no important function in healthy airways, it may be possible to therapeutically suppress MUC5AC production in the airways with minimal toxicity. Strategies to suppress mucin secretion are being developed and might be helpful in acute, severe asthma.
Acknowledgments
This study is supported by grants from the National Institutes of Health, Cystic Fibrosis Foundation, and American Heart Association
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- 2••.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. An authoritative, up-to-date review of the gel-forming mucins of the airway.
- 3.Williams OW, Sharafkhaneh A, Kim V, et al. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther. 2008 doi: 10.1016/j.pharmthera.2008.11.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 7.Widdicombe JG. Airway liquid: a barrier to drug diffusion? Eur Respir J. 1997;10:2194–2197. doi: 10.1183/09031936.97.10102194. [DOI] [PubMed] [Google Scholar]
- 8.Ordonez CL, Khashayar R, Wong HH, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 9••.Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. Direct, quantitative comparison of polymeric mucin gene expression during allergic airway inflammation in mice, along with bioinformatic and functional analysis of Muc5ac core promoter.
- 10.Evans CM, Williams OW, Tuvim MJ, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Nguyen LP, Omoluabi O, Parra S, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. First report of a required permissive role for β-adrenoceptor signaling in mucous metaplasia.
- 12••.Zhu Y, Ehre C, Abdullah LH, et al. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. Identification of a key regulated component of the mucin exocytic machinery. Functional analysis uncovers the presence of Muc5b within nonmetaplastic Clara cells and points to a single regulated exocytic machine that mediates mucin secretion both at baseline and upon stimulation.
- 13•.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. Up-to-date review of the composition, function, and regulation of the airway secretory cell exocytic machinery.
- 14•.Agrawal A, Rengarajan S, Adler KB, et al. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol. 2007;102:399–405. doi: 10.1152/japplphysiol.00630.2006. This study establishes the role of mucus hypersecretion in airway hyperresponsiveness in allergic airway inflammation in mice.
- 15.Singer M, Martin LD, Vargaftig BB, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 16••.Zhen G, Park SW, Nguyenvu LT, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. This study quantitatively measures Muc5ac and Muc5b expression in response to IL-13 in mice in vivo and human bronchial epithelial cells in vitro, identifies genes that are coordinately regulated with mucins, demonstrates downregulation of Foxa2 expression by both IL-13 and EGFR, and dissects key differences in the regulation of MUC5AC by IL-13 and EGFR.
- 17.Lewis CC, Yang JY, Huang X, et al. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med. 2008;177:376–387. doi: 10.1164/rccm.200702-333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Lee CG, Hartl D, Matsuura H, et al. Endogenous IL-11 signaling is essential in Th2- and IL-13-induced inflammation and mucus production. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0053OC. [Epub ahead of print] Demonstrates a novel role for IL-11Rα signaling in allergic mucous metaplasia in mice.
- 19.Grayson MH, Cheung D, Rohlfing MM, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. This study examines in fresh tissue from humans, changes in epithelial gene expression that are usually examined only in experimental animals or in cultured human cells.
- 21.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 22.Kuperman DA, Huang X, Nguyenvu L, et al. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol. 2005;175:3746–3752. doi: 10.4049/jimmunol.175.6.3746. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker L, Niu N, Temann UA, et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol. 2002;27:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 24.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan H, Kaestner KH, Ang SL, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 26•.Deshmukh HS, Shaver C, Case LM, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008;38:446–454. doi: 10.1165/rcmb.2006-0339OC. This study helps elucidate the mechanism of mucous metaplasia induced by an important component of cigarette smoke.
- 27.Perrais M, Pigny P, Copin MC, et al. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;277:32258–32267. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- 28.Wierstra I. Sp1: emerging roles — beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 29.Chu S, Ferro TJ. Sp1: regulation of gene expression by phosphorylation. Gene. 2005;348:1–11. doi: 10.1016/j.gene.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Hellwig-Burgel T, Stiehl DP, Wagner AE, et al. Review: hypoxia-inducible factor-1 (HIF-1) — a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 31.Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohrman A, Miyata S, Gallup M, et al. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1998;1406:251–259. doi: 10.1016/s0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam SJ, Clement CG, De la Garza MM, et al. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Pantano C, Ather JL, Alcorn JF, et al. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. This study shows that activation of NF-κB in airway secretory cells does not lead to mucous metaplasia in mice in vivo, despite earlier reports suggesting regulation of MUC5AC expression by NF-κB.
- 35.Kamachi A, Munakata M, Nasuhara Y, et al. Enhancement of goblet cell hyperplasia and airway hyperresponsiveness by salbutamol in a rat model of atopic asthma. Thorax. 2001;56:19–24. doi: 10.1136/thorax.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163:208–213. doi: 10.1016/j.resp.2008.05.015. This authoritative review brings together evidence and mechanism for the coordinated regulation of multiple aspects of mucociliary clearance by extracellular nucleotides.
- 37•.Ehre C, Zhu Y, Abdullah LH, et al. nPKCepsilon, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol. 2007;293:C1445–C1454. doi: 10.1152/ajpcell.00051.2007. Surprising demonstration of the functional importance of one protein kinase C isoform in regulation of mucin exocytosis in mice in vivo, despite prior focus on the association of the membrane translocation of another isoform with exocytic signaling.
- 38.Rossi AH, Sears PR, Davis CW. Ca2+ dependency of ‘Ca2+-independent’ exocytosis in SPOC1 airway goblet cells. J Physiol. 2004;559:555–565. doi: 10.1113/jphysiol.2004.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Rossi AH, Salmon WC, Chua M, Davis CW. Calcium signaling in human airway goblet cells following purinergic activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L92–L98. doi: 10.1152/ajplung.00081.2006. This study provides a confirmation of the importance of intracellular calcium release rather than calcium entry from the extracellular space in airway polymeric mucin secretion, together with measurement of cytoplasmic calcium transients and pharmacologic dissection of signaling pathways.
- 40.Kemp PA, Sugar RA, Jackson AD. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2004;31:446–455. doi: 10.1165/rcmb.2003-0211OC. [DOI] [PubMed] [Google Scholar]
- 41.Bahra P, Mesher J, Li S, et al. P2Y2-receptor-mediated activation of a contralateral, lanthanide-sensitive calcium entry pathway in the human airway epithelium. Br J Pharmacol. 2004;143:91–98. doi: 10.1038/sj.bjp.0705913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Effros RM, Peterson B, Casaburi R, et al. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99:1286–1292. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 43.Pang ZP, Melicoff E, Padgett D, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;38:116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Fang S, Crews AL, et al. MARCKS regulation of mucin secretion by airway epithelium in vitro: interaction with chaperones. Am J Respir Cell Mol Biol. 2008;39:68–76. doi: 10.1165/rcmb.2007-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogg JC, Hegele RG. Postmortem pathology. In: Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ, editors. Asthma. Lippincott-Raven; New York: 1997. pp. 201–208. [Google Scholar]
- 47.Kuyper LM, Pare PD, Hogg JC, et al. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 48.Wagers SS, Haverkamp HC, Bates JH, et al. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol. 2007;102:221–230. doi: 10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]
- 49.Lundblad LK, Thompson-Figueroa J, Allen GB, et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 51.Dickey BF. Exoskeletons and exhalation. N Engl J Med. 2007;357:2082–2084. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 52••.Reese TA, Liang HE, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. This study provides fundamental insight into the initiation and deviation of inflammatory signaling in response to helminths and allergens.
- 53••.Kiss A, Montes M, Susarla S, et al. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. This study, together with work cited in [54], establishes proteolysis as a mechanism for the initiation of type 2 inflammatory signaling leading to Th2-deviated adaptive immune responses.
- 54••.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. This study extends the findings of [53] by demonstrating that basophil is a key target of proteolytic signaling.


