Abstract
β-Carotene biochemistry is a fundamental process in mammalian biology. Aberrations either through malnutrition or potentially through genetic variation may lead to vitamin A deficiency, which is a substantial public health burden. In addition, understanding the genetic regulation of this process may enable bovine improvement. While many bovine QTL have been reported, few of the causative genes and mutations have been identified. We discovered a QTL for milk β-carotene and subsequently identified a premature stop codon in bovine β-carotene oxygenase 2 (BCO2), which also affects serum β-carotene content. The BCO2 enzyme is thereby identified as a key regulator of β-carotene metabolism.
THE metabolism of β-carotene to form vitamin A is nutritionally important, and vitamin A deficiency remains a significant public health burden. Genetic variation may underlie individual differences in β-carotene metabolism and contribute to the etiology of vitamin A deficiency. Within an agricultural species, genetic variation provides opportunity for production improvements, disease resistance, and product specialization options. We have previously shown that natural genetic variation can be successfully used to inform bovine breeding decisions (Grisart et al. 2002; Blott et al. 2003). Despite numerous reports of quantitative trait loci (QTL), few causative mutations have been identified. We discovered a QTL for milk β-carotene content and report here the identification of a mutation in the bovine β-carotene oxygenase 2 (BCO2) gene responsible for this QTL. The mutation, which results in a premature stop codon, supports a key role for BCO2 in β-carotene metabolism.
The QTL trial consisted of a Holstein-Friesian × Jersey cross in an F2 design and a half-sibling family structure (Spelman et al. 2001). Six F1 sires and 850 F2 female progeny formed the trial herd. To construct the genetic map, the pedigree (including the F1 sires, F1 dams, F2 daughters, and selected F0 grandsires: n = 1679) was genotyped, initially with 237 microsatellite markers, and subsequently, with 6634 SNP markers (Affymetrix Bovine 10K SNP GeneChip). A wide range of phenotypic measures relating to growth and development, health and disease, milk composition, fertility, and metabolism were scored on the F2 animals from birth to 6 years of age.
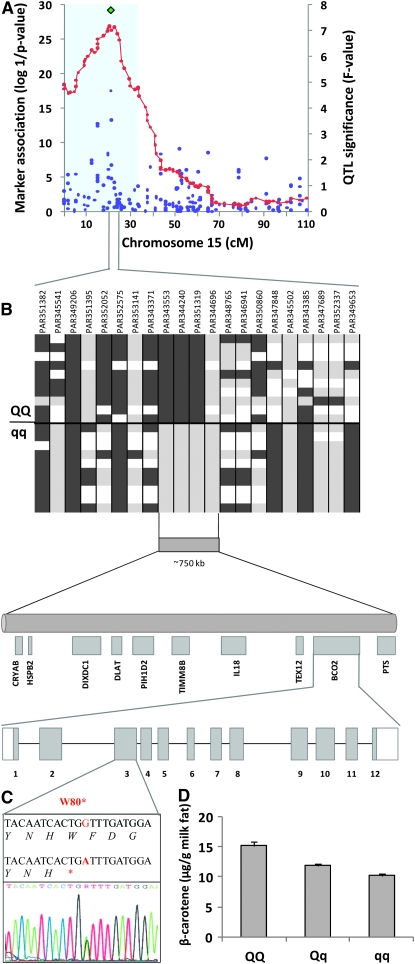
To facilitate the discovery of QTL and genes regulating β-carotene metabolism, milk concentration of β-carotene was measured during week 6 of the animals' second lactation (n = 651). Using regression methodology in a half-sib model (Haley et al. 1994; Baret et al. 1998), a QTL on bovine chromosome 15 (P < 0.0001; Figure 1A) was discovered. The β-carotene QTL effect on chromosome 15 was also significant (P < 0.0001) at two additional time points, in months 4 and 7 of lactation. Three of the six F1 sire families segregated for the QTL, suggesting that these three F1 sires would be heterozygous for the QTL allele (“Q”). To further define the most likely region within the QTL that would harbor the causative mutation, we undertook association mapping, using the 225 SNP markers that formed the chromosome 15 genetic map (Figure 1A). One SNP (“PAR351319”) was more closely associated with the β-carotene phenotype than any other marker (P = 2.522E−18). This SNP was located beneath the QTL peak. Further, the SNP was heterozygous in the three F1 sires that segregated for the QTL, and homozygous in the remaining three sires. On this basis, we hypothesized that the milk β-carotene phenotype would differ between animals on the basis of the genotype of SNP PAR351319.
Figure 1.—
Discovery of BCO2 mutation affecting milk β-carotene concentration. (A) The β-carotene QTL on bovine chromosome 15 (P < 0.0001) is shown by the red line. The maximum F-value at 21 cM was 7.15. The 95% confidence interval is shown by the shaded box. The association of each marker with milk β-carotene is shown by the blue dots, and the association of the BCO2 genotype is shown by the green diamond. A total of 233 informative markers (8 microsatellite markers and 225 single nucleotide polymorphisms) were included on the genetic map for BTA15. QTL detection was conducted using regression methodology in a line of descent model (Haley et al. 1994) and a half-sib model (Baret et al. 1998). Threshold levels were determined at the chromosomewide level using permutation testing (Churchill and Doerge 1998) and confidence intervals estimated using bootstrapping (Visscher et al. 1996). (B) The haplotypes of 10 representative animals for “QQ” and “qq” are shown for the SNP markers encompassing the SNP (“PAR351319”) most closely associated with the milk β-carotene phenotype. Light and dark gray boxes represent homozygous SNPs, while white boxes represent heterozygous SNPs. The genes present within the defined region are also shown. (C) The mutation in the bovine BCO2 gene is shown. The structure of the BCO2 gene is indicated by the horizontal bar, with vertical bars representing exons 1–12. The A > G mutation in exon 3 (red) causes a premature termination codon at amino acid position 80. (D) The mean concentration of β-carotene in the milk fat of “QQ,” “Qq,” and “qq” cows is shown. β-Carotene was measured by absorbance at 450 nm as previously described (Winkelman et al. 1999). Data are means ± SEM. The statistical significance was determined using ANOVA (***P < 0.0001; n = 651).
We then made the following assumptions: that the effect of the QTL was additive, that the Q allele was present in the dam population, allowing the occurrence of homozygous (“QQ”) offspring, and that the QTL was caused by a single mutation, acting with a dominant effect on the milk β-carotene phenotype. Haplotypes encompassing the PAR351319 SNP were determined in the F2 offspring. A comparison of the phenotypic effect of homozygous Q, heterozygous and homozygous q individuals revealed that indeed, animals with the “QQ” genotype had a higher concentration of milk β-carotene than animals with the “qq” genotype (Figure 1D). We predicted that the region of homozygosity was likely to contain the causative gene and mutation. The extent of this region and the candidate genes contained within it are shown in Figure 1B. A total of 10 genes with known function, including BCO2, were located within the region. This information, combined with knowledge of the role BCO2 plays in β-carotene metabolism in other species (Kiefer et al. 2001), made BCO2 a good positional candidate for the QTL. We therefore sequenced the entire coding region (12 exons, NC_007313.3) of the BCO2 gene in each of the six F1 sires. An A > G mutation, which was heterozygous in the three F1 sires that segregated for the QTL, was discovered in exon three, 240 bp from the translation initiation site (Figure 1C). The three remaining sires were homozygous for the G allele, which encodes the 530-amino-acid BCO2 protein (NP_001101987). The A allele creates a premature stop codon resulting in a truncated protein of 79 amino acids. To determine whether this mutation was associated with the QTL, the remainder of the pedigree was genotyped. The BCO2 genotype was significantly associated with the milk β-carotene phenotype (P = 8.195E−29) The AA genotype (referred to as BCO2−/−) was present in 3.4% (n = 28) of the F2 population. The AG and GG genotypes (subsequently referred to as BCO2−/+ and BCO2+/+, respectively) were present in 32.8% (n = 269) and 63.8% (n = 523), respectively, of the F2 population.
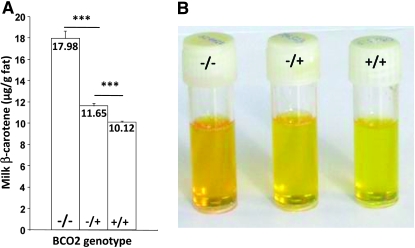
The effect of the premature stop codon on milk β-carotene content was striking. BCO2−/− cows produced milk with 78 and 55% more β-carotene than homozygous (GG) and heterozygous (AG) wild-type animals, respectively (P < 0.0001; Figure 2A). Consequently, the yellow color of the milk fat varied greatly (Figure 2B). The genotype effect on milk β-carotene content was similar at the other two time points measured during lactation (78 and 68% more β-carotene in milk from BCO2−/− cows compared to BCO2+/+ cows; data not shown).
Figure 2.—
Effect of BCO2 genotype on milk β-carotene content. (A) The mean concentration of β-carotene in the milk fat of BCO2−/−, BCO2−/+, and BCO2+/+ cows is shown. β-Carotene was measured by absorbance at 450 nm as previously described (Winkelman et al. 1999). Data are means ± SEM. The statistical significance was determined using ANOVA (***P < 0.0001; n = 651). (B) The effect of the BCO2 genotype on milk fat color is illustrated.
No adverse developmental or health affects as a result of the A allele were observed at any stage throughout the lifespan of the animals. The BCO2−/− cows were fertile and milk yield was normal throughout lactation. Interestingly, quantitative real-time PCR showed fourfold lower levels of the BCO2 mRNA in liver tissue from BCO2−/− cows (data not shown).
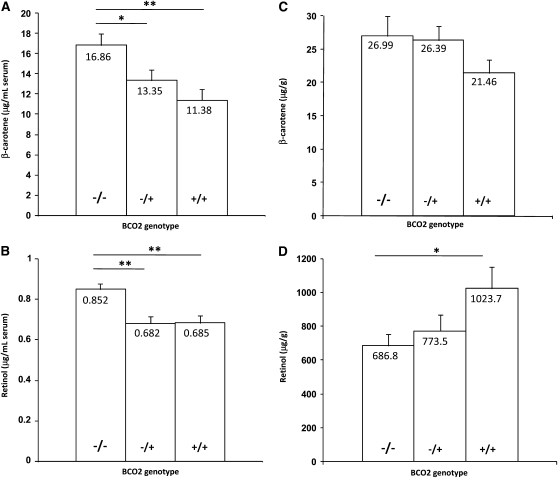
β-Carotene and vitamin A (retinol) concentrations were also measured in serum, liver, and adipose tissue samples, and vitamin A concentration was measured in milk samples from 14 F2 cows of each genotype. Serum β-carotene concentration was higher in BCO2−/− cows compared to the heterozygous and homozygous wild-type cows (P = 0.003; Figure 3A). Thus, the effect of the mutation on β-carotene concentration was similar for both milk and serum, showing that this effect was not confined to the mammary gland. Vitamin A concentration was higher in serum from BCO2−/− cows (P = 0.001; Figure 3B); however, the concentration did not differ in milk (13.1 μg/g fat vs. 14.1 μg/g fat for BCO2−/− and BCO2+/+ cows, respectively; P > 0.1). Liver β-carotene concentration did not differ between genotype groups (Figure 3C), but liver vitamin A was lower in BCO2−/− cows compared to BCO2+/+ cows (P < 0.03; Figure 3D). β-Carotene and vitamin A concentration did not differ between the genotype groups in adipose tissue (data not shown), suggesting tissue-specific effects of the BCO2 enzyme.
Figure 3.—
Effect of the BCO2 genotypes on concentration of β-carotene (A and C), and retinol (B and D), in serum (A and B), and liver (C and D). Subcutaneous adipose tissue biopsies (∼500 mg tissue), liver biopsies (∼100 mg tissue), and serum samples (10 ml) were taken from a subset of 42 cows (14 animals each BCO2−/−, BCO2−/+, and BCO2+/+ genotypes). β-Carotene and retinol measurements were determined using HPLC with commercial standards, on the basis of a published method (Hulshof et al. 2006). Data shown are means ± SEM. Significant differences are indicated by asterisks (*P < 0.05; **P < 0.01; ANOVA, n = 14 per genotype).
While previous studies have shown a key role for β-carotene 15, 15′ monooxygenase (BCMO1) in catalyzing the symmetrical cleavage of β-carotene to vitamin A (von Lintig and Vogt 2000; von Lintig et al. 2001; Hessel et al. 2007) similar evidence for the role of the BCO2 enzyme in β-carotene metabolism is lacking. The physiological relevance of BCO2 has therefore been a topic of debate (Wolf 1995; Lakshman 2004; Wyss 2004). BCO2 mRNA and protein have been detected in several human tissues (Lindqvist et al. 2005), and the in vitro cleavage of β-carotene to vitamin A has been demonstrated (Kiefer et al. 2001; Hu et al. 2006). Our results provide in vivo evidence for BCO2-mediated conversion of β-carotene to vitamin A. BCO2−/− cows had more β-carotene in serum and milk and less vitamin A in liver, the main storage site for this vitamin.
Our results show that a simple genetic test will allow the selection of cows for milk β-carotene content. Thus, milk fat color may be increased or decreased for specific industrial applications. Market preference for milk fat color varies across the world. Further, β-carotene enriched dairy foods may assuage vitamin A deficiency. Milk may be an ideal food for delivery of β-carotene, which is fat soluble and most efficiently absorbed in the presence of a fat component (Ribaya-Mercado 2002).
In conclusion, we have discovered a naturally occurring premature stop codon in the bovine BCO2 gene strongly suggesting a key role of BCO2 in β-carotene metabolism. This discovery has industrial applications in the selection of cows producing milks with β-carotene content optimized for specific dairy products or to address a widespread dietary deficiency. More speculatively, it would be interesting to investigate possible effects of BCO2 variation in humans on the etiology of vitamin A deficiency.
Acknowledgments
Technical assistance from the Cawthron Institute was gratefully received. Farm management and sampling assistance by staff at DairyNZ and the Whareroa Fonterra farm is gratefully acknowledged. The authors are appreciative of helpful discussions with D. Garrick and H. Jacob. This research was supported by a grant from the Foundation for Science, Research and Technology (New Zealand).
References
- Baret, P., S. Knott and P. Visscher, 1998. On the use of linear regression and maximum likelihood for QTL mapping in half-sib designs. Genet. Res. 72 149–158. [DOI] [PubMed] [Google Scholar]
- Blott, S., J. Kim, S. Moisio, A. Schmidt-Küntzel, A. Cornet et al., 2003. Molecular dissection of a quantitative trait locus: a phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics 163 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, G., and R. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisart, B., W. Coppieters, F. Farnir, L. Karim, C. Ford et al., 2002. Positional candidate cloning of a QTL in dairy cattle: identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 12 222–231. [DOI] [PubMed] [Google Scholar]
- Haley, C.S., S. Knott and J. Elsen, 1994. Mapping quantitative trait loci in crosses between outbred lines using least squares. Genetics 136 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessel, S., A. Eichinger, A. Isken, J. Amengual, S. Hunzelmann et al., 2007. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282 33553–33561. [DOI] [PubMed] [Google Scholar]
- Hu, K., C. Liu, H. Ernst, N. Krinsky, R. Russell et al., 2006. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 281 19327–19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshof, P., T. van Roekel-Jansen, P. van de Bovenkamp and C. West, 2006. Variation in retinol and carotenoid content of milk and milk products in the Netherlands. J. Food Comp. Anal. 19 67–75. [Google Scholar]
- Kiefer, C, S. Hessel, J. Lampert, K. Vogt, M. Lederer et al., 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276 14110–14116. [DOI] [PubMed] [Google Scholar]
- Lakshman, M., 2004. Alpha and omega of carotenoid cleavage. J. Nutr. 134 241S–245S. [DOI] [PubMed] [Google Scholar]
- Lindqvist, A., Y. He and S. Andersson, 2005. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J. Histochem. Cytochem. 53 1403–1412. [DOI] [PubMed] [Google Scholar]
- Ribaya-Mercado, J., 2002. Influence of dietary fat on beta-carotene absorption and bioconversion into vitamin A. Nutr. Rev. 60 104–110. [DOI] [PubMed] [Google Scholar]
- Spelman, R., F. Miller, J. Hooper, M. Thielen and D. Garrick, 2001. Experimental design for QTL trial involving New Zealand Friesian and Jersey breeds. Proc. Assoc. Advmt. Anim. Breed. Genet. 14 393–396. [Google Scholar]
- Visscher, P., R. Thompson and C. Haley, 1996. Confidence intervals in QTL mapping by bootstrapping. Genetics 143 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig, J., and K. Vogt, 2000. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 275 11915–11920. [DOI] [PubMed] [Google Scholar]
- von Lintig, J., A. Dreher, C. Kiefer, M. Wernet and K. Vogt, 2001. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation in vivo. Proc. Nat. Acad. Sci. USA 98 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman, A., D. Johnson and A. MacGibbon, 1999. Estimation of heritabilities and correlations associated with milk color traits. J. Dairy Sci. 82 215–224. [DOI] [PubMed] [Google Scholar]
- Wolf, G., 1995. The enzymatic cleavage of beta-carotene: still controversial. Nutr. Rev. 53 134–137. [DOI] [PubMed] [Google Scholar]
- Wyss, A., 2004. Carotene oxygenases: a new family of double bond cleavage enzymes. J. Nutr. 134 246S–250S. [DOI] [PubMed] [Google Scholar]