Abstract
The mammary gland is a complex tissue comprised of a branching network of ducts embedded within an adipocyte-rich stroma. The ductal epithelium is a bi-layer of luminal and myoepithelial cells, the latter being in contact with a basement membrane. During pregnancy, tertiary branching occurs and lobuloalveolar structures, which produce milk during lactation, form in response to hormonal and cytokine signals. Postlactational regression is characterized by extensive cell death and tissue remodeling. These complex developmental events have been difficult to mimic in cell culture although many useful culture models exist. Recently, considerable advances in three-dimensional modelling of the mammary gland have been made with the use of collagen and other biomaterials for the study of branching morphogenesis and tumorigenesis, techniques which may enable rapid advances in our understanding of both basic biology and the study of cancer therapeutics.
Key words: mammary gland, models, extracellular matrix, cell culture, cell-lines, scaffolds, tissue engineering, epithelium, adipocytes
Introduction
Three-dimensional (3D) tissue models promise to overcome many of the shortcomings associated with both in vivo animal experimentation and two-dimensional (2D) tissue culture. Culturing cells with the correct 3D relationship to each other maintains tissue function by establishing appropriate cell-signaling pathways and extracellular matrix interactions. Indeed, several studies have noted distinct patterns in gene expression profiles between cell lines and tissue samples of varying phenotypes including breast tissue1–3 demonstrating the genetic adaptations that can take place over a long period of 2D culture. On the other hand, in vivo transgenic animal models, while undoubtedly representing the gold standard for basic molecular studies and diagnostic work, are expensive to produce and are subject to stringent regulation often resulting in studies utilizing low replicate numbers, with consequences for scientific rigor. There is therefore a significant requirement for permissive 3D models that could contribute to understanding both normal tissue function and changes that occur in disease, particularly cancer. The biochemical and mechanical properties of the surrounding stroma affect processes such as the invasion of neoplastic cells into surrounding tissue and metastasis, or the heightened angiogenic properties in the tumor microenvironment.2 The ultimate aim of such tissue culture models is to utilize human cells and in so doing improve currently available drug discovery platforms or offer autologous tissue replacement therapies.
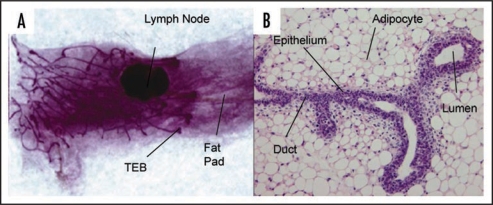
The mammary gland is a complex tissue of many differing cell types. In the most part it is comprised of two epithelial cell subtypes, inner luminal epithelia and outer myoepithelia that together form milk producing acini linked to a branching ductal structure which terminates at the nipple. These structures are invested within a network of adipocytes forming a supporting fatty stroma known as a fat pad (Fig. 1A). The mammary gland holds a unique place in mammalian organ development, in that the last stage of this development occurs in the adult during pregnancy. Following pregnancy it undergoes a dramatic remodeling phase, known as involution, where up to 80% of the total epithelial tissue mass is destroyed by programmed cell death, conserving the epithelial ductal structure within a replenishing adipose stroma. With successive pregnancies the gland will cycle through epithelial proliferation, differentiation, secretion and involution. The unique development and complexity in the regulation of this structure, together with the prevalence of mammary gland neoplasia4 makes it an ideal target for development of 3D culture models.
Figure 1.
Structure of the mouse mammary gland. (A) Carmine alum stained whole mount of mammary gland from 6 week virgin mouse showing branched ductal structure embedded in a fat pad. The growing ends of the ducts terminate in terminal end buds (×3). (B) H&E stained section of paraffin embedded tissue showing a bifurcated TEB (×60).
Advances in biomaterials science and tissue engineering have equipped the biologist with novel scaffolds and techniques to enable highly controlled 3D cell culture in contrast to the heterogeneous environment found in vivo. Several considerations are pertinent to 3D model development. Ultimately the overriding intention of a good model must be to replicate the behavior of an in vivo system as simply as possible, in a reproducible manner. This review with focus on some of the novel experimental approaches taken to date to understand the functions of the mammary gland in health and disease.
Mammary Gland Developmental Stages
The development of the mammary gland can be considered as a three stage process that begins during development of the embryo and continues during puberty to be completed with pregnancy and lactation. In the embryo, mammary glands develop along specialized regions of ectoderm localized to two ventrally located ridges, known as milk lines which themselves overlie specialized regions of mesoderm. Although a detailed analysis of the signaling events that control how these structures arise is beyond the scope of this review, it is largely controlled by the initiation of Wnt signaling pathways in the ectoderm, initiated by the paracrine release of various fibroblastic growth factors (FGFs) and parathyroid hormone related protein (PTHrP).3,5 Ectodermal cells migrate along each milk line6 and coalesce to form thickened regions known as placodes. This structure will later penetrate into the mesenchyme to form a bud and finally a rudimentary branched gland known as a mammary sprout, through coordinated signaling pathways originating from both epidermal and mesenchymal compartments.7 Within the sprout a lumen forms via a process of cavitation,8 in which epithelial cells die via apoptotic mechanisms that could in part be regulated by loss of extracellular matrix contact.9,10 Such processes are complex however and at least two apoptotic mechanisms, one involving classical caspase activation and cell death, and the other not, are known to operate independently.11 The sprout communicates externally via a region of specialized epithelium called the nipple sheath.
From birth the female rudimentary gland continues to grow at a rate proportional to that of the rest of the body.7 The second stage of mammary gland development begins at around 3 weeks of age when estrogen levels begin to rise and club-like thickenings up to 10 cell layers deep, known as terminal end buds (TEBs), appear. Unlike the embryonic stage which is governed solely by locally released mediators, an increase in circulating estrogen triggers TEB cell proliferation resulting in ductal elongation and branching. The structural form of TEBs can be gathered from Figure 1B. The patterning and growth of this structure is multifaceted in scope and involves sensitization of the mammary stroma by both growth factors and estrogen and ensuing paracrine signaling between mesenchymal and epithelial cells. What follows involves coordinated cell proliferation, accommodation of the growing structure via enzymatic digestion of fat pad components, and maintenance of the differentiated state via appropriate cell-matrix interactions (reviewed in ref. 12). At the onset of pregnancy, the dramatic rise in progesterone triggers tertiary branching and the formation of lobuloalveolar structures where milk is produced during lactation. Recapitulating a proportion of these processes will be required if a useful 3D model of the mammary gland is to be established.
Cell-Cell and Cell-Matrix Interactions Regulate Mammary Gland Physiology
Mammary gland epithelium is enveloped by a continuous layer of basement membrane, principally composed of a lattice-type network of proteins including collagen type IV, laminin, nidogen and heparin sulphate proteoglycan.13,14 This extracellular matrix (ECM) provides the correct biochemical surface to maintain epithelial polarity, allowing secretion and ultimately ejection of milk from the cells lining the luminal cavity. It has long been known that the expression of milk protein genes such as β-casein, β-lactoglobulin and whey acidic protein are dependent on cell interactions with basement membrane,15,16 more specifically integrin ligands presented to the laminin component of ECM.17 Milk protein expression is triggered by prolactin stimulation acting through the JAK1/STAT5 signaling pathway.18 However, it has been shown that treatment with prolactin in isolation is insufficient to induce STAT5 signaling and β-casein expression in primary mammary epithelial cell cultures19 and cell lines.20 Indeed work has highlighted matrix-dependent transcriptional regulatory elements in the promoter sequence for β-casein using chloramphenicol acetyltransferase (CAT) fusion reporter genes.17,19
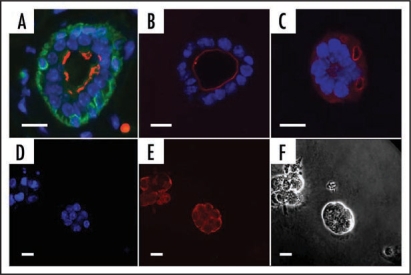
Establishing the correct polarity of the epithelial tissues is critical for tissue function and is a clear requirement of in vitro mammary 3D models, even though it has been demonstrated that isolated epithelial cells in basement membrane can express milk proteins in isolation.17 In a well established procedure, basement membrane preparations containing laminin type 1 impart the correct apical-basal cell polarity to spheroid arrangements of primary luminal epithelial cells in contrast to collagen type I gels which do not21 (See Fig. 2A–F). Interestingly, the resulting “inside-out” epithelial polarity typical of culture within collagen gels can be reversed by the addition of normal, but not neoplastic myoepithelial cells.21 Normal myoepithelial and luminal epithelial cells deposit basement membrane, particularly the α3 and α5 chains of laminin 1, but only myoepithelial cells deposit α1 laminin chains.22 One characteristic of tumor histology is loss of epithelial polarity23 and a reduction of, or complete absence of basement membrane.24,25 Early investigations suggested that this feature was due to altered gene expression for basement membrane proteins rather than any heightened enzymatic matrix breakdown.26 This is further supported by evidence that breast carcinoma cells exhibit a downregulation in the laminin-binding integrin subunits.27,28
Figure 2.
Formation of acini in different matrices. (A–C) Mammary epithelial cells (EpH4) adopt the correct polarity in Matrigel™ but not in collagen gel preparations. (A) Tissue section from virgin mammary gland showing bi-layered epithelium. (B) EpH4 cells in Matrigel. (C) EpH4 cells in Collagen gel. Antibody staining: Green, cytokeratin14, a marker of myoepithelial cells; red, aquaporin 5, a marker of polarized cells that is expressed on the luminal face; blue, DAPI (nucleus—DNA). (D–F) KIM-2 cells form acini when cultured in Matrigel. (D) Nuclear staining (DAPI); (E) E-Cadherin (red) showing cell adhesions. (F) phase contrast. Scale bars = 10 µm.
As well as biochemical signaling from the extracellular matrix, cell-cell signaling mechanisms coordinate to maintain epithelial polarity and differentiation. Gap-junction connexin expression was found to be localized to regions of cell membrane when CID-9 cells were grown on EHS-matrix in contrast to a plastic substrate where it remained cytosolic. Furthermore β-casein expression was dependent on gap-junction mediated inter-cellular signaling and was absent in the presence of functional blocking antibodies of β1 integrins, and inhibitors of cAMP.29 Disrupting Rac1, a GTPase involved in cortical actin remodeling, resulted in the disorganization of laminin deposition by a kidney epithelial cell line (MDCK) and a resultant loss of polarity.30
As well as maintaining tissue architecture, a recent study has highlighted the role of luminal and myoepithelial cell interactions in driving mammary gland branching morphogenesis by observing mammary gland organoids cultured within basement membrane gels in vitro.31 Luminal and myoepitheilial cells were found to be in a constant state of flux, a phenomenon also observed in developing epithelia of the submandibular gland,32 with new ductal growth emanating from the medially located luminal epithelial cell compartment penetrating through gaps in the myoepithelial cell envelope. Ductal elongation was halted when myoepithelial cells restored full coverage or ducts bifurcated with partial coverage. The tips of these ducts, observed in vitro, were characterized by a multi-layered luminal epithelium similar to that of the TEBs that typify the developing gland. What is interesting is that elongating cells retained intimate E-cadherin and β-catenin associations between neighboring cells, while disruption of Rac-1 and Rho-kinase, so far implicated in the maintenance of epithelial polarity in a range of tissues,33,34 selectively reduced and increased the extent of branching respectively. Such studies highlight the important role of the cytoskeleton, acting as a mediator between cell-cell and cell-matrix adhesion complexes and ultimately signaling to the nucleus, modulating gene expression and interacting with cytosolic signaling elements.35,36
The surrounding stroma, which supports the glandular epithelium is more than just a passive supporting structure. It contains many cell types including fibroblasts, adipocytes and inflammatory cells that can influence the epithelium by releasing growth factors and cytokines or directly modulating the extracellular matrix in which the cells reside.37 The gross influence of the stroma was highlighted in early heterotypic recombination experiments where microdissected mammary epithelia were recombined and cultured within salivary gland stroma.15 The resulting epithelial tissue morphology closely resembled dichotomous branching patterns typical of salivary gland. Similar work recombining highly branched mammary epithelia with the epithelium-divested mammary fat pads of sparsely branched glands resulted in a sparsely branched morphology.38
Mammary gland adipose tissue (see Fig. 1B) has historically been seen as a relatively inert energy storage network, however, in the last decade many research groups have highlighted important endocrine and paracrine signaling factors released from adipocytes that can modulate tissue metabolism and homeostasis in addition to extracellular matrix through deposition of proteins and release of MMPs.39,40 Further, these adipokines can influence both the development of the branching network, but also tumor invasion through increased motility, proliferation and adipogenesis.41 Interestingly the bioactive potential of adipocytes is dependent on cell maturation. Like fibroblasts,37 mature adipocytes were found to increase the proliferation in several breast cancer epithelial cell lines although preadipocytes did not show this ability, instead demonstrating a clear upregulation of E-cadherin expression to the mature phenotype.42 In a normal tissue in vitro model, preadipocytes cultured in basement membrane gels enhanced mammary epithelial organoid casein accumulation and luminal area.43 These researchers again reported the absence of any proliferative effect of preadipocytes on the epithelial cell component. Unfortunately a comparison with mature adipocytes could not be made, the authors citing the technical difficulty in establishing mature adipocyte cultures in vitro.
Although a wide range of established mammary cell lines have proven to be invaluable research tools, the behavior of cell lines is not consistent and care should be taken when interpreting results between systems. Unlike EPH4 cells, MDCK cells and NMuMG cells do not require laminin 1 to induce polarity.44,45 Furthermore, EPH4 cells grown in 2D culture do not demonstrate a synergistic differentiation response with the addition of prolactin and ECM proteins, reportedly due to the basolateral location of the prolactin receptor on the polarized 2D cultures.20 Our laboratory has established a conditionally immortal mouse mammary epithelial cell line (KIM-2) from the mammary glands of transgenic mice expressing a temperature sensitive SV40 largeT antigen.68 These cells are bi-potential producing both luminal and myoepithelial progeny in a ratio of approximately 95:5. These cells can be induced to differentiate and express milk proteins. Furthermore, they undergo apotosis when the lactogenic hormone cocktail, that was used to induce differentiation, is removed. KIM-2 cells synthesize and deposit laminin in response to lactogenic hormones and this results in the formation of 3D dome-like structures. KIM-2s also form acini when cultured in Matrigel (Fig. 2D–F). Despite the utility of cell lines, there is a pressing need for better models of mammary gland development that recapitulate branching morphogenesis, lobuloalveolar development and stromal interactions. This is important for both studies of mammary gland biology and breast cancer.
Constructing a 3D Model
Several in vitro culture strategies have so far been developed for studying many of the mechanisms of mammary gland biology thus far discussed. Many adopt a simple deconstructed approach of whole organ culture, explant culture or enzymatic tissue digestions that comprise functional collections of cells known as organoids. These can be recombined with ECM preparations in order to support cells in 3D and maintain the phenotype of the tissue for the duration of the experimental procedure. The choice of this suspension material has centered on the use of broad milieu of basement membrane proteins from the Engelbreth-Holm-Swarm (EHS) tumor or its commercial derivative Matrigel™ or even more complex mixtures of stromal and basement membrane proteins such as Humatrix®.46 Although these have provided the basis of many valuable studies and will continue to do in the future, they possess several disadvantages for use as a biomaterial, including expense and difficulty of isolation, poor definition and batch-to-batch variation, tumor origin and poor mechanical properties.47 Furthermore many of the physiological processes that can be recapitulated within them, such as the establishment of epithelial polarity and the functionally differentiated state do not last beyond several weeks in culture.48,49
The use of purified natural protein preparations for cell culture can provide a defined substrate environment and resultant cellular response. For example, it has been shown that various types of collagen, fibronectin and laminin act in synergy with both epidermal growth factor (EGF) and insulin-like growth factor (IGF) to produce a mitogenic response in mammary epithelial cells.50 Collagen type I gels are widely used in 3D models and are suitable biomedical materials, receiving commercial approval for therapeutic use.51 Culture within or on top of collagen can induce divergent cellular responses compared to basement membrane gels or tissue culture plastic (Fig. 2A–C). MCF-10A normal mammary epithelial cells form ductal-like structures within collagen gels and in Matrigel, alveolar-like structures, while attempts to produce an even mixture of phenotypes by mixing both substrate materials have met with only limited success.49 Epithelial cells express contrasting levels of ECM proteins between 2D plastic culture and collagen gel, with the former favoring the deposition of both collagen type IV and laminin,52 although an earlier study found that glycoproteins synthesized on plastic were largely expelled into culture media and not incorporated within a developing basement membrane.16
A noted feature of seeding cells within 3D hydrated collagen gels is compaction caused by cells exerting traction on the fibril network.53 Although this has been exploited to develop tissue equivalents with high unidirectional mechanical properties, such as tendon,54 the resulting environment would almost certainly be heterogeneous with regard to its mechanical and diffusion properties. However, floating collagen gels do preserve the phenotype of mammary epithelial cells through expression of a range of casein genes55 and milk proteins,52 features expressed at lower levels when cells are cultured within gels attached to a plastic substrate.55 Such differences may be accounted for by the different stress environments within such systems, with attached gels broadly exerting stress in two dimensions.56
The mechanical environment has been shown to be an important regulator of tissue homeostasis, both through direct external load application53,54 and the stiffness of the extracellular matrix per se.55–57 Cells cooperate with local ECM through cell-matrix-cell feedback mechanisms,57 particularly modulating actin cytoskeleton focal adhesion complexes and Rho/ERK signaling.57 For example, low substratum rigidity as been shown to trigger apoptosis in epithelial cells.58 Artificial scaffold systems are ideal tools in which to study such principles in that they can be tailored to match the mechanical conditions encountered by tissues in vivo. This is an exciting area of research considering that the tumor microenvironment is known to be stiffer than normal tissue59 and stem cells have been shown to direct their differentiation pathways depending on the nature of the mechanical environment.60
While careful selection of a basement membrane in clearly critical to maintaining epithelial cell function, the mammary gland is also defined by an extensive fad-pad comprised mainly of a fatty stroma. A successful mammary gland culture would ideally benefit from a coculture system of at least two, but possibly more cell types presented together in a controlled manner. Although 2D transwell membranes remain useful tools, mainly for the ease in which contributing cell populations can be combined and later separated for analysis, the intimate association of epithelia with mesenchyme may have important implications for mammary gland modeling. In an embryonic model, laminin α-1 chain expression within both pulmonary epithelial and mesenchymal cells was found to be dependent on heterotypic cell contact.61 It is tempting to envisage such interactions occurring in the developing mammary gland, particularly considering some of the known mechanisms of branching morphogenesis.31
The development of a fat-pad, a volume of adipose in which epithelial sheets can form tubular structures has already been much advanced through adipose tissue engineering.62–64 To date the thrust of research has centered around the challenges of providing a suitable scaffold that supports proliferation and differentiation, minimizing the loss in mass or volume with culture time, a noted affect of in vivo implantation and adequate 3D seeding and stimulation with exogenous adipogenic factors. While collagen has numerous advantages as a biomaterial, it demonstrates significant mass loss when implanted in vivo. For example, freeze-dried collagen sponges loaded with preadipocytes lost approximately half their wetted mass by as little as 3 weeks of culture.65 The use of composite scaffolds may remedy some of these problems for example, the addition of the mucopolysaccharide hyaluronan to collagen scaffolds improves scaffold integrity by reducing mass loss (unpublished findings from our group). It is also noted to harbor angiogenic properties,66 suitable for supporting the highly vascular nature of adipose67 and is chemically modifiable.68,69
The utilization of mesenchymal progenitors and preadipocyte cell lines in place of mature adipocytes may prove advantageous. Mesenchymal progenitors can differentiate into a wide variety of stromal tissue phenotypes including adipocytes and fibroblasts.70 Preadipocytes exhibit a higher mitotic capacity over adipocytes71 and are easily manipulated in culture without physical rupture. They can also penetrate the confined spaces of pre-fabricated scaffolds due to their smaller physical dimensions and have a lower requirement for oxygen.67 Their effect on epithelial cells is contrasting however. As previously discussed mature adipocytes have a greater proliferative effect on epithelial cell cultures, but preadipocytes may increase tissue organization through greater expression of E-cadherin. EGF, an important component of media that supports mammary epithelial differentiation and proliferation72 enhances triglyceride synthesis in differentiated adipocytes but inhibits its synthesis in preadipocytes.73
Successful fat-pad generation utilizing progenitors should accommodate not only diffuse cell-seeding in 3D, but also generalized delivery of adipogenic stimuli. In practice such homogeneity is difficult to achieve in static cell culture74 although employing dynamic culture systems to improve mass transfer and ultimately the quality and extent of de novo tissue formation may enhance results. As well as the physical attributes of the culture system careful attention must be made to media delivery in coculture systems. Recent work has suggested that 3T3-L1 preadipocytes are sensitive, in a temporal manner to the addition of glucocorticoids. Adipogenesis is commonly initiated by the addition of fetal calf serum (FCS), dexamethasone (Dex) and methylisobutylxanthine (IBMX).71,72 Complete adipogenesis was reported where cells were primed by exposure to Dex 4 days prior to IBMX exposure while much lower rates of adipogenesis were reported with the reciprocal addition of these supplements.75
Numerous natural and synthetic polymeric materials have been investigated as alternatives to ECM protein aggregates in 3D tissue models, which vary in their suitability for application within mammary gland models. Certain materials may be unsuitable for cell encapsulation studies, where cells are dispersed in a material in the liquid phase before solidification, due to solvent toxicity or the necessity for extreme physical conditions to sustain the fluid phase or during polymerization, for example during photo-crosslinking of polyethylene glycol based hydrogels.76 An ideal biomaterial would support both epithelial and stromal cell function and could either be modified to present appropriate ligands so as to mimic extracellular matrix function, or provide a surface that readily allowed the deposition of newly synthesize extracellular matrix by the seeded cells. The arginine-glycine-aspartate peptide sequence (RGD) present on numerous ECM proteins including fibronectin and laminin is an integrin binding motif that has been conjugated to several materials where it is reported to increase cell survival.74,75 In a similar manner peptide sequences specific to laminin have been conjugated to polysaccharide substrates and been shown to support keratinocytes.77,78 However, the addition of the laminin α1 chain peptide sequence AG73 to culture media inhibited laminin mediated submandibular acini development79 and salivary epithelial branching,80 a finding that illustrates the importance of the correct presentation of stimuli in 3D model systems.
A scaffold material that can accommodate gland development should be susceptible to enzymatic breakdown or exhibit degradation properties that can accommodate branching epithelial structures. In the developing mammary gland matrix metalloproteinases (MMPs) are important mediators of branching12 and additionally have a putative role in regulating adipogenesis.81 It is possible that scaffold degradation properties could be optimized to drive tissue formation via drug release.82 Composite scaffolds containing growth factor reservoirs may provide a more controlled stimulus than exogenously applied agents. Recently gelatin microspheres loaded with growth factor have been positioned within collagen matrices to improve adipogenic response and vascularity within in vivo implanted scaffolds,83,84 and fibroblastic growth factor soaked beads have be used to study branching mechanisms in kidney epithelia.85 However, careful optimization of dose and release kinetics for endogenously active agents will no doubt be required if these strategies are to be effective.
The control of internal scaffold architecture through novel fabrication techniques could produce materials optimized for specific tissue phenotypes and controlled development. Soft lithography techniques using elastomeric stamps provide a means by which micro-scaled features can be moulded within soft biomaterial substrates. Such techniques have been used for a number of applications including microwell generation in Matrigel to allow the more controlled study of single cells or tissue structures such as acini with high replicate numbers86 or templates in which to seed two cell components. A recent study by Gillete et al.87 demonstrated the versatility of this technique by stamping various combinations of Matrigel, collagen and alginate gels seeded with different cell types. In this way spatially controlled arrangements of more than one cell type could be developed. In a separate study investigating the control of branching morphogenesis in mammary epithelium, EPH4 cells were cultured within elongated cavities of defined geometry in collagen gels.88 It was found that initiators of branching, in this case markers of epithelial-mesenchymal transition, occurred preferentially at the ends of these cavities and not throughout their long axes. Furthermore if cells were cultured in crescent-shaped cavities, branching cues were expressed to a greater extent on the convex as opposed to the concave surfaces. The authors proposed that such observations were the result gradients in locally secreted inhibitors, confining branching to the most distal tubule positions.
Conclusions
Many challenges remain to be overcome if mammary gland models are to advance significantly beyond the use of natural ECM matrices by many research groups, particularly those working within the fields of developmental biology and pathology and not exposed to the interdisciplinary fields of biomedical materials and tissue engineering. However, recent advances have no doubt presented researchers with novel alternatives that have furthered our understanding of how tissue structures arise in three dimensions. Ultimately a meaningful 3D model system must be a compromise between supplying enough biochemical and mechanical properties to support diverse tissue processes, but also being simple enough to satisfy the constraints of diagnostic procedure, repeatability and quality control. The mammary gland is a unique organ that adapts dramatically during pregnancy undergoing significant stage specific changes in cell frequency, differentiation status and tissue morphology. Perhaps ultimately such models could be tailored to provide environments typical of the virginal, gestational and involuting gland, so enhancing our understanding of disease processes.
Acknowledgements
This work is supported by the Biological and Biotechnology Research Council and the NC3Rs.
Abbreviations
- 2D
two dimensional
- 3D
three dimensional
- FGF
fibroblastic growth factor
- PTHrP
parathyroid hormone related protein
- TEB
terminal end bud
- ECM
extracellular matrix
- CAT
chloramphenicol acetyltransferase
- STAT
signal transducers and activators of transcription
- JAK
Janus associated kinase
- MMP
matrix metalloproteinase
- EHS
Engelbreh-Holm-Swarm
- IGF
insulin-like growth factor
- FCS
fetal calf serum
- IBMX
methylisobutylxanthine
- LIF
leukemia inhibitory factor
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/8321
References
- 1.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 3.Brennan KR, Brown AMC. Wnt Proteins in Mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 4.Trichopoulos D, Adami HO, Ekbom A. Early life events and conditions and breast cancer risk: From epidemiology to etiology. Int J Cancer. 2008;122:481–485. doi: 10.1002/ijc.23303. [DOI] [PubMed] [Google Scholar]
- 5.Robinson GW. Cooperation of signalling pathways in embryonic mammary gland development. Nat Rev Genet. 2007;8:963–972. doi: 10.1038/nrg2227. [DOI] [PubMed] [Google Scholar]
- 6.Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn. 2004;229:349–356. doi: 10.1002/dvdy.10441. [DOI] [PubMed] [Google Scholar]
- 7.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 8.Coucouvanis E, Martin GR. Signals for death and survival: A two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 9.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protine Bim to prevent anoikis. Mol Cell Biol. 2003;25:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 11.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternlicht M, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H. Models for the self-assembly of basement membrane. J Histochem Cytochem. 1986;34:93–102. doi: 10.1177/34.1.3510247. [DOI] [PubMed] [Google Scholar]
- 14.Aeschlimann D, Paulsson M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilzation. J Biol Chem. 1991;266:15308–15317. [PubMed] [Google Scholar]
- 15.Sakakura T, Nishizuka Y, Dawe CD. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:891–893. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 16.Parry G, Lee EY-H, Farson D, Koval M, Bissel MJ. Collagenous substrata relulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Exp Cell Res. 1985;156:487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- 17.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson CJ, Burdon TG. Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway. Rev Reprod. 1996;1:1–5. doi: 10.1530/ror.0.0010001. [DOI] [PubMed] [Google Scholar]
- 19.Streuli CH, Edwards GM, Delcommenne M, Whitelaw BC, Burdon TG, Schindler C, et al. Stat5 as a Target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- 20.Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol. 2009;184:57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane depostion. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slade MJ, Coope RC, Gomm JJ, Coombes RC. The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp Cell Res. 1999;247:267–278. doi: 10.1006/excr.1998.4340. [DOI] [PubMed] [Google Scholar]
- 23.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 24.Kodama J, Shinyo Y, Kusumoto T, Seki N, Nakamura K, Hongo A, et al. Loss of basement membrane heparan sulfate expression is associated with pelvic lymph node metastasis in invasive cervical cancer. Oncol Rep. 2005;14:89–92. [PubMed] [Google Scholar]
- 25.Tosios KI, Kapranos N, Papanicolaou SI. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology. 1998;33:261–268. doi: 10.1046/j.1365-2559.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 26.Dulbecco R, Armstong B, Allen R. Reversion toward an earlier stage of differentiation and loss of polarity during progression of N-methyl-N-nitrosourea-induced rat mammary tumors. Proc Natl Acad Sci USA. 1988;85:9292–9296. doi: 10.1073/pnas.85.23.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koukoulis G, Virtanen I, Korhonen M, Laitinen L, Quaranta V, Gould V. Immunohistochemical localization of integrins in the normal, hyperplastic and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991;4:787–799. [PMC free article] [PubMed] [Google Scholar]
- 28.Pignatelli M, Hanby AM, Stamp GW. Low expression of beta1, alpha2 and alpha3 subunits of VLA integrins in malignant mammary tumors. J Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- 29.El-Sabban ME, Sfeir AJ, Daher MH, Kalaany NY, Bassam RA, Talhouk RS. ECM-induced gap junctional communication enhances mammary epithelial cell differentiation. J Cell Sci. 2003;116:3531–3541. doi: 10.1242/jcs.00656. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 31.Ewald A, Brenot A, Duong M, Chan B, Werb Z. Collective Epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Meyer T, Schwesinger C, Sampogna RV, Vaughn D, Stuart R, Steer D, et al. Rho kinase acts at separate steps in uretic bud and metanephic mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638–647. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 34.Vazei A, Bauer C, Vasioukhin V, Fuchs E. Actin dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 35.Ingber D, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 36.Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70:547–560. doi: 10.1046/j.1432-0436.2002.700908.x. [DOI] [PubMed] [Google Scholar]
- 37.Haslam S, Woodward T. Haslam 2003 Epithelial cell stromal interactions and ECM. Breast Cancer Res. 2003;5:208. doi: 10.1186/bcr615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naylor MJ, Ormandy CJ. Mouse strain-specific patterns of mammary epithelial ductal side branching are elicited by stromal factors. Dev Dyn. 2002;225:100–105. doi: 10.1002/dvdy.10133. [DOI] [PubMed] [Google Scholar]
- 39.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Smas CM. Wdnm1-like, a new adipokine with a role in MMP-2 activation. Am J Physiol Endocrinol Metab. 2008;295:205–215. doi: 10.1152/ajpendo.90316.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyengar P, Combs T, Shah S, Gouon-Evans V, Pollard J, Albanese C, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 42.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 43.Zangani D, Darcy KM, Shoemaker S, Ip MM. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247:399–409. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]
- 44.Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like stuctures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- 45.Ecay TW, Valentich JD. Basal lamina formation by epithelial cell lines correlates with laminin A chain synthesis and secretion. Exp Cell Res. 1992;203:32–38. doi: 10.1016/0014-4827(92)90036-8. [DOI] [PubMed] [Google Scholar]
- 46.Kedeshiana P, Sternlichta MD, Nguyen M, Shaoa Z-M, Barskya SH. Humatrix, a novel myoepithelial matrical gel with unique biochemical and biological properties. Cancer Lett. 1998:215–226. doi: 10.1016/s0304-3835(97)00429-1. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T. Biodegradable polymers for biomedical uses. Prog Polym Sci. 1994;19:663–702. [Google Scholar]
- 48.Ma YL, Fujiyama C, Masaki Z, Sugihara H. Reconstruction of prostatic acinus-like structure from ventral and dorso-lateral prostatic epithelial cells of the rat in three-dimensional collagen gel matrix culture. J Urol. 1997:157. doi: 10.1016/s0022-5347(01)65135-8. [DOI] [PubMed] [Google Scholar]
- 49.Krause S, Maffini MV, Soto AM, Sonnenschein C. A Novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng Part C Methods. 2008;14:261–271. doi: 10.1089/ten.tec.2008.0030. [DOI] [PubMed] [Google Scholar]
- 50.Woodward TL, Xie J, Fendrick JL, Haslam SZ. Proliferation of mouse mammary epithelial cells in vitro: interactions among epidermal growth factor, insulin-like growth factor i, ovarian hormones, and extracellular matrix proteins. Endocrinology. 2000;141:3578–3586. doi: 10.1210/endo.141.10.7701. [DOI] [PubMed] [Google Scholar]
- 51.Falanga V, Sabolinski ML. A bilayered living skin construct (Apligraf®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7:201–207. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 52.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentleman E, Nauman EA, Livesay GA, Dee KC. Collagen composite biomaterials resist contraction while allowing development of adipocytic soft tissue in vitro. Tissue Eng. 2006;12:1639–1649. doi: 10.1089/ten.2006.12.1639. [DOI] [PubMed] [Google Scholar]
- 54.Shi Y, Vesely I. Fabrication of mitral valve chordae by directed collagen gel shrinkage. Tissue Eng. 2003;9:1233–1242. doi: 10.1089/10763270360728143. [DOI] [PubMed] [Google Scholar]
- 55.Eva YH, Parry G, Bissell M. Modification of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howard JC, Varallo VM, Ross DC, Roth JH, Faber KJ, Alman B, et al. Elevated levels of beta-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren's disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16. doi: 10.1186/1471-2474-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paszek M, Zahir N, Johnson K, Lakins J, Rozenberg G, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Chiu W, Wang Y, Wu C, Chen T, Teng C, et al. Deregulation of AP-1 proteins in collagen gel-induced epithelial cell apoptosis mediated by low substratum rigidity. J Biol Chem. 2006;282:752–763. doi: 10.1074/jbc.M604801200. [DOI] [PubMed] [Google Scholar]
- 59.Paszek M, Weaver V. The Tension Mounts: Mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 60.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Schuger L, Skubitz AP, Zhang J, Sorokin L, He L. Laminin alpha1 chain synthesis in the mouse developing lung: requirement for epithelial-mesenchymal contact and possible role in bronchial smooth muscle development. J Cell Biol. 1997;139:553–562. doi: 10.1083/jcb.139.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemmrich K, Van de Sijpe K, Rhodes NP, Hunt JA, Di Bartolo C, Pallua N, et al. Autologous in vivo adipose tissue engineering in hyaluronan-based gels—a pilot study. J Surg Res. 2008;144:82–88. doi: 10.1016/j.jss.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Hemmrich K, von Heimburg D, Cierpka K, Haydarlioglu S, Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73:28–35. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
- 64.Vashi A, Keramidaris E, Abberton K, Morrison W, Wilson J, O'Connor A, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2007:7. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 65.von Heimburg D, Zachariah S, Heschel I, Kühling H, Schoof H, Hafemann B, et al. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429–438. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 66.Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing—Assessment of clinical evidence. Am J Clin Dermatol. 2005;6:392–402. doi: 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- 67.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 68.Prestwich GD, Marecak DM, Marecek JF. Controlled chemical modification of hyaluronic acid: synthesis, applications and biodegradation of hydrazide derivatives. J Control Release. 1998;53:93–103. doi: 10.1016/s0168-3659(97)00242-3. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Kim S, Lee Y. Preparation of porous collagen/hyaluronic acid hybrid scaffolds for biomimetic functionalization through biochemical binding affinity. J Biomed Mater Res. 2007;82:506–518. doi: 10.1002/jbm.b.30756. [DOI] [PubMed] [Google Scholar]
- 70.Caplan AI. Mesenchymal stem-cells. J Ortho Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 71.Ailhaud G, Grimaldi P, Négrel R. Cellular and molecular aspects of adipose tissue development. Ann Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 72.Gordon KE, Binas B, Chapman RS, Kurian KM, Clarkson RW, Clark AJ, et al. A novel cell culture model for studying differentiation and apoptosis in the mouse mammary gland. Breast Cancer Res. 2000;2:222–235. doi: 10.1186/bcr57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adachi H, Kurachi H, Homma H, Adachi K, Imai T, Morishige K, et al. Epidermal growth factor promotes adipogenesis of 3T3-L1 cell in vitro. Endocrinology. 1994;135:1824–1830. doi: 10.1210/endo.135.5.7956906. [DOI] [PubMed] [Google Scholar]
- 74.Daya S, Loughlin A, Macqueen H. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation. 2007;75:360–370. doi: 10.1111/j.1432-0436.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 75.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell. 2008;19:4032–4041. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled β-cell microenvironments. Acta Biomaterialia. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Matsuda A, Kobayashi H, Itoh S, Kataoka K, Tanaka J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials. 2005;26:2273–2279. doi: 10.1016/j.biomaterials.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 78.Ikemoto S, Mochizuki M, Yamada M, Takeda A, Uchinuma E, Yamashina S, et al. Laminin peptide-conjugated chitosan membrane: Application for keratinocyte delivery in wounded skin. J Biomed Mater Res. 2006;79:716–722. doi: 10.1002/jbm.a.30804. [DOI] [PubMed] [Google Scholar]
- 79.Hoffman MP, Nomizu M, Roque E, Amichay N, Lee S, Yamada Y, et al. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J Biol Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- 80.Hosokawa Y, Takahashi Y, Kadoya Y, Yamashina S. Significant role of laminin-1 in branching morphogenesis of mouse salivary epithelium cultured in basement membrane matrix. Develop Growth Differ. 1999;41:207–216. doi: 10.1046/j.1440-169x.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- 81.Chun T, Hotary K, Sabeh F, Saltiel A, Allen E, Weiss S. A Pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 82.Biondi M, Ungaro F, Quaglia F, Netti PA. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 83.Kimura Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 84.Masuda T, Furue M, Matsuda T. Photocured, styrenated gelatin-based microspheres for de novo adipogenesis through corelease of basic fibroblast growth factor, insulin and insulin-like growth factorI. Tissue Eng. 2004;10:523–535. doi: 10.1089/107632704323061889. [DOI] [PubMed] [Google Scholar]
- 85.Chi L, Zhang S, Lin Y, Prunskaite-Hyyryläinen R, Vuolteenaho R, Itäranta P, et al. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- 86.Sodunke T, Turner K, Caldwell S, Mcbride K, Reginato M, Noh H. Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials. 2007;28:4006–4016. doi: 10.1016/j.biomaterials.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Gillette B, Jensen J, Tang B, Yang G, Bazargan-Lari A, Zhong M, et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mat. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 88.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]