Abstract
It is a great pleasure to see these major contributions on peroxynitrite biochemistry to be collected in this series and to be permitted to share a few biased observations here. The Archives of Biochemistry and Biophysics played a major role at a critical junction in my scientific career in 1992. For over a year, our work had been rejected after lengthy review times by Science, Nature and the Journal of Biological Chemistry. In contrast, the editors of Archives allowed us to publish a series of five papers that established peroxynitrite as a biological oxidant, showed how tyrosine nitration can be used to quantify peroxynitrite production and demonstrated that peroxynitrite was quite toxic to bacteria(1–5). The reviews from Archives were insightful rather than being judgmental as to whether the results were of sufficient interest. Rafael Radi has a similar experience with his Archives paper on the iron-independent oxidation of lipids induced by peroxynitrite (6). While judged “not of sufficient interest for the readers“ of journal X, the paper has now been cited over 1200 times. By having the space to fully describe the experimental work rather than being confined to cryptic footnotes, these papers has have received many more citations than other papers that my group has published in high profile journals. Journals such as Archives play an important role in allowing new hypotheses and data to be published of which the importance is not immediately recognized by journals focused on high impact. Access over the web such as by Pubmed gives equal exposure around the world. For new investigators, the difference in where you publish on your career may not be as great as you fear and the benefits of more fully explaining your research in multiple papers in the long run will carry greater weight.
Discovery of Tyrosine Nitration
Like so many discoveries, the five papers we published in Archives resulted from a serendipitous observation I made working late one night in the laboratory. I was characterizing the evolution of oxygen resulting from adding peroxynitrite to hydrogen peroxide. The oxygen evolution persisted longer than the peroxynitrite, so I wanted to test whether superoxide might be generated from hydrogen peroxide via self-propagating reactions involving trace extraneous iron. I added a large excess of bovine superoxide dismutase and was startled to see the solution turn bright yellow. I thought perhaps the peroxynitrite might still be present somehow bound to the SOD. When I acidified the media to drive peroxynitrite decomposition, the solution turned clear. But curiously when I added sodium hydroxide, the solution turned yellow again. Other experiments showed the yellow color was clearly bound to the bovine superoxide dismutase in an irreversible way.
At the time, I played soccer on a Birmingham (Alabama) city league with a crystallographer named Craig Smith, and we recruited Rafael Radi and Harry Ischiropoulos to join the team. Rafael was a post-doc with Bruce Freeman and Harry had just joined my lab as a post doc. After a long hot game, Craig mentioned to me that he could determine where small molecules docked to proteins if the 3D structure of the protein was known. I gave him some yellow SOD and he eventually showed there was an increase of electron density near the only tyrosine in bovine SOD. My group was also working on stroke at the time. Dr. Jun Chen was a surgeon working in my group. He was trying to understand the baroque chemistry of nitric oxide and peroxynitrite that was fast becoming the rest of the lab. He had left a Chinese chemistry textbook open on a lab bench to a page showing the structure of the pH indicator nitrophenol. Nitrophenol turns bright yellow below pH 7.5 due to ionization of the phenol moiety (Fig. 1). Although the text was in Chinese, suddenly it became clear to me that all our data fit with peroxynitrite nitrating the phenolic ring of tyrosine, which resulted in the yellow color on bovine superoxide dismutase. If we had used human superoxide dismutase instead, we would have missed the reaction, because the human sequence lacks tyrosine entirely.
Figure 1.
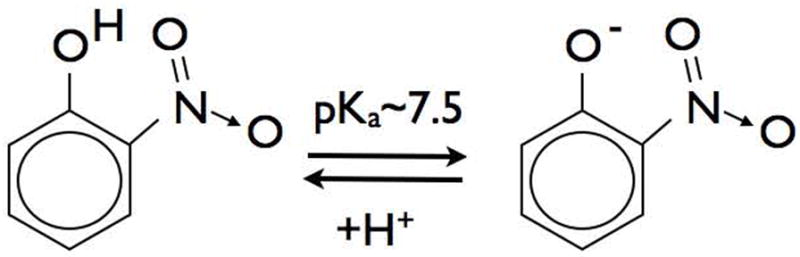
Nitrophenol turns yellow upon ionization at pH’s above 7.5. The biological implications for tyrosine nitration are significant, because nitrotyrosine will turn a hydrophobic residue into a more water soluble negatively charged amino acid at neutral pH.
Harry Ischiropoulos was experienced at isolating alveolar macrophages from rats and we used the nitration reaction catalyzed by superoxide dismutase as a means to detect peroxynitrite. The basic assay was to determine whether alveolar macrophages stimulated to produce superoxide and nitric oxide simultaneously would yield measurable tyrosine nitration in the presence of a large amount of superoxide dismutase. Harry reported the first experiments succeed with a suspension of activated macrophages turning yellow in the presence of a tyrosine analog and superoxide dismutase when we were all having dinner at Rafael Radi’s house. It was a major milestone because it was the first demonstration of peroxynitrite being produced biologically. We separately measured nitric oxide synthesis and its impact on superoxide production and oxygen consumption. All of these measurements were strikingly consistent with nitric oxide being quantitatively converted to peroxynitrite. This was also the first demonstration of tyrosine nitration resulting from an endogenous peroxynitrite formation and being catalyzed by superoxide dismutase. After we had been characterizing the SOD catalyzed nitration, we found that Hirosi Ohshima had found that nitrotyrosine was present in plasma from both smokers and nonsmoking controls by mass spectrometry (7).
The potential in vivo relevance of these results has been recently highlighted by a surprising result concerning acetaminophen poisoning (8). Acetaminophen toxicity is strongly correlated with increased tyrosine nitration and therapeutic approaches to reduce endogenous peroxynitrite formation greatly reduce damage to the liver even when administered many hours after acetaminophen (9–11). Remarkably, both toxicity and tyrosine nitration were greatly diminished in superoxide dismutase-knockout mice (8). One would expect that the loss of superoxide dismutase activity would increase peroxynitrite formation and tyrosine nitration, yet precisely the opposite effect is seen; tyrosine nitration in liver of SOD1-knock out mice is virtually eliminated mice are remarkable resistant at acetaminophen (12).
Linking Nitration to Cell Death
The ability of SOD to catalyze nitration led to my current focus on amyotrophic lateral sclerosis (ALS). In 1993, mutations were discovered in the Cu, Zn SOD1 gene that correlated strongly with a rare but dominant familial inheritance pattern in ALS patients (13). ALS is a fatal disease characterized by the progressive loss of motor neurons over a 1–5 year period. Strong overexpression of the mutant human gene to mice or rats caused motor neuron degeneration in these animals as fast as four months. Our in vitro experiments led us to propose that the loss of zinc from SOD left the copper more exposed and make it more redox active (14,15). This was borne out in our recent determination of the crystal structure of zinc-deficient SOD (16). We showed that zinc-deficient SOD was clearly toxic to motor neurons by a nitric oxide and peroxynitrite dependent mechanism (17). More recently, we established that nitration was a key element in this toxicity by using tyrosine-containing peptides as competitive inhibitors of the endogenous targets of nitration in vivo (18). These peptides did not direct with or scavenge peroxynitrite, but could be delivered to motor neurons in culture in high enough concentrations to reduce endogenous nitration. Urate is also an effective inhibitor of tyrosine nitration, but does not directly scavenge peroxynitrite (19). Our in vitro experiments indicate that the concentrations of peptides or of urate needed to effectively block nitration are in the range of 100 to 300 μM. Raising urate levels to these concentrations is relatively easy to do in humans with little risk of aggravating gout. Urate levels in this range are useful in treating mouse models of multiple sclerosis. Elevated urate levels are correlated with substantially reduced risks of developing multiple sclerosis and Parkinson’s disease (20–25). While there can be considerable controversy about whether peroxynitrite is the real culprit in these experiments, these results suggest that it would be possible to treat peroxynitrite-dependent toxicity. Resolving many of the basic controversies that have obfuscated the field for the past decade may help to promote more effective treatments for many pathological diseases.
The Controversy over Equal Fluxes of Superoxide and Nitric Oxide
Much of the controversy arose over why generating peroxynitrite from large fluxes of superoxide co-generated with nitric oxide donors seemed to yield different results than adding bolus amounts of peroxynitrite (26). This led to several pointed papers questioning whether the reaction of nitric oxide with superoxide actually formed peroxynitrite and whether peroxynitrite could nitrate tyrosine under physiological conditions.
I found these arguments frustrating because we had already demonstrated in series of the Archive papers in 1992 that peroxynitrite was formed by alveolar macrophages in the presence of a large amount of superoxide dismutase (1,3). Furthermore, Alvaro Estévez, Luis Barbeito and their many collaborators had shown that endogenous peroxynitrite formation was responsible for nitration and death of motor neurons under a variety of stressful conditions (27,28). These papers were overlooked, which was unfortunate because they also laid out the combination of experimental controls that are needed to establish the involvement of nitric oxide, superoxide and peroxynitrite in a disease process.
The Radical Chemistry of Peroxynitrite
Yet the controversy is understandable because the chemistry of peroxynitrite is vastly complicated by multiple reactions between the radicals produced during its decomposition. My first encounter with this complexity came when trying to understand why peroxynitrite became much less effective as an oxidant at slightly alkaline pH (29). This paradoxically observation held me back from publishing our first paper on peroxynitrite in PNAS for almost a year and I still did not get the basis for this curious observation correct (30).
Insights from multiple inorganic chemists, including Jim Hurst, Sergei Lymar, Sara Goldstein, Giddy Czapski, and Gabor Merenyi helped identify the key reactions need to understand the subtle but rapid radical interactions (31–33). There are basically three sets of reactions needed to understand peroxynitrite decomposition in the absence of carbon dioxide:
| (1) |
| (2) |
| (3) |
Reaction 1 involves the formation of peroxynitrate (O2NOO−) by the reaction of superoxide with nitrogen dioxide. When superoxide is generated in excess of nitric oxide, this reaction efficiently quenches nitrogen dioxide produced during peroxynitrite decomposition and thus blocks nitration. It is prevented when SOD is present and this is a partial explanation for how SOD paradoxically can increase tyrosine nitration. It also becomes dominant at slightly alkaline pH and explains why peroxynitrite appears to be an ineffective oxidant and oxygen evolution is seen. This is one reason I was studying the reaction of peroxynitrite with hydrogen peroxide so long ago when I stumbled upon SOD-catalyzed tyrosine nitration.
Reaction 2 is more interesting and becomes dominant when nitric oxide is generated about three times faster than superoxide. The nitric oxide reacts with nitrogen dioxide to form N2O3 (dinitrogen trioxide, which a strong nitrosating agent). Under the in vitro conditions used by several investigators, N2O3 reacts quickly with peroxynitrite to form nitrite and two more nitrogen dioxides. This autocatalytic generation of nitrogen dioxide reacts with more nitric oxide and thus turn nitric oxide into a nitrosating agent. These reactions were described nicely by Jack Lancaster’s analysis from the simultaneous generation of superoxide and nitric oxide (34).
Reaction 3 is a natural consequence of every reaction being reversible, but the important result is that the dissociation of peroxynitrite is fast enough to compete with the pH-dependent decomposition of peroxynitrite into nitrogen dioxide and hydroxyl radical.
| (4) |
As the pH becomes more alkaline, the forward rate to form hydroxyl radical and nitrogen dioxide decreases while the dissociation to superoxide and nitrogen dioxide becomes more favorable. The forward and back reactions are nearly equal at about pH 8. Paradoxically, adding preformed peroxynitrite at this pH is equivalent to generating it by adding equal amounts of superoxide and nitric oxide.
It is understandable why the reactions of peroxynitrite, nitric oxide and superoxide were not immediately clear and required years of work to unravel. Yet, the synthesis of these reactions gives a precise, mathematical model to understand peroxynitrite chemistry (31,33,35). Through the tireless efforts of Pal Pacher and Luc Liaudet, we have described how these insights can be extended to physiology and pathology in an intimidating long review (36).
Because it took a decade to understand even the chemistry of peroxynitrite and there is not simple unambiguous inhibitor of peroxynitrite that can be added to cells or tissues, progress lately has been slow. Tyrosine nitration is an excellent marker of peroxynitrite formation in vivo, but has become muddled over concerns as to whether peroxynitrite will nitrate proteins under physiological conditions and because myeloperoxidase can catalyze tyrosine nitration from nitrite and hydrogen peroxide (37,38). Clearly, one cannot claim that all nitration comes exclusively from peroxynitrite. The real issue is whether the observation of tyrosine nitration in vivo is completely independent of peroxynitrite, which seems unlikely. Nitric oxide itself does not directly nitrite proteins over prolonged exposures. One also has to ask how nitrite is formed in vivo. When nitric oxide is removed by reaction with oxy-hemoglobin in red blood cells, nitrate is the product. One underappreciated pathway for generating nitrite is from dinitrogen trioxide from peroxynitrite and nitric oxide. Thus, peroxynitrite also could be a major contributory to nitrosation and potential a major route to form nitrosothiols (39,40).
The prospects for treating human disease
Although no single experiment will uniquely prove whether peroxynitrite is the culprit in a disease process, a combination of approaches can firmly establish its formation and contribution to the disease process. Although relatively high concentrations of scavengers such as urate are necessary to be therapeutically useful in treating peroxynitrite-dependent pathologies, these concentrations can be maintained in human patients for even the treatment of chronic conditions. Nitrotyrosine as well as urate oxidation products can be used as biomarkers for determining whether the experimental treatment reached efficacious concentrations in patients. My hope is that resolving the controversies concerning peroxynitrite biochemistry will renew interests in targeting peroxynitrite in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Arch Biochem Biophys. 1992;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin JC, Tsai M. Arch Biochem Biophys. 1992;298(2):438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 3.Ischiropoulos H, Zhu L, Beckman JS. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 4.Smith CD, Carson M, van der Woerd M, Chen J, Ischiropoulos H, Beckman JS. Arch Biochem Biophys. 1992;299(2):350–355. doi: 10.1016/0003-9861(92)90286-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Gunn C, Beckman JS, Moncada S, Hibbs J, Jr, Marletta M. Peroxynitrite, the product of nitric oxide and superoxide, is bactericidal. Biology of Nitric Oxide II 1992 [Google Scholar]
- 6.Radi R, Beckman JS, Bush KM, Freeman BA. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima H, Friesen M, Brouet I, Bartsch H. Food Chem Toxicol. 1990;28:647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- 8.Zhu JH, Lei XG. Exp Biol Med (Maywood) 2006;231(5):545–552. doi: 10.1177/153537020623100508. [DOI] [PubMed] [Google Scholar]
- 9.Hinson JA, Pike SL, Pumford NR, Mayeux PR. Chem Res Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- 10.Hinson JA, Michael SL, Ault SG, Pumford NR. Toxicol Sci. 1999;53:467–473. doi: 10.1093/toxsci/53.2.467. [DOI] [PubMed] [Google Scholar]
- 11.Hinson JA, Reid AB, McCullough SS, James LP. Drug Metab Rev. 2004;36(3–4):805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 12.Jian-Hong Z, Zhang X, Roneker CA, McClung JP, Zhang S, Thannhauser TW, Ripoll DR, Sun Q, Lei XG. Free Radic Biol Med. 2008;45(5):611–618. doi: 10.1016/j.freeradbiomed.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckman JS, Carson M, Smith CD, Koppenol WH. Nature. 1993;364(6438):584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 14.Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. J Neurochem. 1997;69(5):1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 15.Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS. J Neurochem. 1997;69(5):1945–1953. doi: 10.1046/j.1471-4159.1997.69051945.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts BR, Tainer JA, Getzoff ED, Malencik DA, Anderson SR, Bomben VC, Meyers KR, Karplus PA, Beckman JS. J Mol Biol. 2007;373:377–390. doi: 10.1016/j.jmb.2007.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estevez AG, Spear N, Manuel SM, Barbeito L, Radi R, Beckman JS. Prog Brain Res. 1998;118:269–280. doi: 10.1016/s0079-6123(08)63214-8. [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG. J Biol Chem. 2007;282(9):6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- 19.Teng RJ, Ye YZ, Parks DA, Beckman JS. Free Radic Biol Med. 2002;33(9):1243–1249. doi: 10.1016/s0891-5849(02)01020-1. [DOI] [PubMed] [Google Scholar]
- 20.Koprowski H, Spitsin SV, Hooper DC. Ann Neurol. 2001;49(1):139. doi: 10.1002/1531-8249(200101)49:1<139::aid-ana28>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Scott GS, Hooper DC. Med Hypotheses. 2001;56(1):95–100. doi: 10.1054/mehy.2000.1118. [DOI] [PubMed] [Google Scholar]
- 22.Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. J Immunol. 2000;165(11):6511–6518. doi: 10.4049/jimmunol.165.11.6511. [DOI] [PubMed] [Google Scholar]
- 23.Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H. Proc Natl Acad Sci U S A. 1997;94(6):2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Am J Epidemiol. 2008;167(7):831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry MC, Raman R, Schwarzschild MA, Becerra LM, Thomas RG, Peterson RC, Ascherio A, Aisen PS. Neurodegener Dis. 2009;6(1–2):23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter CD, Teng RJ, Beckman JS. J Biol Chem. 2000;275(42):32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 27.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Science. 1999;286(5449):2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 28.Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. J Neurosci Res. 2002;67(1):21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- 29.Crow JP, Spruell C, Chen J, Gunn C, Ischiropoulos H, Tsai M, Smith CD, Radi R, Koppenol WH, Beckman JS. Free Rad Biol Med. 1994;16:331–338. doi: 10.1016/0891-5849(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 30.Beckman JS, Beckman TW, Chen J, Marshall PM, Freeman BA. Proc Natl Acad Sci (USA) 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coddington JW, Hurst JK, Lymar SV. J Am Chem Soc. 1999;121:2438–2443. [Google Scholar]
- 32.Goldstein S, Czapski G, Lind J, Merényi G. J Biol Chem. 2000;275(5):3031–3036. doi: 10.1074/jbc.275.5.3031. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein S, Merenyi G. Methods Enzymol. 2008;436:49–61. doi: 10.1016/S0076-6879(08)36004-2. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster JR., Jr Chem Res Toxicol. 2006;19(9):1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein S, Lind J, Merenyi G. Chem Rev. 2005;105(6):2457–2470. doi: 10.1021/cr0307087. [DOI] [PubMed] [Google Scholar]
- 36.Pacher P, Beckman JS, Liaudet L. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Vliet A, Eiserich JP, O’Neill CA, Halliwell B, Cross CE. Arch Biochem Biophys. 1995;319(2):341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 38.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. J Biol Chem. 1997;272(12):7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 39.Jourd’heuil D, Miranda KM, Kim SM, Espey MG, Vodovotz Y, Laroux S, Mai CT, Miles AM, Grisham MB, Wink DA. Arch Biochem Biophys. 1999;365(1):92–100. doi: 10.1006/abbi.1999.1143. [DOI] [PubMed] [Google Scholar]
- 40.Wink DA, Cook JA, Kim SY, Vodovotz Y, Pacelli R, Krishna MC, Russo A, Mitchell JB, Jourd’heuil D, Miles AM, Grisham MB. J Biol Chem. 1997;272(17):11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]


