Abstract
Although it is well established that both follicular assembly and the initiation of follicle growth in the mammalian ovary occur independently of pituitary hormone support, the factors controlling these processes remain poorly understood. We now report that neurotrophins (NTs) signaling via TrkB receptors are required for the growth of newly formed follicles. Both neurotrophin-4/5 (NT-4) and brain-derived neurotrophic factor (BDNF), the preferred TrkB ligands, are expressed in the infantile mouse ovary. Initially, they are present in oocytes, but this site of expression switches to granulosa cells after the newly assembled primordial follicles develop into growing primary follicles. Full-length kinase domain-containing TrkB receptors are expressed at low and seemingly unchanging levels in the oocytes and granulosa cells of both primordial and growing follicles. In contrast, a truncated TrkB isoform lacking the intracellular domain of the receptor is selectively expressed in oocytes, where it is targeted to the cell membrane as primary follicles initiate growth. Using gene-targeted mice lacking all TrkB isoforms, we show that the ovaries of these mice or those lacking both NT-4 and BDNF suffer a stage-selective deficiency in early follicular development that compromises the ability of follicles to grow beyond the primary stage. Proliferation of granulosa cells— required for this transition—and expression of FSH receptors (FSHR), which reflects the degree of biochemical differentiation of growing follicles, are reduced in trkB-null mice. Ovaries from these animals grafted under the kidney capsule of wild-type mice fail to sustain follicular growth and show a striking loss of follicular organization, preceded by massive oocyte death. These results indicate that TrkB receptors are required for the early growth of ovarian follicles and that they exert this function by primarily supporting oocyte development as well as providing granulosa cells with a proliferative signal that requires oocyte-somatic cell bidirectional communication. The predominance of truncated TrkB receptors in oocytes and their developmental pattern of subcellular expression suggest that a significant number of NT-4/BDNF actions in the developing mammalian ovary are mediated by these receptors.
Keywords: trkB receptor knockout, Gene targeting, Neurotrophins, Ovarian development, Endocrine cells, Folliculogenesis
Introduction
Neurotrophins (NTs) are essential for the differentiation and survival of various neuronal populations in the central and peripheral nervous systems (Davies, 2000; Snider, 1994). Although it was once believed that NTs were required only within the nervous system, the presence of their high-affinity Trk receptors in several nonneuronal tissues has led to the conclusion that NTs may also be required for the development and function of organs as diverse as those comprising the cardiovascular, immune, endocrine, and reproductive systems (reviewed in Tessarollo, 1998). The critical importance of two NTs, nerve growth factor (NGF) and neurotrophin-3 (NT-3), and their respective TrkA and TrkC receptors in the morphogenesis of such organs was recently demonstrated by the severe defects in thymus, heart, and ovarian development detected in mice lacking the receptors TrkA (García-Suárez et al., 2000) or TrkC (Tessarollo et al., 1997) or the ligands NGF (Dissen et al., 2001) and NT-3 (Donovan et al., 1996). NTs also appear to play a role in vascular development since the complete ablation of the pan NT receptor p75NTR, which recognizes all neurotrophins with similar affinity (Chao and Hempstead, 1995), results in defects of blood vessel formation (von Schack et al., 2001).
Much less is known about the functions that neurotrophin- 4 (NT-4) and brain-derived neurotrophic factor (BDNF), and their TrkB receptors may have outside the nervous system. The recent finding that mice lacking BDNF suffer from specific defects of the cardiac vasculature (Donovan et al., 2000) provided bona fide evidence for the existence of such nonneural functions. Whether the NT-4/BDNF ligand or TrkB receptor complex is also required for normal morphogenesis of other organs has not been established. Within the endocrine system, the developing ovary is a particularly suitable candidate for such TrkB-mediated actions because it expresses both NT-4 and BDNF, as well as TrkB receptors, at a developmental stage during which its functional units, the follicles, become first assembled and begin to grow towards acquiring ovulatory competence (Anderson et al., 2002; Dissen et al., 1995).
Female reproductive capacity requires the ability of the ovary to yield a mature oocyte at ovulation. Oocytes grow surrounded by somatic cells of both epithelial (granulosa) and mesenchymal (theca) origin that together form a functional unit known as the follicle (Matzuk et al., 2002). In rodents, assembly of somatic and germ cells into follicular structures is completed within the first week of postnatal life (Hirshfield, 1991; Malamed et al., 1992). Both follicular formation and the initiation of follicle growth occur independently of pituitary gonadotropin hormone support (Dierich et al., 1998; Kumar et al., 1997; Zhang et al., 2001). Instead, these events are regulated by intraovarian factors, some of which have been identified (Dissen et al., 2001; Dong et al., 1996; Huang et al., 1993) (reviewed in Matzuk et al., 2002). Because the mRNAs encoding BDNF and NT-4 and their TrkB receptor are expressed in neonatal rat ovaries (Dissen et al., 1995), it has been suggested that TrkB signaling may be involved in regulating either follicular assembly or the initiation of follicular growth (Dissen et al., 1995). Alternative splicing of the TrkB pre-mRNA generates both a full-length receptor that uses an intracellular tyrosine kinase domain for signaling, and truncated isoforms containing the identical extracellular and transmembrane domain as the full-length receptor, but lacking the intracellular kinase domain (Klein et al., 1990; Middlemas et al., 1991). Though lacking canonical signaling motifs, these truncated forms are also able to initiate intracellular signaling (Baxter et al., 1997), suggesting that if they are present in the developing ovary, they may contribute to regulating specific ovarian functions. Mice lacking only the full-length TrkB receptor cannot be used to define the role of trkB in postnatal follicular growth because most of the mutant animals die at birth (Klein et al., 1993). A less severe phenotype is, however, observed in mice lacking both full-length and truncated receptor isoforms, as these animals can survive for up to 3 weeks after birth (Rohrer et al., 1999).
In the present study, we report the gene targeting procedure employed to generate these mice and demonstrate that in the absence of both full-length and truncated TrkB receptors, ovarian follicular growth is arrested at an early stage of development. This deficiency is accompanied by reduced granulosa cell proliferation. We further demonstrate that the ovaries from trkB-null mice transplanted under the kidney capsule of wild-type recipient animals, and left in this site for 2 weeks to assess the ability of follicles to grow beyond the preantral stage, are unable to support oocyte survival and follicular growth, resulting in widespread degradation of follicular organization. While most TrkB receptors in the neonatal mouse ovary are truncated and predominantly expressed in oocytes, BDNF and NT-4 are produced by follicular granulosa cells. This distribution suggest that the arrest in follicular growth observed in trkB-null mice is due to impairment of a TrkB-mediated signaling pathway that primarily affects oocyte function, resulting in loss of a germ cell-somatic cell communication process that supports proliferation of follicular somatic cells and hence is required for follicle growth. Because most TrkB receptors expressed in oocytes are truncated, it appears likely that these receptors play a significant role in mediating the supporting actions of NT-4/BDNF on ovarian follicular development.
Materials and methods
Generation of trkB-null mutant mice
The 10 kb of XbaI–BamHI trkB genomic fragment (thick line in Fig. 1A) covering the first coding exon (exon S) was described previously (Xu et al., 2000) and used to construct the trkB targeting vector (Fig. 1A). Exon S covers a 346-bp 5′ untranslated region (open box) and a 211-bp coding region including 31 codons for the signal peptide (black filled box). A ClaI site located at the 19th bp of exon S and a KpnI site located 112 bp downstream of exon S were two critical restriction sites for construction of the targeting vector. To construct the targeting vector, the ClaI–KpnI fragment was replaced with a PGKneo expression unit (Fig. 1A). The targeting vector contains 7 kb of XbaI–ClaI upstream homologous arm and 2.5 kb of KpnI–BamHI downstream homologous arm. A negative selection gene, PGKtk, was inserted downstream of the 2.5-kb homologous arm. Using this construct, trkB mutants were generated following standard procedures. A trkB cDNA fragment (nt1386–2054 in mouse TrkB mRNA) was used as the probe for Northern hybridization. Additional details concerning the postnatal phenotype of these mutant mice have been previously described (Rohrer et al., 1999).
Fig. 1.
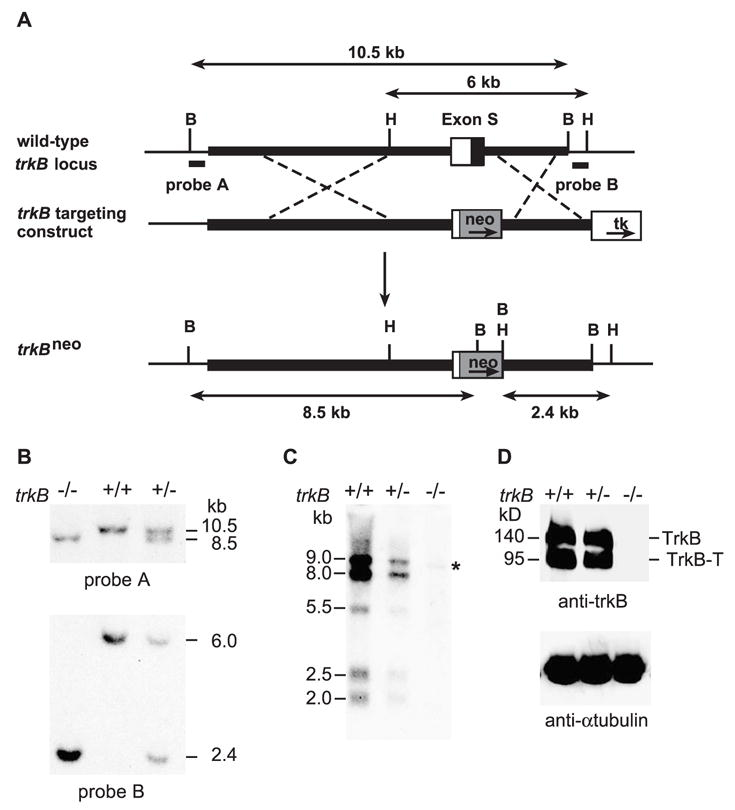
Targeting disruption of the trkB gene. (A) Schematic diagrams of the trkB gene, the targeting construct, and the targeted trkB locus. The probes used for screening and the expected Southern blot fragments are indicated. The homology arms are represented in thick lines. B = BamHI; H = HindIII. (B) Southern blot analyses of a representative tail DNA sample. DNA was digested with BamHI or HindIII and blotted with probe A or probe B. Using probe A, 10.5 and 8.5 kb bands are generated by digestion of the wild-type trkB and the targeted trkB alleles, respectively. Probe B detects 2.4 and 6.0 kb of bands for the wild-type trkB and the targeted trkB alleles, respectively. (C) Northern blot analysis of trkB mRNAs. Half microgram of brain mRNA was loaded onto each lane. An asterisk indicates the presence of an aberrant RNA in the mutant (−/−) lane. (D) Western blot analysis of the TrkB protein. Protein extracts were prepared from the brains of wild-type, heterozygous, and homozygous mice. Fifty micrograms of protein was loaded onto each lane. Alpha tubulin was used as the loading control. TrkB = full-length TrkB receptor; TrkB-T = truncated TrkB receptor.
Western blot analysis
The affinity purified TrkB antibodies (RTB) for Western blot were raised against the TrkB extracellular domain (Huang et al., 1999). They were used at a 1:200 dilution. Nonspecific binding to the membrane containing the electrophoresed proteins was blocked with 5% dry milk in TBST buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween).
Immunohistochemistry
Immunoreactive TrkB receptor forms and BDNF were detected in 14-μm cryostat sections from ovaries immersed in Zamboni’s fixative either overnight (Dissen et al., 1995) or for 2 h at 4°C. The potassium phosphate buffers used for the immunohistofluorescence procedures outlined below were those described earlier (Jung et al., 1997). The full-length TrkB protein was detected with a rabbit antiserum (TrkB-in, 1:500, kindly provided by Dr. David Kaplan, Montreal Neurological Institute, McGill University, Montreal, Canada), directed against amino acids 482–501 in the cytoplasmic domain of TrkB (Fryer et al., 1996). Truncated TrkB-T1 receptors were identified with rabbit polyclonal antibodies (SC-119, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA). BDNF-containing cells were identified using rabbit polyclonal antibodies against human BDNF (AB1779SP, 1:2000, Chemicon International, Temacula, CA). Before incubation with these primary antibodies, the sections were treated with avidin-blocking solution, as described (Jung et al., 1997). To verify the localization of truncated TrkB-T1 receptors to the cell membrane of oocytes, a double immunolabeling experiment was performed using the TrkB-T1 antibodies described above and a rat monoclonal antibody (Mab KMC8, 5 μg/ml, BD Pharmigen, San Diego, CA) against mouse CD9, a member of the tetraspanin family of membrane proteins that is selectively expressed in the plasma membrane of oocytes (Zhu et al., 2002). After an overnight incubation at 4°C with the primary antibodies, all TrkB immunoreactions were developed by incubating the sections for 1 h at room temperature with biotinylated goat antirabbit gamma globulin (1:250, Vector Laboratories, Inc., Burlingame, CA) followed by 1 h at room temperature with fluorescein (FITC) streptavidin (1:400, Jackson ImmunoResearch, West Grove, PA). CD9 immunoreactions were developed with Texas Red-labeled goat antirat gamma globulin 1 h at room temperature (1:250, Jackson ImmunoResearch), added to the sections simultaneously with the biotinylated antibodies against TrkB-T1. In the case of BDNF, the immunoreactions were developed using the biotinylated tyramine enhancement method, as previously described (Jung et al., 1997). In brief, after an overnight incubation with the primary antibodies, the sections were incubated with biotinylated goat antirabbit gamma globulin (1:2000, 1 h at room temperature), followed by 1 h incubation in avidin–biotin complex (ABC, Elite Vectastain, Vector Laboratories). After extensive rinsing, the sections were incubated for 20 min at room temperature with biotinylated tyramine (BT, 5 μl/ml, PerkinElmer Life Sciences, Inc., Boston, MA) containing 0.001% hydrogen peroxide. Thereafter, the sections were washed again several times and incubated for 2 h at 37°C with FITC-streptavidin at a 1:1000 dilution. Cell nuclei were stained with the vital dye Hoechst (Molecular Probes, Eugene, OR) as reported (Dissen et al., 2001). To identify NT-4 immunoreactive cells, the ovaries were immersed in Bouin’s fixative for 4 h, embedded in paraffin, and sectioned at 5 μm. The sections were then subjected to an antigen retrieval procedure (Dissen et al., 2001), followed by incubation with rabbit polyclonal antibodies against human NT-4 (SC 545, 1:250, Santa Cruz Biotechnology, Santa Cruz, CA). NT-4 immunoreactions were developed as described for light microscopy immunohistochemistry (Anesetti et al., 2001). Several controls were used, including sections from ovaries of trkB −/− mice in the case of the TrkB immunoreactions, sections from ovaries of BDNF −/− animals (Bennett et al., 1999) in the case of BDNF staining, and sections incubated with antibodies preadsorbed with the peptide used to raise the antibodies in the case of NT-4 (SC545P, 10 μg peptide/μg antibody, Santa Cruz Biotechnology, 2 h at room temperature). Confocal images were acquired as reported (Dissen et al., 2001) using a Leica TCS SP confocal system (Leica Microsystems, Heidelberg, Germany) equipped with a ×40 Plan Apochromat objective (1.25 numerical aperture). Hoescht and FITC were imaged using the 361-nm line of a UV laser and the 488-nm line of an Argon laser for excitation. The two fluorophores were imaged sequentially to exclude the contribution of Hoescht into the FITC channel and processed as described (Dissen et al., 2001).
Morphometric analysis
The ovaries of 7-day-old trkB +/+, trkB −/−, and trkB +/− mice were fixed in Kahle’s fixative, embedded in paraffin, serially sectioned at 6 μm, and stained with Weigert’s iron hematoxylin and counterstained with picric acid-methyl blue, as reported (Dissen et al., 2001). Every third section was imaged on a Zeiss Axioplan (Carl Zeiss, Jenna, Germany), using a CoolSnap camera (Roper Scientific, Stillwater, MN). Follicles were counted using the manual count feature of MetaMorph (Universal Imaging Co., West Chester, PA). Only follicles in which the nucleus of the oocyte was visible were counted, and the total number of follicles per ovary was determined as reported (Dissen et al., 2001). All counts were performed without previous knowledge of the animal’s genotype.
Ovarian transplantation
Eight- to 10-week-old intact adult female mice C57BL/6J were used as recipients for ovarian transplantation. The recipient mice were anesthetized with a subcutaneous injection of a combination of ketamine hydrochloride (30 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), xylazine (2 mg/kg; Phoenix Scientific Inc., St. Joseph, MO), and acepromazine maleate (1 mg/kg; Burns Veterinary Supply, Rockville Centre, NY). The kidneys were sequentially exteriorized through dorsolateral incisions, and one donor ovary was inserted under the renal capsule of each kidney with the help of fine watchmakers forceps. The donor ovaries, derived from 4- to 5-day-old trkB +/+, trkB +/−, and trkB −/− animals were dissected out, cleaned of adherent tissue, and kept in DMEM (Sigma-Aldrich, St. Louis, MO) until transplantation. After receiving the ovarian grafts, the recipient mice were ovariectomized. The incision was closed with 4-0 Biosyn monofilament synthetic absorbable sutures (Tyco Healthcare Group LP., Norwalk, CT) and 9 mm Autoclips (Becton Dickinson and Company, Sparks, MD). After surgery, each mouse received subcutaneous injections of the antibiotic Enrofloxacin (85 mg kg−1 day−1; Bayer Corp., Shawnee Mission, KA) for 3 days and the analgesic Ketoprofen (2.5 mg kg−1 day−1; Fort Dodge Animal Health) for 2 days. Two weeks after transplantation, the ovaries were collected and processed for morphologic examination as described above.
Assessment of proliferation
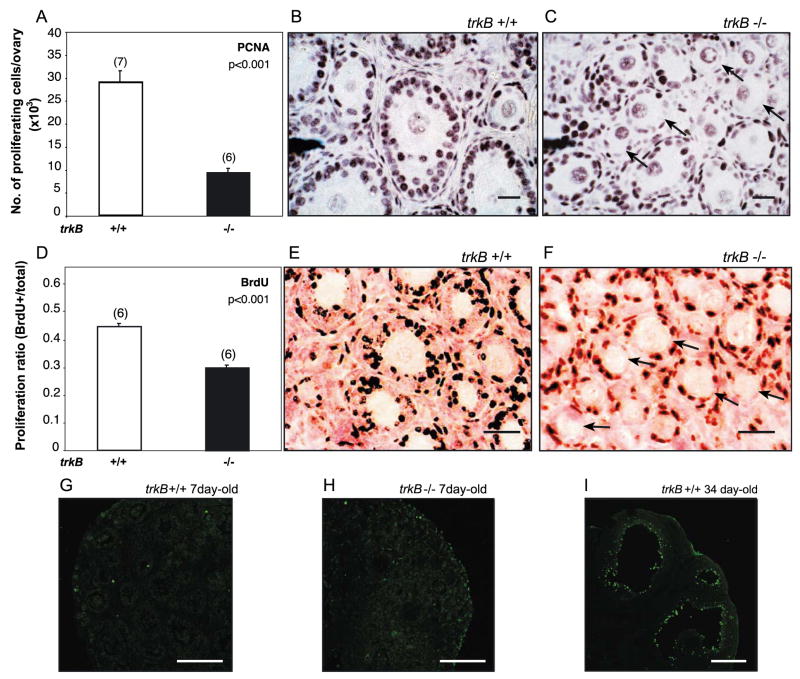
The ovaries from 7-day-old trkB +/+ and trkB −/− mice were fixed in Carnoy’s fixative, embedded in paraffin, sectioned at 4 μm, and subjected to immunohistochemistry for PCNA, as reported (Dissen et al., 2001), using a monoclonal antibody to PCNA (Mab PC-10, 1:1000; Santa Cruz Biotechnology) and developing the immunoreaction with a diaminobenzidine/H2O2/nickel chloride solution, followed by counterstaining with Gill’s hematoxylin (1:10 dilution). As an independent measure of cell proliferative activity, the ovaries from 7 day-old trkB +/+ and trkB −/− mice were explanted into an organ culture system (Mayerhofer et al., 1997) and incubated with bromodeoxy-uridine (BrdU, 100 μM; Roche Diagnostics Co., Indianapolis, IN) for 24 h at 37°C in an atmosphere of 60% oxygen, 35% nitrogen, and 5% CO2, as reported (Dissen et al., 2001). Thereafter, the ovaries were fixed and sectioned as above before BrdU immunohistochemistry, which was performed using a monoclonal antibody to BrdU (Mab B-2531, 1:1000; Sigma-Aldrich). Every fifth section was imaged with a Sony DKC-5000 digital camera (Sony, Co., Tokyo, Japan) attached to a Nikon Eclipse E600 microscope using a Plan ×40 NA 0.65 objective (Nikon Inc., Melville, NY). Positive cells were counted using the manual feature of MetaMorph. In the case of PCNA staining, only positive granulosa cells were counted; in the case of BrdU staining, the sections were counterstained with Nuclear Fast Red (Vector Laboratories, undiluted for 10 min at room temperature followed by a 10-s wash in water containing 0.5% acetic acid) for accurate visualization of nonproliferating, BrdU-negative cells.
Assessment of apoptosis
Apoptotic ovarian cells were detected in 14 μm cryostat sections using the in situ cell death detection kit coupled with fluorescein detection (Roche Diagnostics Co.), following the manufacturer’s instructions. Ovaries from 7-day-old trkB +/+, trkB −/− mice, and adult ovaries from a 34-day-old mouse were immersion fixed for 24 h at 4°C in Zamboni’s fixative, rinsed in PBS (24 h at 4°C), and then cryoprotected in 20% sucrose-PBS overnight at 4°C before embedding them in OCT compound (Miles Inc., Elkhart, IN), and dry ice freezing.
Relative quantitative PCR and RealTime PCR
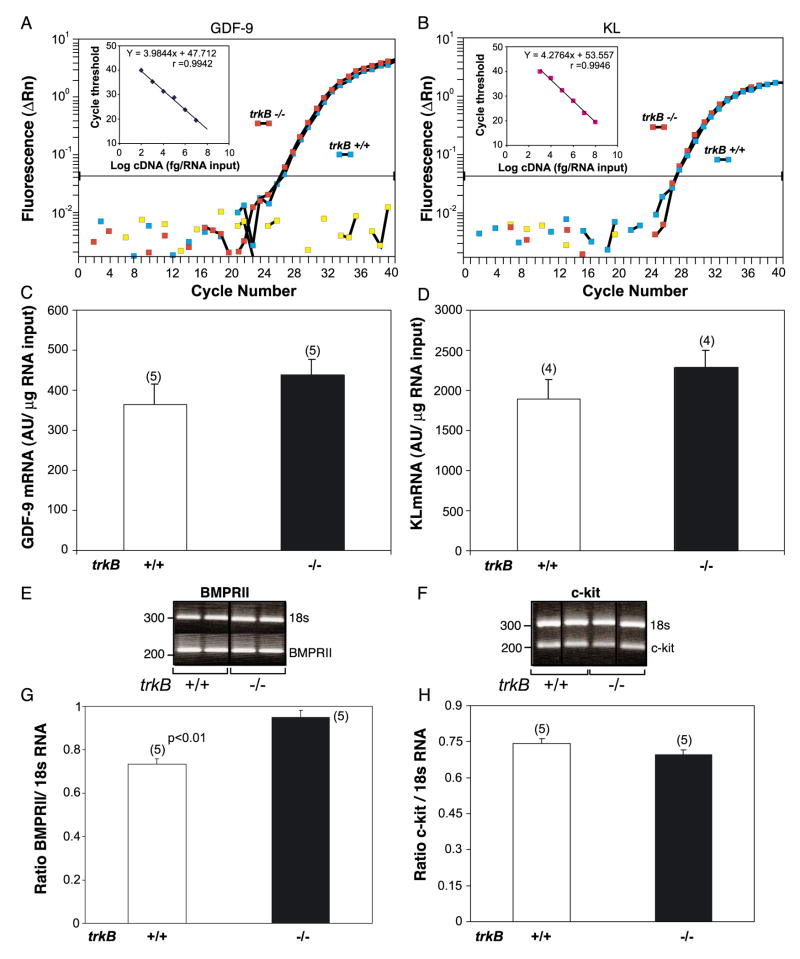
To determine if the absence of TrkB receptors alters the expression of receptor-ligand systems currently known to be required for early follicular growth, the mRNAs encoding these proteins were measured using quantitative PCR procedures. Once follicles acquire two or more layers of granulosa cells, these cells begin to express FSH receptors (FSHR). To determine if this differentiation process is delayed in trkB −/− mice, we also measured FSHR mRNA by quantitative PCR. The relative quantitative PCR procedure of Ambion (Austin, TX) was used to detect the mRNAs encoding the receptors c-kit and bone morphogenetic protein receptor type II (BMPRII). C-kit, the c-kit ligand (KL) receptor, is predominantly expressed in oocytes (Manova et al., 1990), and BMPRII, a putative GDF-9 receptor (Vitt et al., 2002) (see below), is present in granulosa cells (Shimasaki et al., 1999). Before carrying out the amplification procedure, the optimal gene-specific primer concentrations, linear range of the PCR reaction, and optimal primer concentration for the amplification of 18S ribosomal RNA used as an internal standard were determined. The primers used for c-kit receptor mRNA amplification (GenBank accession number NM 021099) were a 5′ forward primer (5′-GTGCCAACCAAGACAGACAA- 3′) corresponding to NT 2216–2235 and a 3′ reverse primer (5′-GGCTGCCAAATCTCTGTGAA-3′) complementary to nucleotides (NT) 2407–2426 in the c-kit mRNA sequence (Qiu et al., 1988). BMPRII mRNA (GeneBank accession number NM_007561) was detected with a 5′ forward primer 5′-TTTGGTAGATAGGAGGGAACG- 3′ and a reverse primer 5′-ATTTGCCATCTTGTGTTGACT- 3′ corresponding to NTs 2674–2690 and 2888–3009, respectively, in BMPRII mRNA (Bepu et al., 1997). The PCR reactions were carried out in a 25-μl volume containing 0.5 μl of reverse transcription reaction, 10 pmol (c-kit) or 15 pmol (BMPRII) of genespecific primers, and 25 pmol of 18S ribosomal RNA primers at a primer/competimer ratio of 1:1. The PCR amplification protocol consisted of 30 cycles of denaturing at 94°C (30 s), annealing at 55°C (30 s), and extension at 72°C (1 min). Equal volumes of the PCR reactions were electrophoresed on 2% agarose gels stained with ethidium bromide, the gels were imaged in a Gel Doc 2000 (Bio-Rad Laboratories, Hercules, CA), and the images were quantitated using the image analysis Quantity One software (Bio-Rad Laboratories).
The mRNAs encoding KL (Huang et al., 1993) and the putative BMPRII ligand GDF-9 (Vitt et al., 2002) were detected by RealTime PCR using a procedure previously described (Romero et al., 2002). GDF-9 mRNA is expressed exclusively in oocytes (Dong et al., 1996; McGrath et al., 1995) and is recognized by BMPRII (Vitt et al., 2002). To detect it, we used a 5′ forward primer (5′-CGATGGTGGACCTGCTGTTT- 3′) corresponding to NT 435–454 and a 3′ reverse primer (5′-GGAGGAAGAGGCAGAGTT GTT C-3′) complementary to NT 515–535 in mouse GDF-9 mRNA (GenBank accession number NM_008110). The internal fluorescent oligodeoxynucleotide probe (5′-CCGGGTGACTGCCATGGAACACTT-3′; Perkin Elmer Applied Biosystems, Foster City, CA) corresponds to NT 463–486 in mouse GDF-9 mRNA and was covalently linked to the fluorescent dye FAM at the 5′-end and the quencher dye TAMRA at the 3′-end. The KL primers used for amplification were a 5′ forward primer (5′-GTTGCAGCCAGCTCCCTTAG-3′) corresponding to NT 562–581 and 3′ reverse primer (5′GTCCATTGTAGG- CCCGAGTCT-3′) complementary to NT 627–647 in mouse c-kit ligand mRNA (GenBank accession number NM_013598). The internal fluorescent oligodeoxynucleotide probe (5′-AGGAAAGCCGCAAAGGCCCCTG- 3′) corresponds to NT 604–625 in the KL mRNA. All primers were selected with the assistance of the program Primers Express (Perkin Elmer Applied Biosystems). The primers and PCR conditions used to quantify FSHR mRNA by RealTime PCR were those recently described (Romero et al., 2002). The 18S ribosomal RNA was used as a normalizing unit for each reaction using a set of primer’s purchased as a kit (TaqMan Ribosomal RNA Control Reagents Kit, Perkin Elmer Applied Biosystems).
Measurement of serum gonadotropins
LH and FSH were measured by radioimmunoassays using reagents provided by the NIH National Pituitary Agency. The binding in the LH assay was 30% and the sensitivity is 0.7 ng/ml. The standard curve had a correlation coefficient of r2 = 0.999. The FSH assay had a binding of 21%, a sensitivity of 0.4 ng/ml, and a correlation coefficient of r2 = 0.995.
Data analysis
The differences in follicle numbers and gonadotropin concentrations were analyzed using one-way ANOVA and the appropriate post hoc test (Student’s t test to compare two groups and Student–Newman–Keuls multiple test for individual means to compare more than two groups).
Results
Generation of mice lacking both full-length and truncated TrkB receptors
Since the initially reported trkB knockout mice still express truncated TrkB receptors (Klein et al., 1993), the truncated TrkB protein may result in dominant negative effects due to the capacity of its extracellular domain to interfere with NT-3 signaling (Conover et al., 1995). In addition, these mutant mice cannot be used to study potential functions of the truncated TrkB receptor. To define the function of TrkB in the ovary, we used mice carrying a new trkB mutant allele, trkBneo. To generate this mutant, the first coding exon of the trkB gene was replaced with the neo selection gene (Fig. 1A). This replacement blocks transcription of the trkB gene and prevents the membrane expression of any TrkB protein that may still be present by removing the signal peptide encoded in the first exon. The trkBneo allele was identified by Southern blot analysis with both 5′ and 3′ probes (probes A and B) (Fig. 1B). As revealed by Northern hybridization, mice homozygous for the trkBneo allele expressed a small amount of an aberrantly sized mRNA (Fig. 1C, asterisk). Despite the presence of this mRNA, Western blots with a polyclonal antiserum against the TrkB extracellular domain unambiguously demonstrated that no TrkB receptors were expressed in the trkBneo mutant mice (Fig. 1D). Therefore, the trkBneo allele is a null locus in which both the full-length and truncated TrkB receptors are eliminated. This trkB mutant has been previously used to examine the role of TrkB in the development of the retina (Rohrer et al., 1999).
Most TrkB receptors in the infantile mouse ovary are truncated and expressed in oocytes
To identify the cells expressing TrkB receptors in the developing mouse ovary, we used specific antibodies in immunohistochemical reactions visualized by confocal microscopy (Fig. 2). We examined ovaries from 7-day-old mice because at this time follicular assembly is completed and gonadotropin-independent follicular growth is well under way. Although the use of frozen sections from ovaries fixed in a paraformaldehyde-based fixative resulted in oocyte shrinking as compared with ovaries fixed in a denaturing fixative and embedded in paraffin (see for instance Fig. 4), the basic morphological characteristics of the follicles remained distinctly evident. Primordial follicles are the initial result of follicular assembly; they contain an oocyte surrounded by a single layer of flattened pregranulosa cells (types 1 and 2; Peters, 1969) (Fig. 2, short arrows). Primordial follicles become primary follicles (type 3a) (Peters, 1969) by a process that results in the differentiation of the flattened granulosa cells into a cuboidal morphology (Fig. 2A, long arrow) (Hirshfield, 1991; Peters, 1969). Granulosa cell proliferation and oocyte growth begin at this point resulting in the formation of larger (type 3b) primary follicles first and secondary follicles with two (type 4) or more layers of granulosa cells (type 5 and larger) subsequently (Fig. 2, large single and double arrowheads, respectively).
Fig. 2.
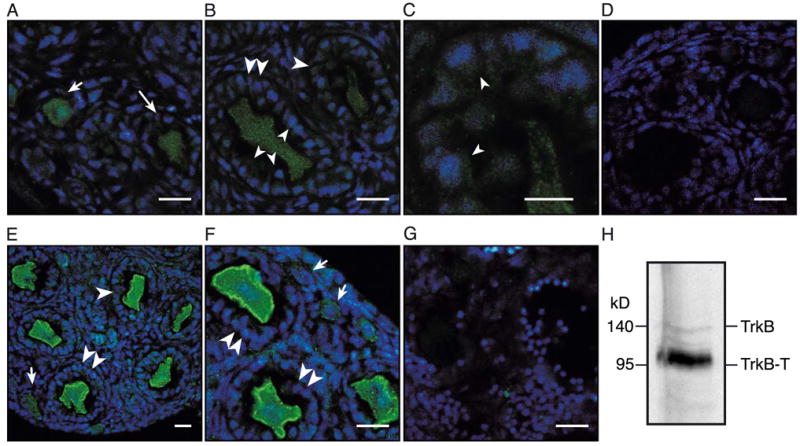
Detection of full-length and truncated TrkB receptor isoforms in the developing mouse ovary by immunohistofluorescence-confocal microscopy and Western blot analysis. Ovaries from 7-day-old trkB +/+ and trkB −/− mice were used. Three animals of each genotype were examined. In all panels, TrkB immunoreactive cells are seen in green, and cell nuclei stained with the DNA-binding dye Hoechst are shown in blue. (A–D) Low abundance of full-length TrkB receptors detected with polyclonal antibodies against the intracellular domain of rat TrkB. (A) Detection of the receptors in oocytes of primordial follicles (single layer of flattened pregranulosa cells, short arrow) and small, nongrowing primary (type 3a) follicles (one single layer of cuboidal granulosa cells, long arrow). (B) Persistence of low levels of full-length TrkB receptor immunoreactivity in oocytes of growing primary (type 3b) follicles (one single cuboidal layer of cells, large single arrowhead) and secondary follicles (two layers of cells, large double arrowheads) and appearance of the receptors in granulosa cells of the same follicles (small arrowheads). (C) Higher magnification image showing full-length TrkB receptor immunoreactivity in granulosa cells of a growing primary follicle (small arrowheads). (D) Lack of TrkB immunoreactivity in the ovary of a trkB −/− mouse. (E–G) Abundance of truncated TrkB-T1 receptors in oocytes of the 7-day-old mouse ovary detected with antibodies that recognize the unique intracellular domain of this receptor isoform. (E and F) T1 receptors are expressed at low levels in oocytes of primordial follicles (short arrows) but are abundant in oocytes of growing primary (type 3b) follicles and secondary follicles (large single and double arrowheads, respectively). (G) T1 receptors are absent in the ovary of a trkB −/− mice. (H) Western blot analysis of the TrkB protein. A protein extract from 30 TrkB heterozygous ovaries was loaded onto the lane. Note that in agreement with the immunohistochemical results, the majority of the TrkB receptors present in the ovary are truncated. Scale bars = 10 μM.
Fig. 4.
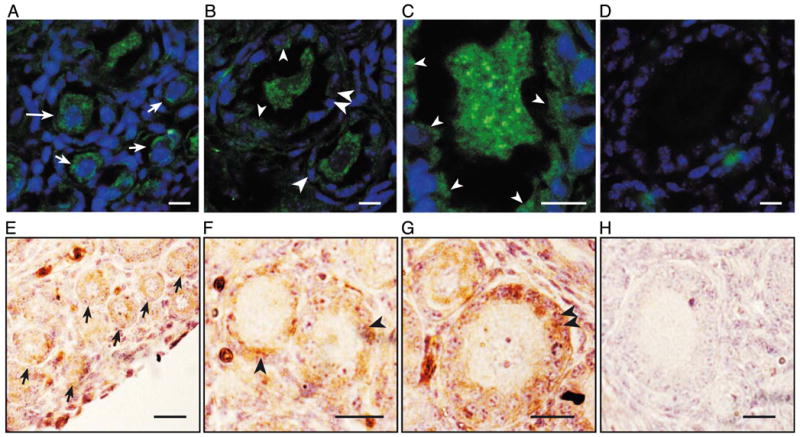
Detection of the TrkB ligands, NT-4 and BDNF, in the developing mouse ovary by immunohistofluorescence-confocal microscopy and conventional light microscopy. Ovaries from 7-day-old trkB +/+ and trkB −/− mice were used. Three animals of each genotype were examined. In panels A– D, immunoreactive cells are seen in green and cell nuclei stained with the DNA-binding dye Hoechst are shown in blue. In panels E– H, NT-4 immunoreactive cells are brown and cell nuclei stained with Gill’s hematoxylin are purple. (A–D) BDNF is present in both oocytes and granulosa cells of newly formed follicles. (A) Oocytes of primordial (short arrows) and small, nongrowing primary follicles (long arrow) contain moderate levels of BDNF immunoreactive material. (B) The oocytes of some growing primary (large single arrowhead) and secondary follicles (large double arrowheads), as well as granulosa cells of some follicles (small arrowheads) contain BDNF. (C) Higher magnification image showing BDNF immunoreactivity in granulosa cells of a growing primary follicle (small arrowheads). (D) Section from a BDNF −/− mouse ovary showing lack of BDNF immunoreactivity. (E–H) Like BDNF, NT-4 is also present in oocytes and granulosa cells of newly formed follicles. (E) NT-4 is detected in oocytes of primordial follicles (short arrows); (F) in growing type 3b primary follicles (large single arrowheads), and (G) secondary follicles (large double arrowheads), NT-4 immunoreactivity is predominantly seen in granulosa cells. (H) Section incubated with NT-4 antibodies preadsorbed with the NT-4 peptide used to raise the antibodies. Scale bars in A–D = 10 μm. Scale bars in E–H = 20 μm.
Antibodies that recognize the full-length TrkB receptor demonstrated low levels of immunoreactive material in oocytes of primordial follicles (Fig. 2A, short arrow), nongrowing primary follicles (Fig. 2A, long arrow), and growing primary and secondary follicles (Fig. 2B, large single and double arrowheads, respectively). In all cases, only a fraction of the receptors appeared to be associated with the cell membrane. Full-length TrkB receptors were also detected, albeit at low levels in granulosa cells of growing primary and secondary follicles (Figs. 2B and C, small arrowheads). The image in panel C is an enlarged view of a portion of panel B highlighting the presence of low levels of full-length TrkB receptor immunoreactivity in granulosa cells (small arrowheads) of a primary follicle. The ovaries from trkB −/− mice, used as controls, showed no detectable TrkB immunoreactivity demonstrating the specificity of the staining (Fig. 2D).
Besides the full-length TrkB receptor, alternative splicing of the trkB pre-mRNAs also generates two truncated protein isoforms (Klein et al., 1990; Middlemas et al., 1991). These isoforms, termed T1 and T2 (Middlemas et al., 1991), lack the tyrosine kinase domain and have unique short intracellular domains. We used an antibody that specifically recognizes T1, the predominant truncated receptor expressed in various tissues (Shelton et al., 1995), to determine if this form is expressed in the mouse ovary. In contrast to the low levels of full-length TrkB receptor detected in growing follicles, the T1 receptor was abundantly expressed in oocytes of both growing primary and secondary follicles (Figs. 2E and F, large single and double arrowheads, respectively). The oocytes from primordial follicles showed a much lower level of T1 expression (Figs. 2E and F, short arrows). Likewise, no significant T1 expression was seen in granulosa cells at any stage of follicular development (Figs. 2E and F). The specificity of the TrkB T1 immunoreactivity was demonstrated by its absence in the ovaries from trkB −/− mice (Fig. 2G). To biochemically define the presence of truncated TrKB receptors in the developing mouse ovary and verify the relative levels of expression of full-length and truncated receptors suggested by the immunohistochemical studies, we prepared protein extracts from ovaries dissected from 7-day-old mice and performed Western blots using antibodies specific to the TrkB extracellular domain. Consistent with the immunohistochemical observations, the immunoblot analysis showed that most TrkB receptors in the infantile mouse ovary are truncated (Fig. 2H).
We next performed double immunohistofluorescence staining to determine if TrkB-T1 receptors are in fact localized to the oocyte’s cell membrane. Using single confocal optical sections (approximately 0.5 μm in thickness), we found that CD9—a cell transmembrane protein selectively expressed in mammalian oocytes (Zhu et al., 2002)—is present in oocytes at all stages of development (Fig. 3), including those in primordial follicles (short arrows, red color), growing primary (large single arrow-heads), and secondary follicles (large double arrowheads). The yellow color in these two cases reflects the colocalization of CD-9 (red) with TrkB-T1 immunoreactivity (green, see below). In contrast to CD9, TrkB-T1 receptors are most abundantly expressed in oocytes of primary and secondary follicles (Fig. 3A). Surprisingly, TrkB-T1 receptors were not localized to the cell membrane of oocytes in either primordial (Figs. 3A and B, examples denoted by short arrows) or small nongrowing primary follicles (Fig. 3C). Receptors targeted to the cell membrane were first observed in oocytes of growing primary follicles (Fig. 3D) and became a distinct feature of secondary follicles (Fig. 3E). Figs. 3F–H illustrate this localization by separately showing the cellular distribution of TrkB-T1 immunoreactive material (panel F) and CD9 (panel G) in an oocyte of a growing primary follicle. The merged images are shown in panel H.
Fig. 3.
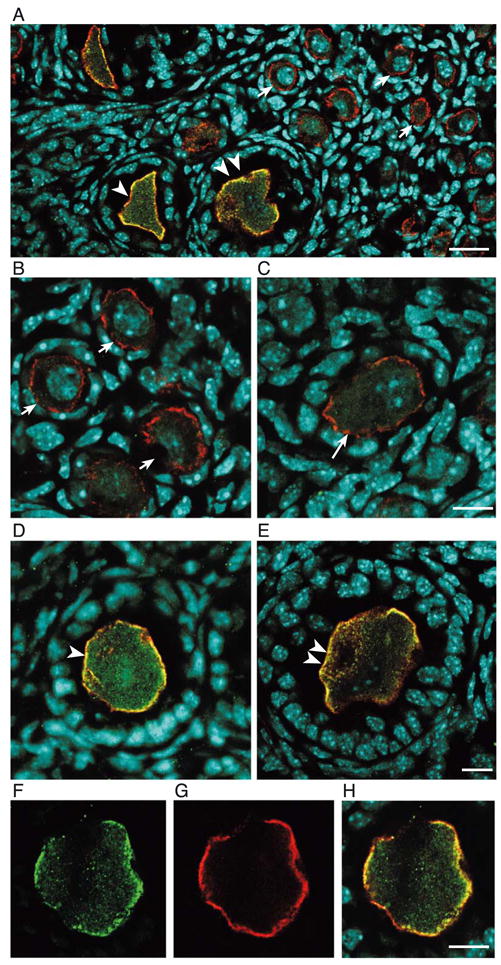
Truncated TrkB-T1 receptors become abundantly expressed in oocytes and targeted to the oocyte’s cell membrane when primary follicles initiate growth. (A) View of a wild-type 7-day-old mouse ovary showing that the membrane-specific protein CD9 (red color) is present in oocytes at all stages of development, whereas TrkB-T1 (green color) becomes targeted to the oocyte’s cell membrane only in growing primary and secondary follicles (large single and double arrowheads, respectively). TrkB-T1 abundance is also markedly increased in these follicles as compared with primordial follicles. (B and C) Oocytes of primordial (short arrows) and small primary follicles (long arrow) contain low levels of TrkB-T1 receptors, which are not targeted to the cell membrane. (D and E) TrkB-T1 immunoreactivity becomes abundant and targeted to the cell membrane in oocytes of growing primary (large single arrowhead) and secondary follicles (large double arrowhead). (F–H) images demonstrating that TrkB-T1 receptors (F, green color) in a growing primary follicle are localized to the same peripheral region of the oocyte that contains an abundance of CD9 (G, red color). The overlap of both immunoreactivities (yellow color) is illustrated by the merged images depicted in H. Scale bars in A = 20 μm; scale bars in B–H = 10 μm.
The infantile mouse ovary expresses both NT-4 and BDNF, the two known TrkB ligands
We next determined the expression sites of BDNF and NT-4 in the ovary. Figs. 4A and B show that BDNF is expressed at low levels in the oocytes of primordial (short arrows), nongrowing, and growing primary follicles (long arrows and large single arrowheads, respectively) and secondary (large double arrowheads) follicles. More noticeable, however, is the appearance of BDNF in granulosa cells of growing primary and secondary follicles (Figs. 4B and C, large single and double arrowheads). Fig. 4C shows a higher magnification view of a growing primary follicle highlighting the presence of BDNF in granulosa cells (small arrowheads). No BDNF immunoreactivity was detected in the ovary from a BDNF −/− mouse (Fig. 4D).
Because we could not reliably localize NT-4 by immunohistofluorescence in Zamboni-fixed ovaries, we used a denaturing fixative and thin paraffin-embedded section for this purpose. As shown in Figs. 4E–H, NT-4 shows a developmental pattern of expression similar to that of BDNF. Initially, NT-4 immunoreactivity is present in the oocytes of primordial follicles (Fig. 4E, short arrows) but switches to granulosa cells when the follicles reach the primary and secondary stages (Figs. 4F and G, large single and double arrowheads, respectively). Preadsorption of the primary antibody with its antigenic peptide (NT-4) completely eliminated the immunoreactive reactions in both cases (Fig. 4H). Thus, the developing mouse ovary contains the complete ligand receptor system required for neurotrophin-dependent, TrkB-mediated cellular signaling.
Lack of TrkB receptors delays ovarian follicular growth but it does not affect the size of the primordial follicle population present at the end of the first week of postnatal life
The original TrkB KO mice lack the full-length TrkB receptor but still express truncated TrkB receptor isoforms (Klein et al., 1993). They show developmental defects of the central and peripheral nervous system and die at birth (Klein et al., 1993). Although the mutant mice described in the present report survive longer, they show similar neurological defects and are smaller than their littermates (Rohrer et al., 1999). Thus, potential defects in early postnatal ovarian development may be caused by extra-ovarian deficiencies in either the neural or endocrine control of the ovary. A neural defect can be ruled out because animals lacking the ovarian innervation do not show a deficit in preantral follicular development (Lara et al., 1990). To determine if the hypothalamic–pituitary unit is affected in trkB KO animals, we measured serum LH and FSH levels in 7-day-old trkB +/+, trkB +/−, and trkB −/− mice. The results showed that while LH levels were normal in the mutant mice, serum FSH levels were reduced by about 25% in both trkB +/− and trkB −/− mice (Figs. 5A and B). Next, we performed a morphometric analysis of the ovary to compare the degree of follicular growth achieved in wild-type and TrkB-deficient mice at this postnatal age. To determine if the reduction in serum FSH observed in trkB −/− and +/− mice affects follicular growth independently of the presence or absence of TrkB receptors, we also analyzed the ovaries of trkB +/− mice and compared them to the glands of trkB +/+ animals.
Fig. 5.
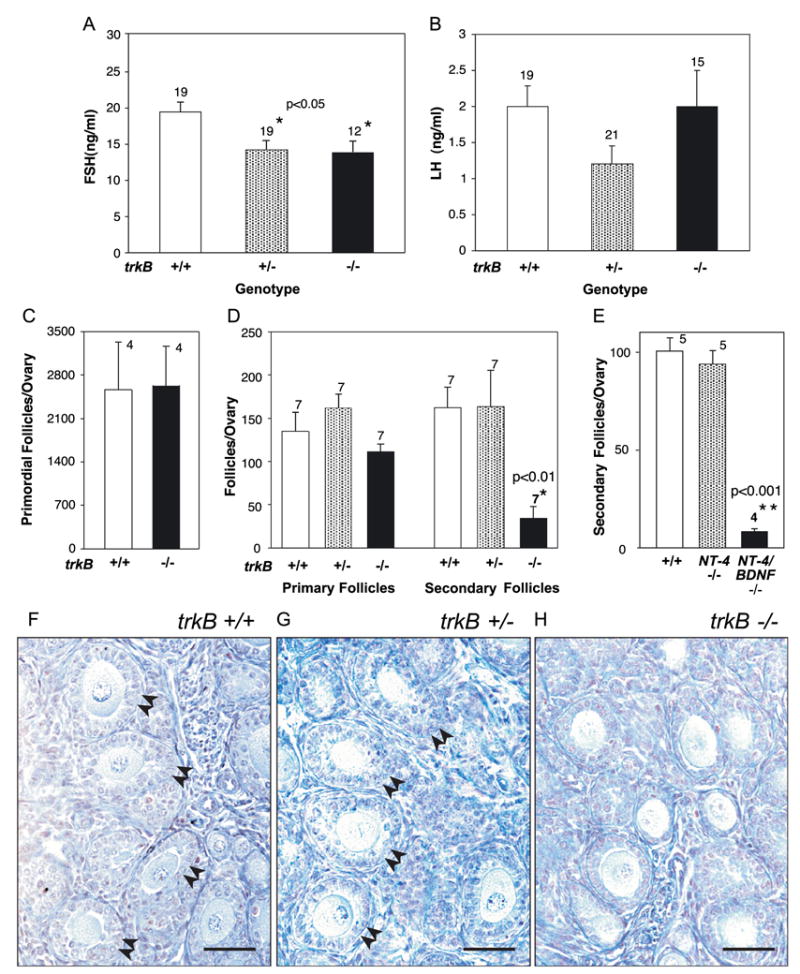
Infantile ovarian follicular development is delayed in a gonadotropin hormone-independent manner in TrkB-deficient mice and mice lacking both the NT-4 and BDNF genes. (A and B) Serum FSH levels (A), but not LH (B), are mildly reduced in both trkB +/− and trkB −/− mice, but only trkB −/− animals show a defect in follicular development. (C) The number of primordial follicles is similar in the ovaries of 7-day-old trkB −/− and wild-type mice. (D) Selective reduction of secondary but not primary follicles in the ovaries of trkB −/− mice. (E) Similar reduction in the number of secondary follicles in the ovaries of 10-day-old mice lacking both NT-4 and BDNF, as compared to the ovaries of wild-type controls and ovaries from mice lacking NT-4. (F–H) Microphotographs of ovaries from 7-day-old trkB +/+, trkB +/−, and trkB −/− mice illustrating the deficiency in early follicular growth associated with the loss of TrkB-mediated signaling. The genotype of each animal was determined by PCR analysis of tail DNA before sectioning the ovaries. The ovaries from both trkB +/+ and trkB +/− mice show numerous secondary follicles (examples denoted by double arrowheads), in contrast to the ovaries from trkB −/− mice that show a normal number of primary follicles and a reduced population of secondary follicles. Scale bars = 50 μm. In A– E, bars are means and vertical lines represent SEM. Numbers above bars indicate number of animals per group.
We observed that the number of primordial follicles per ovary was similar in trkB +/+ and trkB −/− ovaries (Fig. 5C), indicating that TrkB receptor signaling is not required for follicular assembly. The number of primary follicles per ovary was also similar in trkB −/− ovaries as compared to both wild-type and trkB +/− mice, but the number of secondary (two-layer stage) follicles was strikingly reduced in the trkB −/− animals (Fig. 5D, microphotographs in Figs. 5F–H). To determine if the deficiency in secondary follicles is caused by the loss of NT-4 and/or BDNF-initiated signaling, we quantified the number of secondary follicles in mice lacking either NT-4 (Conover et al., 1995) or both genes (Conover et al., 1995). The results showed that the loss of NT-4 in mice with an intact BDNF or TrkB signaling complex does not alter the number of secondary follicles per ovary (Fig. 5E). Instead, the concomitant loss of the NT-4 and BDNF genes results in a deficiency in secondary follicle formation similar to that detected in trkB −/− mice (Fig. 5E). Thus, in the absence of NT-4, there is a compensatory activation of ovarian TrkB receptors by BDNF, the remaining ligand.
Absence of TrkB receptors during the first week of postnatal life results in decreased proliferation of granulosa cells
The growth of primary into secondary follicles requires proliferation of granulosa cells (Hirshfield, 1991; Oktay et al., 1995). To determine if the deficiency in follicular growth seen in trkB −/− mice is related to a reduction in granulosa cell proliferation, we estimated the proliferative activity of these cells by two independent methods, the immunohistochemical detection of the proliferation marker PCNA and the incorporation of BrdU into cell nuclei after exposing the ovaries from trkB +/+ and trkB −/− mice to the nucleoside for 24 h in organ culture. Fig. 6 shows that both the total number of proliferating granulosa cells per ovary, estimated by PCNA immunohistochemistry (Figs. 6A–C), and the proliferation ratio estimated by the ratio of BrdU positive/BrdU negative cells (Figs. 6D–F) were significantly reduced in trkB −/− ovaries. The microphotographs depicted in panels B and C (PCNA staining) and E and F (BrdU staining) illustrate these changes in addition to demonstrating that within individual follicles, there are fewer proliferating granulosa cells in trkB −/− than wild-type ovaries (examples denoted by arrows). The greater difference in number of proliferating cells determined by PCNA immunostaining (panel A) as compared with the difference in proliferation ratio assessed by BrdU incorporation (panel D) may be related to the detection of PCNA in mitogenically inactive cells (Jaskulski et al., 1998). Because up-regulation of PCNA expression in quiescent cells can be elicited by growth factors, such an increase would be more likely to occur in granulosa cells with an intact growth factor system than in trkB −/− ovaries.
Fig. 6.
Granulosa cell proliferation at the end of the first postnatal week of life is decreased in TrkB-deficient mice, without a concomitant increase in apoptotic cell death. (A–F) Decreased number of cell nuclei positive for the proliferation markers PCNA (A–C) and BrdU (D–F) in granulosa cells of trkB −/− ovaries analyzed at the end of the first postnatal week of life (day 7). (A) Number of PCNA-positive granulosa cells per ovary. (D) Ratio of BrdU positive/total granulosa cells per ovary. Bars are means and numbers on top of bars are number of animals per group. Vertical lines are SEM. (B and C) Microphotographs of ovarian sections from trkB +/+ and trkB −/− mice stained with antibodies to the proliferation marker PCNA and counterstained with Gill’s hematoxylin. (E and F) Microphotographs of ovarian sections from trkB +/+ and trkB −/− mice stained with antibodies to BrdU and counterstained with nuclear fast red. Arrows denote follicles showing a predominance of nonproliferating granulosa cells. Scale bars = 50 μm. (G and H) The stunted development of ovarian follicles associated with the loss of TrkB receptors is not accompanied by increased apoptotic cell death, as determined by a TUNEL fluorescence assay. (I) Follicles from a prepubertal 34-day-old ovary showing numerous apoptotic cell nuclei in the granulosa cell compartment of atretic antral follicles. The extensive apoptosis seen in these cells (green nuclei) is in contrast with the almost complete absence of apoptotic nuclei in the follicles of infantile ovaries (7-day-old) mice having either a normal complement of trkB receptors (+/+, G) or lacking all trkB receptor forms (−/−, H). The images depicted represent observations made in ovaries from three 7-day-old mice per genotype and two wild-type late juvenile mice. Scale bars = 25 μm.
Absence of TrkB receptors during the first week of life does not increase ovarian cell apoptosis
Disruption of the trkB gene results in apoptotic cell death in the nervous system (Klein et al., 1993). To determine if the absence of TrkB receptors during the first week of postnatal life resulted in cell death of either granulosa cells or oocytes, we performed TUNEL assays on ovarian sections from 7-day-old wild-type and trkB −/− mice. The results showed that apoptotic cell death in these young ovaries was minimal in both wild-type and KO ovaries (Figs. 6G and H) in contrast to the abundance of apoptotic granulosa cells in atretic antral follicles of older prepubertal animals (Fig. 6I). Thus, the relative inability of trkB-deficient ovaries to sustain the growth of primary follicles does not seem to be brought about by an increased cell death of either oocytes or granulosa cells. In this sense, the ovaries from trkB −/− mice behave like the ovaries of normal animals in which granulosa cells of preantral follicles undergo almost no apoptosis (Hirshfield, 1991).
Absence of TrkB receptors during the first week of postnatal life does not reduce the expression of growth factors required for early follicular growth
Previous studies have shown that the oocyte-derived growth factor GDF-9 and the granulosa cell product KL do not influence follicular formation but are required for the initiation of follicle growth (Dong et al., 1996; Huang et al., 1993). Both the genetic ablation of GDF-9 (Dong et al., 1996) and natural mutations in the steel panda gene encoding KL (Huang et al., 1993; Kuroda et al., 1988) result in arrest of follicle development at the primary 3b stage, that is, at the same stage where an arrest of follicular development becomes apparent in trkB −/− mice. In the absence of GDF-9, granulosa cells proliferation is reduced (McGrath et al., 1995), just as it happens in trkB −/− mice. While KL acts via the receptor tyrosine kinase c-kit, which is predominantly expressed in oocytes (Manova et al., 1990), a recent report (Vitt et al., 2002) showed that GDF-9 is recognized by bone morphogenetic protein receptor II (BMPRII), a receptor expressed in granulosa cells (Shimasaki et al., 1999). To determine if the absence of TrkB signaling might result in reduced expression of these proteins, the encoding mRNAs were quantified by either RealTime PCR (the ligands GDF-9 and KL) or Relative Quantitative PCR (the receptors BMPRII and c-kit). As shown in Fig. 7, the abundance of the two mRNAs expressed in oocytes, the ligand GDF-9 (panels A and C) and the receptor c-kit (panels F and H), was essentially the same in trkB −/− and wild-type ovaries, indicating that the supportive effect of NTs acting via TrkB receptors on early follicular growth does not require the intermediacy of these oocyte- derived molecules. A similar analysis was performed for the mRNAs encoding the granulosa cell located GDF-9 receptor BMPRII and the granulosa cell-secreted c-kit ligand KL. Because the total number of granulosa cells per ovary was reduced by 23% in trkB −/− mice, the BMPRII and KL mRNA values detected in trkB −/− ovaries were corrected by this factor. The results showed that the content of both mRNAs remained essentially unchanged in the KO ovaries, with a small increase being statistically significant (P < 0.01) for BMPRII (Figs. 7E and G, B and D). Thus, neither of these oocyte-granulosa cell communication complexes seems to be a downstream component underlying the supportive effect of TrkB receptor activation on follicular growth.
Fig. 7.
The ovarian content of the mRNAs encoding GDF-9 and its presumed receptor, BMPRII, and KL and its c-kit receptor is not altered in trkB −/− mice. (A and B) Representative RealTime PCR amplification profiles of GDF-9 mRNA (A) and KL mRNA (B) from the ovary of trkB −/− mice compared with that of wild-type littermates. The insets depict the standard curves use to estimate the amount of each mRNA present in the two groups. The curves were generated using serial dilutions of reverse transcription reactions derived from one μg of total RNA from 7-day-old mouse ovaries (equivalent to 10−1– 10−8 μg total RNA). The values obtained were normalized using the values of 18S ribosomal RNA amplified with each reaction as an internal control. The primers and probe for ribosomal RNA were those provided in the TaqMan ribosomal RNA Control Reagents Vic probe Kit (Perkin Elmer Applied Biosystems). The PCR reactions were carried out as described (Romero et al., 2002) using an ABI Prism 7700 sequence Detection System (Perkin Elmer Applied Biosystems), in a total volume of 10 μl, containing 2 μl of reverse transcription reaction, 5 μl of TaqMan Universal PCR Master Mix (Perkin Elmer Applied Biosystems), and 3 μl primer mix (250 nM of each gene-specific and ribosomal fluorescent probes and 300 nM of each gene-specific unlabeled primer plus 80 nM of each unlabeled ribosomal primer). (C and D) Ovarian content of GDF-9 (C) and KL (D) mRNA detected by RealTime PCR in wild-type and trkB −/− 7-day-old mice. (E and F) Representative ethidium bromide-stained gels showing BMPRII (E) and c-kit (F) PCR products amplified from total ovarian RNA using the Relative Quantitative PCR conditions described in Materials and methods. (G and H) Relative content of BMPRII (G) and c-kit (H) mRNAs estimated by Relative Quantitative PCR. Values are shown as ratios between the gene-specific signal and the signal provided by 18S ribosomal RNA coamplified with each gene of interest. Bars are mean ± SEM and numbers on top of bars are number of mice per group. Because the KL and BMPRII mRNAs are expressed in granulosa cells, the values obtained in trkB −/− mice were corrected to take into consideration the reduction in granulosa cell number observed in these animals.
Absence of trkB receptors results in decreased expression of FSH receptors
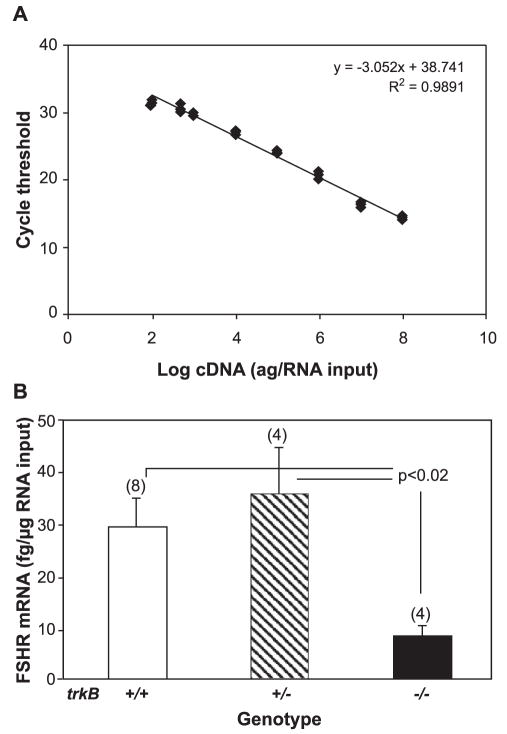
As ovarian follicles grow beyond the primary stage, granulosa cells acquire FSH receptors (Oktay et al., 1997) and become responsive to the gonadotropin (Sokka and Huhtaniemi, 1990). Experimental induction of FSHR synthesis at this stage of development allows secondary follicles to acquire additional layers of granulosa cells in response to FSH (Romero et al., 2002). The finding that follicular growth in mice lacking the FSHR gene progresses only to the secondary two-layer stage (Kumar et al., 1997) indicates that this is indeed the developmental phase during which the growth of ovarian follicles become gonadotropin dependent. RealTime PCR measurement of FSHR mRNA demonstrated that in keeping with the deficiency in secondary follicles observed at the end of the first week of postnatal life in TrkB-deficient mice, the content of FSHR mRNA (corrected as in the case of BMPRII and KL) is strikingly reduced in the ovaries of these animals as compared with either trkB +/+ or trkB +/− mice (Fig. 8). The FSHR mRNA content in trkB +/− ovaries was identical to that of trkB +/+ mice. It then follows that in the absence of TrkB signaling, follicles fail to reach the stage in which they will continue to grow under pituitary gonadotropin control.
Fig. 8.
Reduced FSHR mRNA content in the ovaries of 7-day-old trkB −/− mice as compared with wild-type and trkB +/− animals. (A) Linear regression analysis of the standard curve used to calculate the mRNA values shown in B. The curve depicted resulted from the amplification of different amounts (0.1 –100,000 fg/tube) of in vitro transcribed sense FSHR RNA standard. (B) Mean FSHR mRNA content in the ovaries from trkB +/+, trkB +/−, and trkB −/− mice. Vertical lines are SEM, and numbers above bars are number of mice per group.
Sustained absence of TrkB receptors results in oocyte death, interruption of follicle growth, and widespread degradation of follicular organization
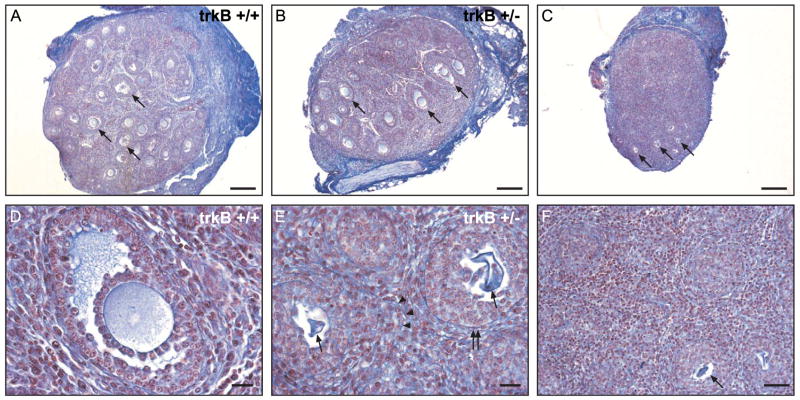
Because TrkB-deficient mice are in poor health, it is difficult to study the development of the ovary after the first week of postnatal life. To overcome this limitation, we transplanted the ovaries of 4- to 5-day-old trkB +/+, trkB +/−, and trkB −/− mice under the kidney capsule of adult female C57BL/6J mice and evaluated the morphological aspect of the ovaries 2 weeks after transplantation. The ovaries (n = 4) from trkB +/+ mice contained numerous follicles in different stages of development (Fig. 9A), including follicles that had reached the antral stage (examples denoted by arrows). Likewise, oocyte development appeared normal as evidenced by the presence of healthy oocytes at different stages of development (Fig. 9A). The morphological aspect of trkB +/− ovaries (n = 6) was indistinguishable from that of wild-type ovaries. They also had antral follicles in various stages of growth and containing healthy oocytes (Fig. 9B, examples denoted by arrows). One such follicle is shown at higher magnification in panel D. In striking contrast, the ovaries of trkB −/− mice (n = 6) were essentially depleted of oocytes (Fig. 9C). The few oocytes still present appeared to be in advanced stages of degeneration (examples denoted by arrows in Fig. 9C and in the higher magnification image shown in Fig. 9E). Although trkB −/− exhibited remnants of follicles that had progressed beyond the primary stage (example denoted by double arrows in Fig. 9E), structural dissolution was evident in several follicles containing a dying oocyte. In these follicles, the integrity of the basal lamina appeared compromised so that the granulosa cells were no longer contained within individual follicles (arrowheads in Fig. 9E). In the absence of oocytes, the identity of individual follicles disappeared (Fig. 9F). Surprisingly, no overt morphological signs of granulosa cell death were detected in follicles that had loss or were in the process of losing, their oocyte (Figs. 9E and F).
Fig. 9.
Sustained absence of TrkB receptors results in oocyte death, interruption of follicle growth and loss of follicular organization. To examine the consequences of the absence of TrkB receptors on follicular growth beyond the first week of life the ovaries of 4- to 5-day-old trkB +/+, trkB +/−, and trkB −/−, mice were transplanted under the kidney capsule of adult female C57BL/6J mice and their morphological aspect was evaluated 2 weeks after transplantation. (A) Ovary from a trkB +/+ showing numerous follicles in different stages of development, including follicles that had reached the antral stage (arrows). (B) Ovary from a trkB +/− mouse exhibiting a degree of follicular development indistinguishable from that of wild-type ovaries. (C) Ovary from a trkB −/− mouse showing marked depletion of oocytes and loss of follicular boundaries. The few oocytes still present appear to be in advanced stage of degeneration (arrows). (D) Higher magnification image illustrating the presence of normal antral follicles containing healthy oocytes in trkB +/− ovaries. (E) High magnification image of a trkB −/− ovary demonstrating the presence of dying oocytes (arrows) in follicles still intact (double arrow) and follicles undergoing dissolution as evidenced by the loss of basal lamina continuity (arrowheads). (F)Widespread loss of follicular structure in the absence of oocytes. Even in the absence of oocytes or follicular structure, the granulosa cells appear normal. Scale bars in A–C = 200 μm; in D and E = 20 μm; in F = 50 μm.
Discussion
The present study provides evidence for the involvement of TrkB receptors in the development of an endocrine organ. Using trkB-null mice that lack all forms of the receptor, we show that during the early stages of the deficiency (assessed in ovaries in situ at the end of the first postnatal week of life), follicular growth is impaired at the stage during which primary follicles (type 3b) begin to grow. Transplantation of trkB −/− ovaries under the kidney capsule of adult mice revealed that the sustained absence of TrkB receptors results in oocyte death, absence of antral follicle formation, and dissolution of follicular boundaries.
Morphometric analysis of 7-day-old trkB −/− ovaries demonstrated that the deficiency selectively affects the development of secondary follicles, which requires mitogenic activation of granulosa cells (Lintern-Moore and Moore, 1979; Oktay et al., 1995), but not the presence of gonadotropin hormones (Dierich et al., 1998; Kumar et al., 1997; Zhang et al., 2001). Granulosa cells of trkB −/− ovaries exhibit a marked decrease in proliferative activity, without obvious apoptosis, indicating that the growth defect is primarily related to a reduction in cell proliferation and not caused by an increase in programmed cell death. Because mice carrying a deletion of both the NT-4 and BDNF genes show a similar deficiency of follicular growth, it is clear that the defect is caused by the loss of NT-4 or BDNF signaling through TrkB receptors. In addition, the normal number of secondary follicles detected in mice lacking only the NT-4 gene suggests that in the absence of this ligand, BDNF assumes a compensatory role.
Although the number of secondary follicles in TrkB-deficient mice was reduced, this deficiency was not accompanied by significant changes in the number of primordial or primary follicles. Because a selective reduction in secondary follicles would be expected to be accompanied by accumulation of primary follicles, the lack of such an increase suggests that the transition of primordial to primary follicle may be also compromised in trkB KOs. However, the subtlety of this change makes it unlikely that a reduction in the formation of primary follicles is the primary mechanism by which the lack of TrkB receptors impairs the formation of secondary follicles.
The ovarian phenotype observed at the end of the first postnatal week of life in trkB KO mice is similar to that of mice lacking GDF-9, an oocyte-derived growth factor member of the TGF-β superfamily (Elvin et al., 2000). Like in trkB KOs, the growth of primary one-layer (type 3b) into secondary two-layer (type 4) follicles is impaired in GDF-9-deficient mice (Dong et al., 1996; Elvin et al., 1999b). Also like in TrkB-deficient mice, GDF-9-null mutants show a defect in granulosa cell proliferation, but no increase in apoptotic cell death (Elvin et al., 1999b). Furthermore, the oocyte expression of both truncated TrkB receptors (this paper) and GDF-9 (Elvin et al., 1999a) becomes first evident in primary type 3a follicles, increasing markedly thereafter. It does not appear, however, that the two systems are functionally or hierarchically related because GDF-9 mRNA expression was normal in trkB KOs as was the expression of BMPRII, a serine or threonine kinase receptor recently shown to be a receptor for GDF-9 (Vitt et al., 2002). In fact, when the BMPRII mRNA values were corrected to reflect the smaller number of granulosa cells present in trkB −/− ovaries, a significant increase in the mRNA content became apparent.
A fundamental difference between the ovaries of trkB −/− mice and those of mice lacking GDF-9 was revealed by ovarian transplantation experiments. We used this approach to overcome the limitation imposed by the poor health of trkB −/− mice, which makes it difficult to assess the consequences of trkB gene deletion on ovarian development beyond the first week of postnatal life. The transplantation of 4- to 5-day-old wild-type ovaries into adult, ovariectomized wild-type mice demonstrated that, as shown by others ((Liu et al., 2002) and references therein), follicular development progresses unabated in the transplanted ovaries. We also observed that follicular growth in transplanted trkB +/− ovaries was indistinguishable from that of wild-type animals. In marked contrast, the ovaries from trkB −/− mice exhibited a dramatic depletion of the oocyte population and widespread arrest of follicle growth. The few remaining oocytes showed signs of advanced degeneration in the presence of apparently healthy granulosa cells. These changes are not seen in GDF-9-deficient mice, in which oocytes grow more rapidly than in wild-type animals and remain healthy despite the arrested follicular growth (Carabatsos et al., 1998).
Because in transplanted trkB-null ovaries oocyte loss is not accompanied by similar alterations in granulosa cell viability, it appears warranted to conclude that oocytes represent the primary target of ligand-dependent TrkB receptor activation. These results and those obtained with in situ 1-week-old ovaries suggest that once oocytes begin to acquire functional TrkB receptors during the transition from primary to secondary follicles, they become increasingly dependent on TrkB-mediated signaling for survival and development. Initially, the oocytes might not be able to sustain cell–cell communication pathways necessary for granulosa cell proliferation (Vanderhyden et al., 1992), and the growth of primary follicles is delayed. Later on, oocyte survival is compromised and this ultimately leads to arrest of follicular growth. Follicular boundaries are then lost resulting in widespread dissolution of follicular organization. Noteworthy, the aforementioned changes occur in transplanted trkB −/− ovaries in the face of strong gonadotropin stimulation. This postulated sequence of events is in keeping with recent findings demonstrating that oocytes promote the differentiation and growth of granulosa cells and determine the rate of follicular development (Eppig et al., 2002). Because the elimination of oocytes before the time of folliculogenesis by either pharmacological (Hirshfield, 1991) or genetic (Soyal et al., 2000) means results in failure of follicular formation, it appears clear that oocytes are essential for folliculogenesis, subsequent follicle growth and follicle survival.
In 7-day-old trkB −/− ovaries, in situ follicular growth is arrested at the primary 3b stage, but the follicles that could still be discerned in transplanted trkB −/− appeared to have progressed beyond the secondary stage. We interpret this difference as due to both the transplantation procedure and the hormonal environment to which the ovaries are subjected when grafted into ovariectomized hosts. Within 12 h after transplantation, there is a massive apoptotic loss of follicles, which is followed by recruitment of a new cohort of primordial follicles for growth (Liu et al., 2002). Because the host mice are ovariectomized, growth of these new follicles proceeds under the influence of elevated FSH and LH levels, which can hyperstimulate granulosa cell proliferation despite an initially reduced complement of gonadotropin receptors resulting from the absence of TrkB receptors (see below).
The distribution of NT-4 and BDNF in granulosa cells and TrkB receptors in oocytes is similar to the KL or c-kit signaling system, in which KL is produced by granulosa cells (Joyce et al., 1999; Manova et al., 1993), and binds to c-kit receptors expressed in the oocyte (Manova et al., 1990). However, the ovaries of trkB-null mice show normal levels of both KL and c-kit mRNA, indicating that TrkB-mediated signaling does not require changes in expression of the KL or c-kit signaling complex. It thus appears that activation of ovarian TrkB receptors regulates oocyte survival and follicle growth via unidentified pathways that function in parallel to those activated by KL in the oocyte and GDF-9 in granulosa cells. These two systems are also relatively independent from each other, as animals lacking GDF-9 have elevated KL mRNA levels, but a normal c-kit expression (Elvin et al., 1999b). FSHR mRNA levels were strikingly reduced in in situ trkB −/− ovaries, indicating that in the absence of TrkB receptors, the attainment of a developmental stage in which the follicles will acquire FSHRs, and thus become gonadotropin dependent, is hampered. This selective deficiency in FSHR gene expression in the face of a normal BMPRII and KL mRNA levels (which like FSHRs are also expressed in granulosa cells) indicates that the absence of trkB signaling results in a growth defect affecting a specific component of the ovary instead of a generalized disruption of tissue development. That the reduction in FSHR gene expression is not a consequence of the lower serum FSH levels observed in trkB −/− mice is indicated by the fact that a similar decrease in serum FSH occurs in trkB +/− and yet follicular development was only impaired in the former. The decrease in FSH levels might be related to loss of a supportive TrkB effect on the release of hypothalamic gonadotropin hormone-releasing hormone, which controls pituitary FSH secretion.
The remarkable developmental patterns of cellular expression observed for both the TrkB receptors and their NT-4 and BDNF ligands suggest that ovarian TrkB-dependent signaling requires the interaction of granulosa cells and oocytes. Although both NT-4 and BDNF are initially detected in oocytes of primordial follicles, their site of expression switches to granulosa cells at the outset of follicular growth, indicating that somatic cells provide the bulk of NT signals activating ovarian TrkB receptors at this critical stage of follicular growth. Because low levels of full-length TrkB receptors are detected in oocytes at all phases of follicular development, but they can be detected in granulosa cells only in growing follicles, it might be concluded that NT-4 and BDNF exert part of their facilitatory effects on granulosa cell proliferation via paracrine and autocrine loops that affect granulosa cells both directly and through intermediacy of the oocyte.
Noteworthy, the majority of the TrkB receptors detected in the infantile mouse ovary are truncated, exclusively expressed in oocytes of growing follicles (type 3b onwards), and strikingly more abundant than full-length receptors, suggesting that they may play a significant role in the control of oocyte functions. The unexpected finding that truncated TrkB receptors become targeted to the oocyte’s cell membrane only when primary follicles initiate growth and the dramatic loss of oocytes observed in transplanted trkB −/− supports this idea and suggests that truncated TrkB receptors might also be important for oocyte survival. The mechanisms underlying these effects are unknown. Binding of NT-4 or BDNF to TrkB T1 receptors may activate signaling pathways (Baxter et al., 1997; Kryl and Barker, 2000) coupled to the production of proliferating factors by the oocyte (Vanderhyden et al., 1992) and/or required for oocyte survival. Alternatively, TrkB T1 receptors in oocytes may bind NTs and present them to the full-length receptors expressed in granulosa cells, which in turn would provide signals (Carabatsos et al., 2000; Cecconi and Rossi, 2001; Mehlmann et al., 2002) required for oocyte survival and development. Further investigation is required to clarify this issue.
We previously reported that the lack of NGF diminishes both the differentiation of primordial into primary follicles and growth of primary into secondary follicles and that this defect is related to a deficiency in mesenchymal cell proliferation (Dissen et al., 2001). By now showing that NT-4 or BDNF facilitates the growth of primary follicles and that the absence of TrkB receptors results first in reduced granulosa cell proliferation and then oocyte death, the present findings lend credence to the concept that neurotrophins support the development of nonneural target tissues, such as the ovary, by providing signals mediated by their high-affinity receptors. Based on these observations, we propose that neurotrophins are intraovarian factors that contribute with KL and GDF-9 to the control of early follicular development (Matzuk et al., 2002). While current evidence suggest that KL and NGF promote the differentiation of primordial follicles into primary follicles (Dissen et al., 2001; Parrott and Skinner, 1999), the present data indicate that NT-4 and BDNF may work in concert with GDF-9, KL, and NGF in promoting the transition of primary follicle into secondary stage. Our findings also suggest that truncated TrkB receptors expressed in oocytes may play a hitherto unsuspected role in mediating these effects. The loss of oocytes seen in transplanted trkB −/− ovaries suggests that a functional NT-4/BDNF-TrkB signaling complex is essential for oocyte survival during preantral follicle development.
Acknowledgments
We thank Ms. Maria Costa for performing the immunohistochemical reactions and Pablo Ojeda for morphometric analysis of follicular development. We are grateful to Dr. Les Dees (Department of Veterinary Anatomy and Public Health, Texas A&M University, College Station, Texas 77843) for measuring serum LH and FSH levels. This work was supported by NIH grants HD-24870, RR-00163 for the operation of the Oregon Regional Primate Research Center, NICHD through cooperative agreement U54 HD18185 as part of the Specialized Cooperative Centers Program in Reproduction Research (SRO), and the Howard Hughes Medical Institute and NINDS PO1-16033 (LFR). LFR is an investigator of the Howard Hughes Medical Institute. A.P. is a graduate student of the Biochemistry Graduate Program, Faculty of Chemistry and Pharmaceutical Sciences, University of Chile, supported by a fellowship from NICHD TW/HD00668 Fogarty International Training in Population and Health grant. C.R. was a visiting scientist supported by a fellowship from NICHD TW/HD00668 Fogarty International Training and Research in Population and Health grant.
References
- Anderson RA, Robnson LLL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab. 2002;87:890–897. doi: 10.1210/jcem.87.2.8221. [DOI] [PubMed] [Google Scholar]
- Anesetti G, Lombide P, D’Albora H, Ojeda SR. Intrinsic neurons in the human ovary. Cell Tissue Res. 2001;306:231– 237. doi: 10.1007/s004410100451. [DOI] [PubMed] [Google Scholar]
- Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, Medina-Selby A, Coit D, Valensuela P, Feinstein SC. Signal transduction mediated by the truncated trkB receptor isoforms, trkB. T1 and trkB.T2. J Neurosci. 1997;17:2683– 2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Bepu H, Minowa O, Miyazono K, Kawabata M. cDNA cloning and genomic organization of the mouse BMP type II receptor. Biochem Biophys Res Commun. 1997;235:499– 504. doi: 10.1006/bbrc.1997.6816. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373– 384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocytye-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226:167– 179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- Cecconi S, Rossi G. Mouse antral oocytes regulate preantral granulosa cell ability to stimulate oocyte growth in vitro. Dev Biol. 2001;233:186– 191. doi: 10.1006/dbio.2001.0209. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, Friedman B, Wiegand S, Vejsada R, Kato AC, DeChiara TM, Yancopoulos GD. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–241. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Davies AM. Neurotrophins: neurotrophic modulation of neurite growth. Curr Biol. 2000;10:R198–R200. doi: 10.1016/s0960-9822(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Newman Hirshfield A, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology. 1995;136:4681– 4692. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Newman Hirshfield A, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078– 2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531– 535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Hahn R, Tessarollo L, Hempstead BL. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat Genet. 1996;14:210–213. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531– 4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999a;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999b;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-β superfamily members in female fertility. Mol Cell Endocrinol. 2000;159:1 –5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99:2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21– 40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- García-Suárez O, Germanà A, Hannestad J, Ciriaco E, Laurà R, Naves J, Esteban I, Silos-Santiago I, Vega JA. TrkA is necessary for the normal development of the murine thymus. J Neuroimmunol. 2000;108:11 – 21. doi: 10.1016/s0165-5728(00)00251-4. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100– 109. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Farinas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskulski D, Gatti C, Travali S, Calabretta B, Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1998;263:10175– 10179. [PubMed] [Google Scholar]
- Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev Biol. 1999;214:342– 353. doi: 10.1006/dbio.1999.9437. [DOI] [PubMed] [Google Scholar]
- Jung H, Shannon EM, Fritschy JM, Ojeda SR. Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Res. 1997;780:218– 229. doi: 10.1016/s0006-8993(97)01152-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647– 656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113– 122. [PubMed] [Google Scholar]
- Kryl D, Barker PA. TTIP is a novel protein that interacts with the truncated T1 TrkB neurotrophin receptor. Biochem Biophys Res Commun. 2000;279:925– 930. doi: 10.1006/bbrc.2000.4058. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matsuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201– 204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Terada N, Nakayama H, Matsumoto K, Kitamura Y. Infertility due to growth arrest of ovarian follicles in Sl/SIt mice. Dev Biol. 1988;126:71– 79. doi: 10.1016/0012-1606(88)90240-0. [DOI] [PubMed] [Google Scholar]
- Lara HE, McDonald JK, Ojeda SR. Involvement of nerve growth factor in female sexual development. Endocrinology. 1990;126:364– 375. doi: 10.1210/endo-126-1-364. [DOI] [PubMed] [Google Scholar]
- Lintern-Moore S, Moore GPM. The initiation of follicle and oocyte growth in the mouse. Biol Reprod. 1979;20:773– 778. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17:605–611. doi: 10.1093/humrep/17.3.605. [DOI] [PubMed] [Google Scholar]
- Malamed S, Gibney JA, Ojeda SR. Ovarian innervation develops before initiation of folliculogenesis in the rat. Cell Tissue Res. 1992;270:87– 93. doi: 10.1007/BF00381883. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057– 1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, Besmer P, Bachvarova RF. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157:85– 99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178– 2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Costa ME, Ojeda SR. A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology. 1997;138:3320– 3329. doi: 10.1210/endo.138.8.5335. [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee SJ. Ooctye-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jones TLZ, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343– 1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143– 153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53:295– 301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748– 3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol. 1969;62:98– 116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Qiu F, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, Besmer P. Primary structure of c-kit: Relationship with the CSF-1/PDGF receptor kinase family-oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003– 1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photo-receptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919– 8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology. 2002;143:1485–1494. doi: 10.1210/endo.143.4.8711. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesions. J Neurosci. 1995;15:477– 491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Zachow RJ, Li D, Kim H, Iemura SI, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A. 1999;96:7282– 7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627– 638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sokka T, Huhtaniemi I. Ontogeny of gonadotrophin receptors and gonadotrophin-stimulated cyclic AMP production in the neonatal rat ovary. J Endocrinol. 1990;127:297– 303. doi: 10.1677/joe.0.1270297. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amieh A, Dean J. FIGα, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645– 4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Tessarollo L. Pleiotrophic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125– 137. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci U S A. 1997;94:14776– 14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC, Telfer EE, Eppig JJ. Mouse oocytes promote proliferation of granulosa cells from preantral and antral follicles in vitro. Biol Reprod. 1992;46:1196– 1204. doi: 10.1095/biolreprod46.6.1196. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJW. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67:473–480. doi: 10.1095/biolreprod67.2.473. [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888– 6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172– 183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Zhu GZ, Miller BJ, Boucheix C, Rubinstein E, Liu CC, Hynes RO, Myles DG, Primakoff P. Residues SFQ (173–175) in the large extracellular loop of CD9 are required for gamete fusion. Development. 2002;129:1995– 2002. doi: 10.1242/dev.129.8.1995. [DOI] [PubMed] [Google Scholar]