Abstract
Bestrophin 1 (Best1) controls intracellular Ca2+ concentration, induces Ca2+-activated Cl- conductance, and increases proliferation of colon carcinoma cells. Here, we show that expression of Best1 in mouse renal collecting duct (CD) cells causes i) an increase in cell proliferation, ii) a loss of amiloride-sensitive Na+ absorption, iii) induction of Ca2+-dependent Cl- conductance (CaCC), and iv) epithelial-to-mesenchymal transition. During conditions of high proliferation or when we exposed CD cells to serum or TGF–β1, we observed upregulation of Best1, increased CaCC, redistribution of the epithelial-to-mesenchymal transition marker β-catenin, and upregulation of vimentin. In contrast, suppression of Best1 by RNAi inhibited proliferation, reduced CaCC, and downregulated markers of EMT. CaCC and expression of Best1 were independent of the cell cycle but clearly correlated to cell proliferation and cell density. During renal inflammation in LPS-treated mice or after unilateral ureteral obstruction, we observed transient upregulation of Best1. These data indicate that repression of cell proliferation, CaCC, and expression of Best1 occurs during mesenchymal-to-epithelial transition once CD cells polarize and terminally differentiate. These results may suggest a role for Best1 in renal fibrosis and tissue repair.
Principal cells of the renal collecting duct (CD) reabsorb Na+ through epithelial Na+ channels (ENaC), while stimulation of luminal purinergic receptors enhances intracellular Ca2+ and inhibits ENaC by means of hydrolysis of phosphatidylinositol bisphosphate (P1P2).1–4 In intact micro-perfused CDs, no evidence was found for a luminal Ca2+-dependent Cl- conductance (CaCC).2,5 In contrast, CaCC was readily detectable in M1 mouse CD cells and primary cultured renal epithelial cells.6,7 This raises the question as to what degree available CD culture models represent native renal tubular function. Discrepancies between properties in intact tubules and isolated epithelial cells are well recognized and may be caused by epithelial-to-mesenchymal transition (EMT) occurring during primary cell culture.
Transition from an epithelial to a mesenchymal phenotype during cell culture of epithelial cells is well known and has been examined previously.8 Also during development, both EMT and the reverse process, namely mesenchymal-to-epithelial transition (MET) are fundamental processes. EMT contributes to degeneration of mature epithelial structures and to generation of fibroblasts associated with accumulation of extracellular matrix during renal inflammation (reviewed in9). EMT also takes place during morphogenesis, wound healing and tissue repair, and is observed during tumor progression. The most important stimulus for EMT is TGF-β.9,10 In fact, TGF-β is able to induce a genetic program of cell plasticity that involves key pathways and regulators of epithelial dedifferentiation, cytoskeletal reorganization, and proliferation.11 We recently detected an EMT-like process in T84 colonic carcinoma cells, which induced enhanced proliferative activity of this cell line.12 Notably, ion channels, such as the cell cycle regulated hEag1 potassium channel as well as the putative Ca2+-dependent Cl- channel bestrophin 1 (Best1), were upregulated during EMT. Both channels supported proliferation in fast growing T84 cells.12
Best1 was shown to form a Ca2+-activated Cl- channel but, surprisingly, also regulates intracellular Ca2+ signaling.13–15 We identified Best1 as a component important for the activation of endogenous Ca2+-activated Cl- channels in airway cells and other epithelial tissues.16–18 A novel family of Ca2+ activated Cl- channels (TMEM16) has been identified recently, with biophysical properties strikingly similar to endogenous CaCC.19–21 Strong evidence was presented that TMEM16A is, in fact, the endogenous Ca2+-activated Cl- channel, and studies are underway to examine the role of Best1 in activation of TMEM16A.19–21 Here, we asked whether expression of Best1 is upregulated during dedifferentiation of collecting duct cells, and if Best1 induces proliferation, EMT, and enhanced Ca2+-activated Cl- conductance.
RESULTS
Coincidence of CaCC and EMT in CD Cells
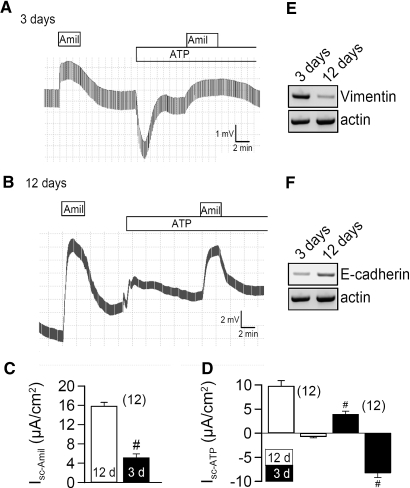
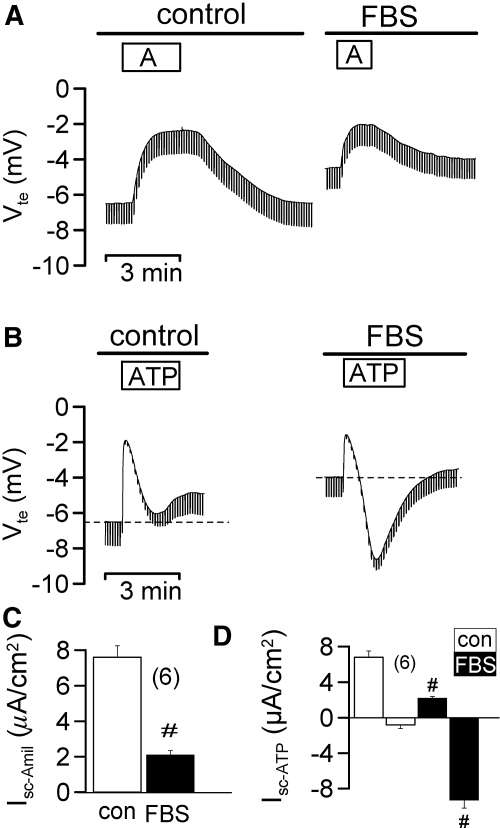
M1 CD cells grown on permeable supports for 3 d in the presence of 10% bovine serum formed tight monolayers (Rte = 228 ± 23 Ωcm2; n = 37), when mounted in a perfused micro Ussing chamber, and expressed amiloride sensitive Na+ channels (ENaC), as indicated by amiloride (10 μM)-induced positive voltage deflection (Figure 1A). Application of luminal ATP (100 μM) activated a luminal Ca2+ dependent Cl- conductance (CaCC; negative voltage deflection) and inhibited ENaC. When M1 cells were grown for 12 d in the absence of serum, they formed higher resistance monolayers (Rte = 874 ± 56 Ωcm2; n = 28) and showed augmented amiloride sensitive short circuit currents (Isc-Amil). Under these conditions, ATP no longer activated CaCC but only induced a transient K+ secretion, as indicated by positive voltage deflections (Figure 1, B through D). As these properties are similar to those of native CD,2 M1 cells may undergo MET upon polarization of filter membranes. In fact, expression of the marker for EMT, vimentin, was reduced in polarized cultures, while E-cadherin expression was enhanced (Figure 1, E and F). In contrast, acute application of 1% serum (FBS) to polarized M1 cells inhibited Isc-Amil and enabled ATP to activate CaCC (Figure 2). Thus, proliferation and loss of polarization of collecting duct cells correlates with upregulation of CaCC and probably with EMT.
Figure 1.
Ion conductances in M1 cells are determined by cell polarization. Original recordings of transepithelial voltages and effects of luminal amiloride (Amil, 10 μM) and ATP (10 μM) measured in little polarized (A) and highly polarized (B) M1 monolayers. (C) Summary of the equivalent amiloride sensitive short circuit currents (Isc) in M1 cells grown to low (3 d; black bars) or high (12 d; white bars) polarization. (D) Summary of ATP-activated short circuit currents (Isc after ATP-stimulation − Isc under control conditions = ΔIsc or Isc -ATP). In poorly polarized monolayers, ATP activates a Cl- secretion (negative voltage deflection), while highly polarized cells activate a K+ secretion (positive voltage deflection). # indicates significant difference between M1 cells at low and high polarization (unpaired t test). n = number of monolayers. (E) The EMT marker vimentin is expressed at higher levels in poorly polarized cells (RT-PCR; n = 2). (F) Higher levels of E-cadherin are found in differentiated cells (RT-PCR; n = 2).
Figure 2.
M1 cells are less polarized under growth conditions. Original recordings of transepithelial voltages and effect of luminal application of 1% fetal bovine serum (FBS) on amiloride (A) and ATP (B) sensitive transport. FBS attenuates Na+ absorption but induces Ca2+-dependent Cl- secretion by ATP (negative voltage deflection). Summary of amiloride sensitive transport (C) and ATP-activated short circuit currents (Isc after ATP-stimulation − Isc under control conditions = Isc-ATP) (D) in the absence (white bars) or presence (black bars) of FBS. # indicates significant difference between absence and presence of FBS (unpaired t test). (n) = number of monolayers.
Induction of EMT Enhances Expression of Best1 and CaCC
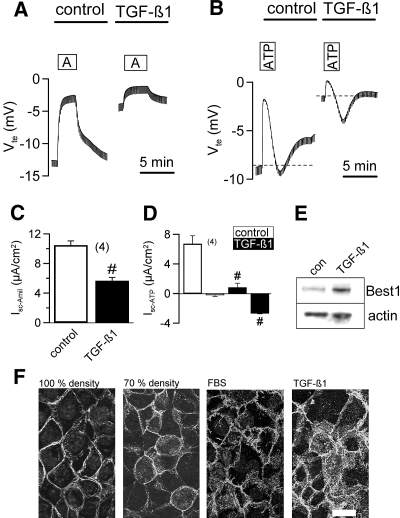
TGF-β1 has been described as a major factor for EMT.9 We incubated highly polarized M1 cells with TGF-β1 (5 ng/ml, 5 h) and observed a remarkable decrease in amiloride sensitive Na+ absorption and induction of Ca2+-dependent Cl- secretion, similar to the treatment with FBS (Figure 3, A through D). Inhibition of ENaC by TGF-β1 was slightly attenuated by 15.3 ± 2.1% (n = 5) upon treatment with siRNA for Best1. Notably, incubation with TGF-β1 for 6 h upregulated expression of Best1 in CD cells (Figure 3E). In contrast, other cytokines like IL1-β (100 ng/ml), IL-8 (10 ng/ml), IFN-γ (100 ng/ml), and TNF-α (100 ng/ml) did not activate CaCC but abolished amiloride sensitive transport as reported earlier (data not shown).22 Moreover, activation of CaCC by TGF-β1 was not affected by inhibition of PI3K (Ly294002; 10 μM), MAPK kinase or Erk1,2 (SB203840; 25μM, U0126; 5 μM), or interfering with the NO pathway (SNAP; 30 μM, L-NAME; 100 μM) (data not shown). Redistribution and cytosolic location of β-catenin is a hallmark of EMT and is often observed during tumor invasion.25 Redistribution of β-catenin to the cytosol was also observed in proliferative M1 cells (70% density) or after treatment with FBS or TGF-β1 (Figure 3F). Moreover, exposure to FBS induced expression of the EMT marker vimentin but decreased E-cadherin expression (data not shown). Thus, TGF-β1 promotes EMT, increased Best1-expression, and activation of CaCC.
Figure 3.
TGF-β1 activates Ca2+-dependent Cl- secretion in M1 cells. Original recordings of transepithelial voltages and effects of incubation with 0.5 nM TGF-β1 on amiloride- (A) and ATP- (B) sensitive transport. TGF-β1 attenuates Na+ absorption and induces Ca2+-dependent Cl- secretion by ATP (negative voltage deflections). Summary of amiloride sensitive Isc (C) and ATP-activated short circuit currents (Isc after ATP-stimulation − Isc under control conditions = Isc-ATP) (D) in the absence (white bars) or presence (black bars) of TGF-β1. (E) Western blot indicates upregulation of Best1 by TGF-β1 (n = 2). (F) Expression of β-catenin in M1 collecting duct cells under various conditions. Cytosolic staining of β-catenin is found at low cell density and after incubation with FBS or TGF-β1, suggesting EMT under these conditions (n = 5 for each series; scale bar = 20 μm). # indicates significant difference between absence and presence of TGF-β1 (unpaired t test). (n) = number of monolayers.
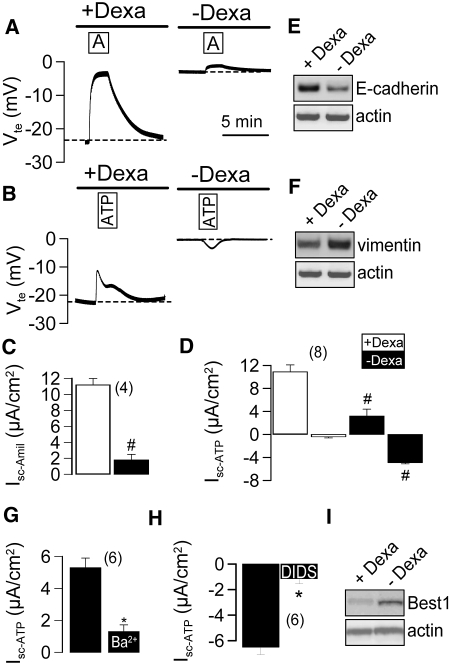
Glucocorticoids such as dexamethasone are known to induce expression of epithelial Na+ channels and have been reported to reduce cell proliferation.26 We compared transport properties of M1 cells that had been grown in the presence of low (0.05 μM) or high (1 μM) dexamethasone concentration (Figure 4, A and B). High dexamethasone concentrations induced a large ENaC and a luminal ATP activated K+ conductance as demonstrated by inhibition of the K+ conductance with 5 mM Ba2+ (Figure 4, C and D). Both ENaC and K+ conductance were reduced in the absence of dexamethasone, while 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) -inhibited CaCC was readily detectable (Figure 4, C and D). Moreover, dexamethasone-induced expression of E-cadherin (Figure 4E) reduced expression of the EMT marker vimentin, as indicated by semiquantitative RT-PCR (Figure 4F). Isc-ATP in dexamethasone-treated cells was due to a Ba2+-sensitive K+ conductance, while Isc-ATP in the absence of dexamethsone was due to a DIDS-sensitive Cl- conductance (Figure 4, G and H). Western blot analysis of Best1 clearly indicated downregulation of Best1-expression by dexamethasone (Figure 4I).
Figure 4.
Dexamethasone suppresses Ca2+ dependent Cl- secretion in M1 cells. (A and B) Original recordings of transepithelial voltages in M1 cells grown on permeable supports in the absence or presence of dexamethasone (5 μM). Summary of amiloride-sensitive Isc (C) and ATP-activated short circuit currents (Isc after ATP-stimulation − Isc under control conditions = Isc-ATP) (D) in the absence (black bars) or presence (white bars) of dexamethasone. (E and F). Dexamethasone induces expression of E-cadherin but reduces expression of the EMT marker vimentin (n = 2 for each RT-PCR). (G) Positive voltage deflections induced by ATP (Isc-ATP) in Dexa-treated cells are due to Ba2+-sensitive K+ conductance. (H) Negative voltage deflections induced by ATP (Isc-ATP) in the absence of Dexa are due to DIDS-sensitive Cl- conductance. # indicates significant difference between absence and presence of dexamethasone (unpaired t test). (n) = number of monolayers. (I) Western blot indicates downregulation of Best1 by dexamethasone (n = 2).
CaCC and Best1 Are Expressed Only in Highly Proliferating CD Cells
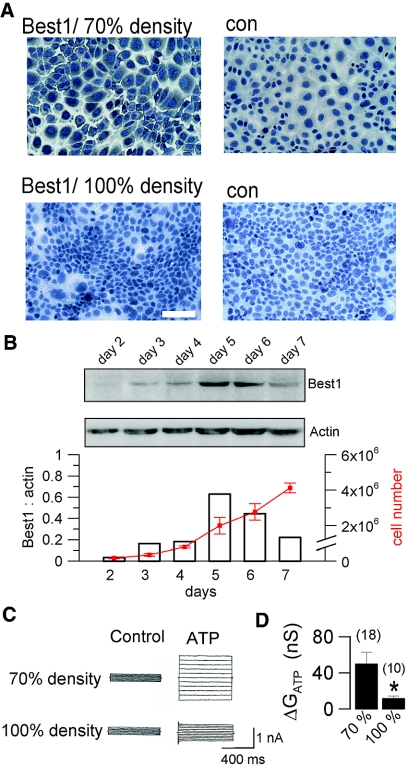
Best1 has been shown previously to induce both proliferation and CaCC in colonic carcinoma cells.12 We found pronounced Best1-expression in cytosolic compartments of highly proliferative M1 cells at a cell density of approximately 70%, which was almost completely downregulated in confluent monolayers (Figure 5A). Expression of Best1 reached a peak during the log phase of proliferation but was turned off after cells reached confluence (Figure 5B). Patch clamp experiments indicated largely reduced activation of CaCC at high cell density, indicating a clear correlation between cell density, Best1 expression, and CaCC (Figure 5, C and D). Notably, the reduced ATP response in confluent monolayers was not due to a loss of P2Y2 receptors (Supplement 1A).
Figure 5.
Expression of Best1 and Ca2+ activated Cl- currents are proliferation dependent. (A) Immunohistochemistry of Best1 in mouse M1 cells grown at 70% density (log phase; upper panels) and to complete confluence (lower panels) (n = 5 for each series; scale bar = 50 μm). (B) Expression of Best1 (western blots in duplicates) during 7 d of culture and cell proliferation (n = 3). C) Activation of whole cell Cl- currents by ATP in cells grown to 70% and 100% confluence, respectively. Cells were voltage clamped from −50 to + 50 mV. (D) Summary of the ATP-induced whole cell Cl- conductances at 70% and 100% density. # indicates significant difference between high and low density (unpaired t test). (n) = number of experiments.
Bestrophins Support Proliferation of M1 Cells
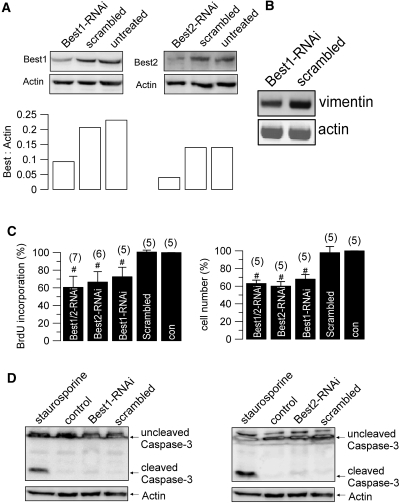
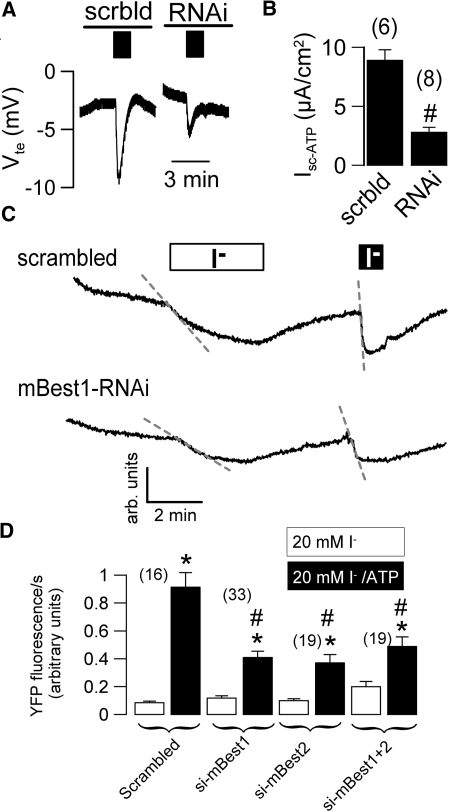
To further examine whether Best1 affects proliferation of CD cells, we used RNAi to suppress expression of Best1 and the paralog Best2, respectively (Figure 6A). Cell proliferation was assessed by BrdU incorporation and cell counting, and was suppressed by approximately 40% with either Best1-RNAi or Best2-RNAi (Figure 6, A and C). Notably, expression of the EMT marker vimentin was reduced in Best1-siRNA treated cells (Figure 6B). No evidence was found for induction of apoptosis by RNAi, as indicated by a lack of caspase-3 cleavage (Figure 6D). Activation of CaCC by ATP (Isc-ATP) was attenuated in RNAi-treated cells, as demonstrated in Ussing chamber recordings (Figure 7, A and B). This was further confirmed by reduced iodide-quenching of the fluorescence signal from the halide sensitive dye YFP, in Best1-RNAi- and Best2-RNAi-treated cells (Figure 7, C and D). In additional experiments, overexpression of Best1 increased cell proliferation by 18 ± 4.5%, as measured by cell number (n = 3). Taken together, bestrophins support proliferation of CD cells and are essential for generating a Ca2+ activated Cl- conductance. Notably, common CaCC (and Best1) inhibitors, such as DIDS, also inhibited cell proliferation by approximately 30%, supporting the role of Best1 and CaCC for cell proliferation (Supplement 1B).
Figure 6.
Bestrophins control cell proliferation. (A) Western blots for Best1 (left panel) and Best2 (right panel) and ratios for both Best1 and Best2 to β-actin expression (lower panels) in M1 cells. Expression of both Best1 and Best2 was inhibited by siRNAs (n = 2 for each series). (B) Expression of the EMT marker vimentin is reduced in Best1-siRNA treated cells (RT-PCR; n = 2). (C) Proliferation of M1 cells assessed by BrdU incorporation and cell counting. Both Best1-RNAi and Best2-RNAi inhibit proliferation. (D) Western blotting of cleaved and uncleaved caspase 3 in M1 cells (n = 3). Activation of protein kinase C by staurosporine but not siRNA for Best1 or Best2 induced caspase 3 cleavage and cell apoptosis. # indicates significant difference between different treatments (unpaired t test). (n) = number of dishes.
Figure 7.
RNAi-suppression of Best1 inhibits Ca2+ dependent Cl- currents in M1 cells. (A) Original recordings of transepithelial voltages and effects of luminal ATP (black bars; 10 μM) measured in M1 cells treated with scrambled RNA or RNAi for Best1. (B) Summary of Isc-ATP obtained in experiments with M1 cells as shown in (A). Original recordings (C) and summary (D) of the fluorescence produced by the halide sensitive yellow fluorescence protein (YFP) and effect of siRNA-knockdown of Best1 and Best2. Cl- conductance was assessed by isotonic application of 20 mM iodide in the absence (white bar) or presence (black bar) of ATP (100 μM). ATP activated a Ca2+-activated Cl- conductance and enhanced I- uptake and fluorescence quenching. ATP-induced fluorescence quenching was reduced by siRNA-knockdown of Best1 and Best2. # indicates significant difference between different treatments (unpaired t test). (n) = number of dishes.
Since expression of Best1 was largely dependent on cell proliferation, we asked whether expression of Best1 was cell cycle-dependent. Yet synchronization of the cells into early G1, G1/S, and M-phase,12 did not reveal any major changes in Best1-expression or CaCC during cell cycling (Supplement 2). Staining of Best1 during early G1, G1/S, and M-phase detected a perinuclear staining during eG1, which changed into a cytosolic staining during G1/S (Supplement 3A). This finding was supported by western blotting, which showed an increase of Best1 in the perinuclear fraction of the cell lysate during eG1 (Supplement 3B).
Expression of Best1 is Controlled by Cell Density and a Soluble Factor and Upregulated during Tissue Repair
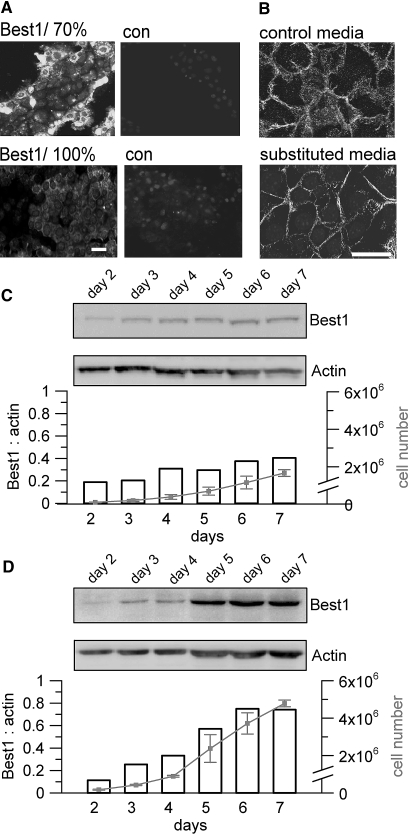
As pointed out, expression of Best1 is downregulated in dense and polarized monolayers. Notably, M1 cells at the edge of a cell island demonstrated particularly strong staining for Best1 (Figure 8A). β-catenin staining was typically broad, but M1 cells changed to a membrane restricted β-catenin expression when grown in a media that was substituted by 50% media from confluent monolayers (Figure 8B). Under these conditions both cell proliferation and Best1-expression were largely reduced (Figure 8C). In contrast, when grown in a medium that was frequently refreshed (2 times per day) Best1-expression was no longer downregulated when reaching confluence and proliferation was enhanced (Figure 8D).
Figure 8.
Cell density controls expression of Best1. (A) Immunohistochemistry of Best1 in mouse M1 cells. When grown to 70% density, cells at the edge of an island show high levels of Best1 expression, while cells in the center of islands and monolayers grown to 100% confluence downregulate Best1 expression (n = 5 for each series). (B) β-catenin localization in cells grown in normal media (control media) or cells exposed to conditioned medium from confluent monolayers (substituted media) (n = 3 for each series). Membrane-limited expression of β-catenin suggests MET in cells exposed to conditioned medium (scale bars = 20 μm). (C) Reduced cell proliferation and Best1-expression in cells grown in media substituted by supernatant (50 vol%) from confluent M1 cultures. (D) Enhanced proliferation and Best1-expression in cells grown in media that was frequently (twice per day) replaced (n = 2 for each series).
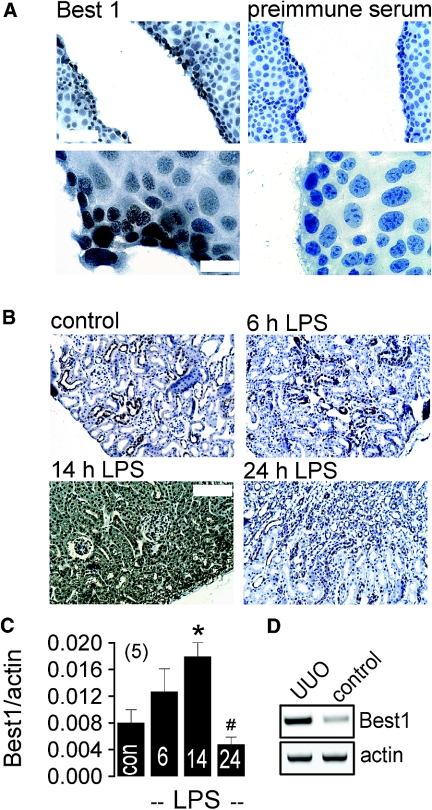
These results suggest that EMT is induced by soluble factors such as TGF-β1, while MET occurs during polarization possibly engaging autocrine mechanisms. EMT in renal epithelial cells could be important for the replacement of aged cells and during posttraumatic tissue repair. In fact, in a scratch assay using densely grown M1 cells, we detected upregulation of Best1 in the proliferating cells facing the tissue defect (Figure 9A). Moreover intraperitoneal injection of LPS, which is known to induce renal inflammation and inhibition of the epithelial Na+ channel ENaC,22,27 caused upregulation of tubular Best1 expression and increase in Best1-mRNA as detected by real-time RT-PCR (Figure 9, B and C). Western blot analysis confirmed a transient increase in renal Best1-expression in LPS-treated animals, while expression of other ion channels such as CFTR did not change (Supplement 4A). In another model for renal damage using unilateral ureter obstruction (UUO), we also found significant upregulation of Best1 by 65 ± 8.2%, as demonstrated by semiquantitative RT-PCR (n = 5 animals for each series) (Figure 9D). These data indicate that Best1 is upregulated during EMT transition. Since EMT is considered a contributor of renal fibrosis, attenuating expression or function of Best1 could be of therapeutic potential.
Figure 9.
Upregulation of Best1 during tissue repair. (A) Immunocytochemistry of Best1 in a scratch assay with densely grown M1 cells. Upregulation of Best1 in proliferating cells facing the monolayer defect, at lower (upper panel, scale bar = 100 μm) and higher (lower panel, scale bar = 20 μm) magnification (n = 5). (B) Immunohistochemistry showing time dependent expression of renal Best1 expression in mice treated with LPS (intraperitoneal injection of 10 mg/kg; n = 5 mice). (C) Summary of real time RT-PCR showing time dependent increase of renal Best1-expression after LPS injection. *,# indicate significant difference between control and 14 h, and 14h and 24 h after LPS injection, respectively (unpaired t test). (n) = number of animals. (D) Semiquantitative RT-PCR indicates upregulation of Best1-expression in renal epithelial cells after UUO (n = 4 animals for each series).
DISCUSSION
Here, we supply evidence that expression of Best1 in mouse renal collecting duct (CD) cells increases cell proliferation, causes Ca2+ dependent Cl- conductance and EMT, but is inversely correlated to amiloride-sensitive Na+ absorption. The data indicate that collecting duct cells retain their ability to express Ca2+ activated Cl- channels. Thus, in acutely isolated intramedullary CD cells, and various CD cell lines such as IMCD-K2, IMCD-K3, M1, and MDCKII, nucleotide-sensitive CaCC were identified.6,28–30 This is in contrast to data obtained from isolated perfused CD tubules that are regarded as the gold standard for epithelial transport studies. In isolated perfused CD tubules no evidence was found for a significant role of CaCC in either the luminal or basolateral membrane.2,5,31,32 The present results suggest that CD cells in culture undergo a rapid dedifferentiation program that can be characterized as EMT. Depending on the growth conditions, M1 cells transform either into rapidly proliferating nonpolarized cells or into a highly polarized nonproliferating epithelium. Thus, M1 cells undergo MET when grown under polarized conditions in a serum-free medium substituted with dexamethasone.
Notably, glucocorticoids and aldosterone-induced transcription factors such as ATF3 impinge directly on the activity of transport proteins and cellular differentiation.33–36 Interestingly, a related transcription factor, ATF2, was shown to be activated through TGF-β.37 In the present study, we found that dexamethasone is able to antagonize the effects of pro-inflammatory cytokines, such as TGF-β1. EMT is a process whereby renal tubular cells lose their epithelial characteristics and gain a fibroblast like phenotype. This process is induced by TGF-β mediated activation of the transcription factor E2A and has a potential role in renal fibrosis.38 However, as cellular responses to TGF-β are variable, TGF-β may cause either growth arrest or EMT, depending on the cell type and context of stimulation.
TGF-β1 can signal through NFκB to suppress Na+ transport in principal cells of the CD by downregulating the serum and glucocorticoid-regulated kinase SGK1, apart from other mechanisms for inhibition of ENaC in renal and airway epithelial cells.27,39,40 Notably, membrane expression of TGF-β strongly depends on cell polarization, which may explain why in the present study only cells at the edge of a monolayer expressed Best1 and proliferated, since they are able to expose their basolateral TGF-β receptors to serum factors.41 Similar observations were done in a colonic carcinoma cell line (T84), that changed from a polarized growth pattern to fast growing cells with augmented intracellular Ca2+ signaling.12 Thereby, ER-localized bestrophins may enhance cell proliferation and couple receptor mediated intracellular Ca2+ signaling to membrane localized Ca2+-dependent ion channels such as TMEM16A.18–21 As Ca2+ activated Cl- channels and probably Best1 expression are common features of epithelial cells undergoing dedifferentiation during primary cell culture,42 the present results suggest a novel role for both during EMT and probably embryonic development (Supplement 4B).
CONCISE METHODS
Animals, Cell Culture, cDNAs, siRNAs, and Transfection
Mice (C57B6) received NaCl (control) or LPS (Escherichia coli, serotype 0111:B4; Sigma, St. Louis, MO) (10 mg/kg intraperitoneal), and were killed 6, 12, and 24 h (n = 5 per group) after injection.22 Mice were treated with unilateral ureteral obstruction (UUO) in the laboratory of Prof. Dr. Mack (University of Regensburg, Germany) as described previously.23 M1 cells (mouse collecting duct cells) were grown at 37°C in 5% CO2 as described previously. 6 M1 cells were grown on permeable supports or plastic dishes to highly polarized monolayers in the absence or presence of serum (FBS) or TGF-β1. To verify EMT, cells were stained for β-catenin and semiquantitative RT-PCR was performed to examine expression of vimentin and E-cadherin, using β-actin as an internal standard. We purchased three different batches of duplexes of 25-nucleotide RNAi from Invitrogen (Karlsruhe, Germany). The sense strands of the RNAi used to silence the Best1 gene were 5′-UCCAGUACAUGUCACGUUCCAUGGG-3′, and for Best2 gene were 5′-UUUGCUCCGA AACACAUUCAGCUCC-3′, scrambled sequence RNAi ds-oligomer, not homologous to any known gene (BLOCK-ITTM Fluorescence Oligo) served as control. We transfected M1 cells using Lipofectamine™2000 (Invitrogen).
Western Blotting and Antibodies
M1 cells were homogenized in lysis buffer (mmol/L: NaCl 150, Tris 50, DTT 100, 1% NP-40, and 1% protease inhibitor cocktail) (Sigma), separated by 7% SDS-PAGE, and transferred to Hybond-P (Amersham Biosciences, Freiburg, Germany). Primary antibodies were either rabbit anti-mouse best1, rabbit anti-mouse best2, or rabbit anti caspase 3 antibody (Davids Biotechnology, Regensburg, Germany). Proteins were visualized using goat anti-rabbit IgG conjugated to horseradish peroxidase (Acris Antibodies, Hiddenhausen, Germany) and ECL (Amersham Biosciences).
Immunohistochemistry
M1 cells were fixed for 2 h with 4% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4. Sections were incubated overnight at 4°C with rabbit anti-mouse Best1 antibodies diluted 1:10,000 in Tris buffer containing Triton X-100 (0.8%) and goat serum to prevent nonspecific binding. Sections were incubated with horseradish peroxidase linked goat anti-rabbit secondary antibodies (Amersham Pharmacia Biotech, Freiburg, Germany). The avidin biotin peroxidase complex (ABC) technique was used to visualize the labeling with 3,3-diaminobenzidine. The ABC technique involves application of a biotin-labeled secondary antibody followed by the addition of the ABC.24 Sections were counterstained with Mayer's hematoxylin. Alternatively a fluorescence-labeled secondary antibody was used (diluted 1:1,000; Acris Antibodies, Hiddenhausen, Germany).
Patch Clamping, Ussing Chamber, and Iodide Quenching
We performed patch-clamp experiments in the fast whole-cell configuration as described recently.12 In intervals, membrane voltages (Vc) were clamped in steps of 10 mV from −50 to + 50 mV relative to resting potential. We calculated the membrane conductance Gm from the measured current (I) and Vc values according to Ohm's law. M1 cells grown to confluence on permeable supports (Millipore, Schwalbach, Germany) were mounted into a perfused micro Ussing chamber. The luminal and basolateral surfaces of the epithelium were perfused continuously with buffer solution at a rate of 5 to 10 ml/min (chamber volume 2 ml). We carried out all experiments at 37°C under open circuit conditions. We determined transepithelial resistance (Rte) by applying short (1-s) current pulses (ΔI = 0.5 μA) and continuously recorded the corresponding changes in transepithelial voltage (Vte) and basal Vte. Values for the Vte were referred to the serosal side of the epithelium. We calculated Rte according to Ohms law (Rte = ΔVte/ΔI) and the equivalent short-circuit current (Isc) according to Ohms law from Vte and Rte (Isc = Vte / Rte). We induced YFPI152L fluorescence by excitation (wave length 500 nm) using an poly-chromatic illumination system for microscopic fluorescence measurement (VisiChrome; Visitron Systems, Puchheim, Germany) and light emission was measured at 535 ± 15 nm with a photomultiplier detector (SF, Zeiss, Munich, Germany). We induced quenching of YFPI152L fluorescence via I- influx by replacing 20 mM extracellular Cl- with I-.
Proliferation Assays and Statistics
We seeded M1 cells at a density of 5000 cells/0.35 cm2 on 96-well plates (Sarstedt, Nuembrecht, Germany). We quantified cell proliferation by measuring 5-bromo-2′-deoxyuridine (BrdU) incorporation and performed cell counting as described recently.12 To measure the effect of RNAi treatment on cell proliferation, we seeded M1 cells at a density of 10,000 cells/0.35 cm2 in 96-well plates and treated wells with the siRNA. We used BLOCK-IT™ Fluorescence Oligos as controls. After 48 h, we quantified cell proliferation by measuring BrdU incorporation and by cell number. All compounds used were of the highest available grade of purity and were from Sigma (Taufkirchen, Germany) or Calbiochem (Darmstadt, Germany). The t test (for paired or unpaired samples as appropriate) was used for statistical analysis. P < 0.05 was accepted as significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by grants DFG SFB699A7, DFG KU 756/8-2, Else Kröner-Fresenius-Stiftung P36/05//A44/05, and TargetScreen2 (EU-FP6-2005-LH-037365). We gratefully acknowledge critical discussions and support of chemicals by Dr. Klaus Höcherl (Department of Physiology, University of Regensburg, Regensburg, Germay). We kindly acknowledge the generous supply of mRNA from UUO-treated animals and control mice by Prof. Mack (Department of Nephrology, University of Regensburg).
Published online ahead of print. Publication date available at www.jasn.org.
F.A. and M.S. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Schlatter E: Regulation of ion channels in the cortical collecting duct. Ren Physiol Biochem 16: 21–36, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J: Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Kunzelmann K, Bachhuber T, Regeer RR, Markovich D, Sun J, Schreiber R: Purinergic inhibition of the epithelial Na+ channel ENaC via hydrolysis of PIP2. FASEB J 18: 142–163, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD: Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Schlatter E, Greger R, Schafer JA: Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl- transport. Pflugers Arch 417: 317–323, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C: ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol (Lond) 524 Pt 1: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartzell HC, Putzier I, Arreola J: Calcium-activated chloride channels. AnnuRevPhysiol 67: 719–758, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Greenburg G, Hay ED: Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95: 333–339, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavadil J, Bottinger EP: TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Pennison M, Pasche B: Targeting transforming growth factor-beta signaling. Curr Opin Oncol 19: 579–585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP: Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA 98: 6686–6691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzner M, Martins JR, Barro Soria R, Ousingsawat J, Scheidt K, Schreiber R, Kunzelmann K: Eag1 and Bestrophin 1 are upregulated in fast growing colonic cancer cells. J Biol Chem 283: 7421–7428, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau KW, Nathans J: Structure-function analysis of the bestrophin family of anion channels. J Biol Chem 278: 41114–41125, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Tsunenari T, Yau KW, Nathans J: The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci USA 2002 Mar 99: 4008–4013, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, Marmorstein AD, Strauss O: Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J 20: 178–180, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Barro Soria R, Spitzner M, Schreiber R, Kunzelmann K: Bestrophin 1 enables Ca2+-activated Cl- conductance in epithelia. J Biol Chem 281: 17460–17467, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barro Soria R, Schreiber R, Kunzelmann K: Bestrophin 1 and 2 are components of the Ca2+-activated Cl- conductance in mouse airways. BBA 1783: 1993–2000, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Milenkovic VM, Schreiber R, Barro Soria R, AlDehni F, Kunzelmann K: Functional assembly and purinergic activation of bestrophins. Pflugers Arch (in press1). 2009 [DOI] [PubMed]
- 19.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U: TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Schroeder BC, Cheng T, Jan YN, Jan LY: Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ: TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M: Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D: A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM: Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92: 3439–3443, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarino M: Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 39: 2153–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A: Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 27.de Seigneux S, Leroy V, Ghzili H, Rousselot M, Nielsen S, Rossier BC, Martin PY, Feraille E: NF-kappa B inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem. 2008 [DOI] [PubMed]
- 28.Qu Z, Wei RW, Hartzell HC: Characterization of Ca2+-activated Cl currents in mouse kidney inner medullary collecting duct cells. Am J Physiol Renal Physiol 285: F326–F335, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Boese SH, Aziz O, Simmons NL, Gray MA: Kinetics and regulation of a Ca2+-activated Cl- conductance in mouse renal inner medullary collecting duct cells. Am J Physiol Renal Physiol 286: F682–F692, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Delles C, Haller T, Dietl P: A highly calcium-selective cation current activated by intracellular calcium release in MDCK cells. J Physiol 486: 557–569, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neil RG, Sansom SC: Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol 82: 281–295, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Imai M, Yoshitomi K: Electrophysiological study of inner medullary collecting duct of hamsters. Pflugers Arch 416: 180–188, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Stockand JD: New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Amanatullah DF, Zafonte BT, Pestell RG: The cell cycle in steroid hormone regulated proliferation and differentiation. Minerva Endocrinol 27: 7–20, 2002 [PubMed] [Google Scholar]
- 35.Verrey F: Early aldosterone action: Toward filling the gap between transcription and transport. Am J Physiol 277: F319–F327, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Dauwalder T, Adam G, Fakitsas P, Verrey F: Effect of the aldosterone-induced proteins ATF3 and Gremlin2 on distal nephron transport activity and differentiation. Acta Physiologica 192-S663: PM02–06-L1443, 2008 [Google Scholar]
- 37.Beier F, Ali Z, Mok D, Taylor AC, Leask T, Albanese C, Pestell RG, LuValle P: TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol Biol Cell 12: 3852–3863, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slattery C, McMorrow T, Ryan MP: Overexpression of E2A proteins induces epithelial-mesenchymal transition in human renal proximal tubular epithelial cells suggesting a potential role in renal fibrosis. FEBS Lett 580: 4021–4030, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Lang F, Klingel K, Wagner CA, Stegen C, Warntges S, Friedrich B, Lanzendorfer M, Melzig J, Moschen I, Steuer S, Waldegger S, Sauter M, Paulmichl M, Gerke V, Risler T, Gamba G, Capasso G, Kandolf R, Hebert SC, Massry SG, Broer S: Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy [see comments]. Proc Natl Acad Sci USA 97: 8157–8162, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF: Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Murphy SJ, Dore JJ, Edens M, Coffey RJ, Barnard JA, Mitchell H, Wilkes M, Leof EB: Differential trafficking of transforming growth factor-beta receptors and ligand in polarized epithelial cells. Mol Biol Cell 15: 2853–2862, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunzelmann K, Milenkovic VM, Spitzner M, Barro Soria R, Schreiber R: Calcium dependent chloride conductance in epithelia: Is there a contribution by Bestrophin? Pflügers Arch 454: 879–889, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.