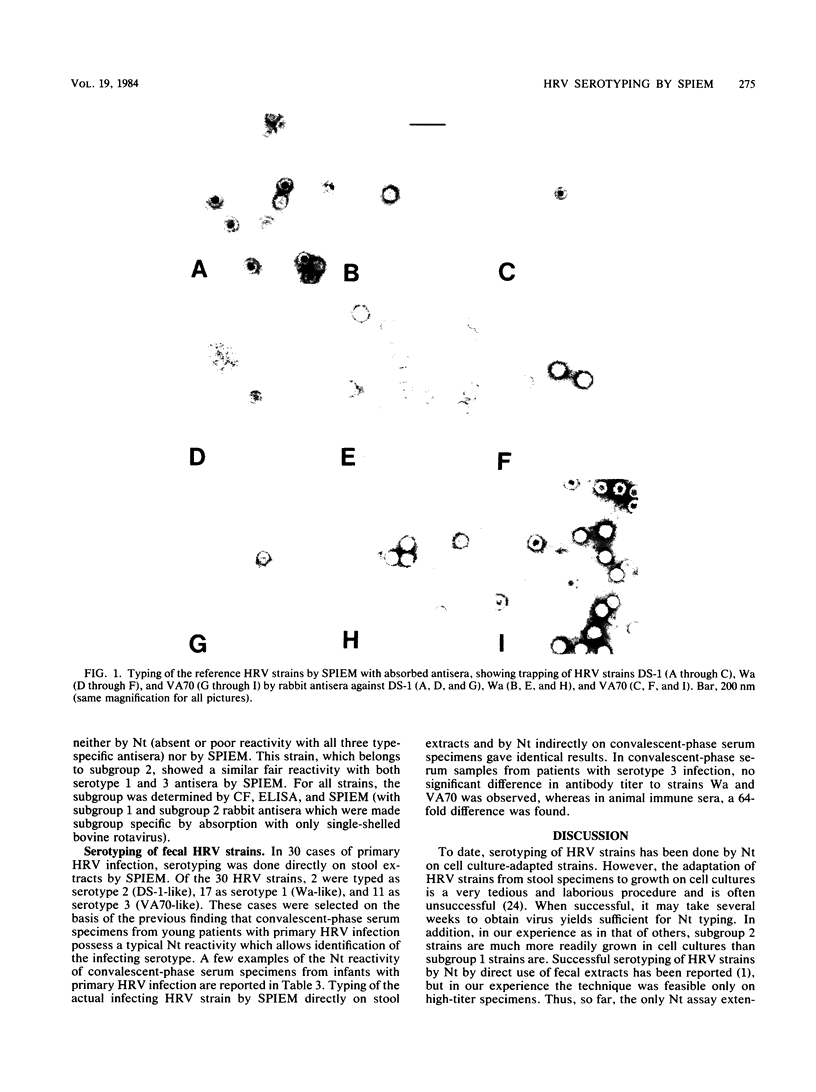
Abstract
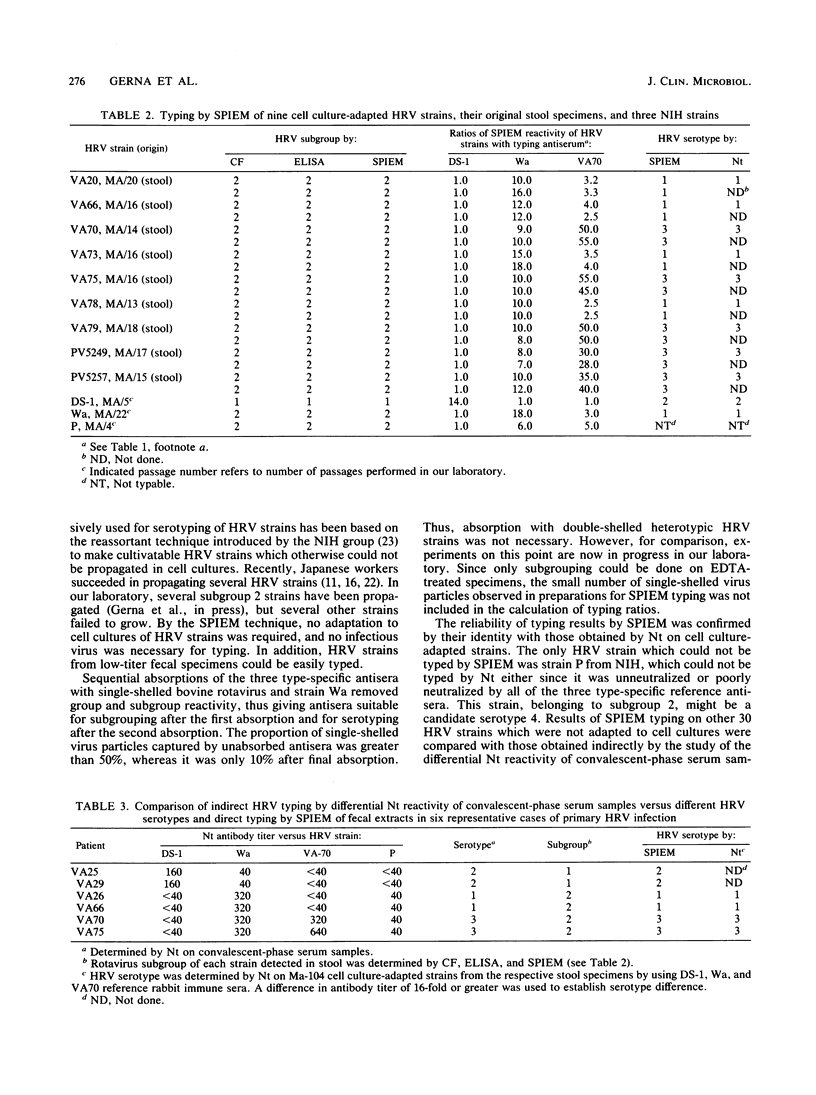
Nine cell culture-adapted, as well as 30 clinical, human rotavirus (HRV) strains from fecal extracts of children with primary HRV infection were typed by rapid solid-phase immune electron microscopy with protein A and absorbed DS-1 (HRV serotype 2), Wa (serotype 1), and VA70 (assumed serotype 3) rabbit immune sera. As a reference typing test for cell culture-adapted strains, the neutralization assay was used, whereas for noncultivatable strains typing was done for comparison, indirectly, based upon the differential neutralization reactivity of convalescent-phase serum samples from patients with primary HRV infection versus the three reference HRV serotypes. Typing results by solid-phase immune electron microscopy for all strains examined were in complete agreement with those obtained by the neutralization assay, both on cell culture-adapted strains with the three reference rabbit antisera and on three reference HRV strains with human convalescent-phase serum samples. Since adaptation to growth in cell cultures of clinical HRV strains from stool specimens is a time-consuming procedure and is often unsuccessful, solid-phase immune electron microscopy is preferred over the neutralization assay, giving results in about 16 h and also allowing typing of HRV strains from stool specimens low in virus particles. In addition, HRV strains reacting differently from the three reference serotypes may be easily selected by solid-phase immune electron microscopy for further characterization, as was the case for one strain in this study.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Cukor G. Viral gastroenteritis. N Engl J Med. 1981 Feb 12;304(7):397–406. doi: 10.1056/NEJM198102123040705. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Muñz O., Serafin F., Romero P. Shift in the prevalent human rotavirus detected by ribonucleic acid segment differences. Infect Immun. 1980 Feb;27(2):351–354. doi: 10.1128/iai.27.2.351-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Cattaneo E., Revello M. G., Battaglia M. Grouping of human adenoviruses by early antigen reactivity. J Infect Dis. 1982 May;145(5):678–682. doi: 10.1093/infdis/145.2.678. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Kapikian A. Z., Kalica A. R., Flores J., Jones R. Rescue and serotypic characterization of noncultivable human rotavirus by gene reassortment. Infect Immun. 1982 Jul;37(1):104–109. doi: 10.1128/iai.37.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Kjeldsberg E., Mortensson-Egnund K. Comparison of solid-phase immune electron microscopy, direct electron microscopy and enzyme-linked immunosorbent assay for detection of rotavirus in faecal samples. J Virol Methods. 1982 Feb;4(1):45–53. doi: 10.1016/0166-0934(82)90053-2. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T., Konno T., Suzuki H., Kapikian A. Z., Ebina T., Ishida N. Isolation of human rotavirus subgroups 1 and 2 in cell culture. J Clin Microbiol. 1982 Oct;16(4):727–730. doi: 10.1128/jcm.16.4.727-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. P., Marissens D., Marbehant P., Zissis G. Prevalence of subgroup 1, 2, and 3 rotaviruses in Belgian children suffering from acute diarrhea (1978-1981). J Med Virol. 1983;11(1):31–38. doi: 10.1002/jmv.1890110105. [DOI] [PubMed] [Google Scholar]
- Nicolaieff A., Obert G., van Regenmortel M. H. Detection of rotavirus by serological trapping on antibody-coated electron microscope grids. J Clin Microbiol. 1980 Jul;12(1):101–104. doi: 10.1128/jcm.12.1.101-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obert G., Gloeckler R., Burckard J., van Regenmortel M. H. Comparison of immunosorbent electron microscopy, enzyme immunoassay and counterimmunoelectrophoresis for detection of human rotavirus in stools. J Virol Methods. 1981 Sep;3(2):99–107. doi: 10.1016/0166-0934(81)90006-9. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Shinozaki T., Fujii R., Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69(2):155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Svensson L., von Bonsdorff C. H. Solid-phase immune electron microscopy (SPIEM) by use of protein A and its application for characterization of selected adenovirus serotypes. J Med Virol. 1982;10(4):243–253. doi: 10.1002/jmv.1890100404. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Beards G. M., Flewett T. H. Serotyping and subgrouping of rotavirus strains by the ELISA test. Arch Virol. 1982;73(3-4):219–230. doi: 10.1007/BF01318076. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H., Woode G. N., Bridger J. C., Snodgrass D. R., Herring J. A. Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization. Arch Virol. 1977;53(4):287–294. doi: 10.1007/BF01315627. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Three human rotavirus serotypes demonstrated by plaque neutralization of isolated strains. Infect Immun. 1982 Nov;38(2):781–784. doi: 10.1128/iai.38.2.781-784.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25(10):1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Zissis G., Brandt C. D., Rodriguez W. J., Kim H. W., Parrott R. H., Urrutia J. J., Mata L., Greenberg H. B. Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med. 1978 Nov 23;299(21):1156–1161. doi: 10.1056/NEJM197811232992103. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P. Different serotypes of human rotaviruses. Lancet. 1978 Jan 7;1(8054):38–39. doi: 10.1016/s0140-6736(78)90380-x. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P. Enzyme-linked immunosorbent assays adapted for serotyping of human rotavirus strains. J Clin Microbiol. 1980 Jan;11(1):1–5. doi: 10.1128/jcm.11.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P., Kapsenberg J. G., Enders G., Mutanda L. N. Human rotavirus serotypes. Lancet. 1981 Apr 25;1(8226):944–945. doi: 10.1016/s0140-6736(81)91639-1. [DOI] [PubMed] [Google Scholar]