Abstract
Lhx8 is a member of the LIM-homeobox transcription factor family and preferentially expressed in oocytes and germ cells within the mouse ovary. We discovered that Lhx8 knockout females lose oocytes within 7 days after birth. At the time of birth, histological examination shows that Lhx8-deficient (Lhx8−/−) ovaries are grossly similar to the newborn wild-type ovaries. Lhx8−/− ovaries fail to maintain the primordial follicles, and the transition from primordial to growing follicles does not occur. Lhx8−/− ovaries misexpress oocyte-specific genes, such as Gdf9, Pou5f1, and Nobox. Very rapid loss of oocytes may partly be due to the drastic downregulation of Kit and Kitl in Lhx8−/− ovaries. We compared Lhx8−/− and wild-type ovaries using an Affymetrix 430 2.0 microarray platform. A total of 80 (44%) of 180 of the genes downregulated more than 5-fold in Lhx8−/− ovaries were preferentially expressed in oocytes, whereas only 3 (2%) of 146 genes upregulated more than 5-fold in the absence of Lhx8 were preferentially expressed in oocytes. In addition, the comparison of genes regulated in Lhx8−/− and Nobox−/− newborn ovaries discovered a common set of 34 genes whose expression level was affected in both Lhx8- and Nobox-deficient mice. Our findings show that Lhx8 is a critical factor for maintenance and differentiation of the oocyte during early oogenesis, and it acts in part by downregulating the Nobox pathway.
Keywords: developmental biology, gamete biology, gene regulation, KIT, KITL, meiosis, LHX8, NOBOX, oocyte development, oogenesis, ovary, primordial follicle
Lhx8 transcription factor disrupts early oogenesis and likely effects its action by disrupting expression of transcription factor Nobox and Kit ligand and Kit receptor signaling pathway
INTRODUCTION
In mice, female primordial germ cells migrate to the genital ridge and divide by mitosis, and oocytes cluster to form germ cell clusters [1]. Shortly after birth, germ cell clusters break down and pregranulosa cells envelop individual oocytes to form primordial follicles [1, 2]. During this process, large numbers of oocytes in the clusters are eliminated by unknown mechanisms, and approximately one third of the oocytes survive to form primordial follicles [1, 2]. Oocyte elimination in mice continues for several days after birth, and oocyte numbers stabilize by unknown mechanisms to form the finite pool of primordial oocytes. Selected primordial follicles are continuously recruited to progress into primary and growing follicles, but the rest of the primordial follicles remain quiescent [3]. The transition from primordial to primary follicles is critical for early folliculogenesis.
Transcription of numerous oocyte-specific genes, such as zona pellucida 1 (Zp1), Zp2, and Zp3, growth differentiation factor 9 (Gdf9), and Pou5f1 (also known as Oct4) commences during early folliculogenesis [4, 5]. Several transcription factors are known to affect formation of ovarian follicles. FIGLA (Factor in the germline alpha), NOBOX (Newborn ovary homeobox), and SOHLH1 (Spermatogenesis and oogenesis helix-loop-helix 1) are critical for the formation and maintenance of primordial follicles. FIGLA is a basic helix-loop-helix transcription factor that regulates expression of the zona pellucida genes [6, 7]. NOBOX is a homeobox transcription factor that is necessary for expression of several key oocyte-specific genes, including Gdf9 and Pou5f1 [5, 8]. SOHLH1 is another germ cell-specific basic helix-loop-helix transcription factor that is upstream of Lhx8 (LIM-homeobox protein 8) [9].
Lhx8 transcripts localize to oocytes of germ cell clusters and primordial, primary, and antral follicles in the mouse ovary [9]. However, the physiological function of Lhx8 in early folliculogenesis has not been described. In the present study, we find that Lhx8 deficiency accelerates postnatal oocyte loss in the ovary and causes infertility in female mice. Moreover, Lhx8 deficiency affects the expression of numerous oocyte-specific genes in the ovary, and LHX8 can bind to the promoter region of Nobox via LHX8 DNA-binding elements. Lhx8 is critical in early follicle formation and oocyte differentiation, and functions in part by regulating the Nobox pathway.
MATERIALS AND METHODS
Animal Breeding
All murine experiments were carried out on C57BL/6;129S6/SvEv hybrid background. Litters were weaned at 3 wk, and breeding pairs set up at 6 wk of age. One mating pair was placed per cage and inspected daily for the presence of litters. All procedures described within were reviewed and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee, and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Histology and Histomorphometric Analysis
Testes were fixed in Bouin solution (Sigma-Aldrich, St. Louis, MO), and ovaries were fixed in 10% buffered formalin or 4% paraformaldehyde. Fixed tissues were embedded in paraffin, serially sectioned (5 μm), and stained with hematoxylin and eosin or with periodic acid-Schiff (PAS). Histomorphometric analysis was performed as previously described [9]. In brief, every fifth section was derived from the long axis of the ovary and photographed, and oocytes containing nuclei were scored. The total numbers of oocytes from all of the sections were summed, and the mean number per ovary was determined. No correction factor was used. Fisher exact t-test was used to calculate P values. Germ cell cysts were defined as two or more oocytes that were not individually separated by stromal cells. Primordial follicles were defined as small oocytes (<20 μm) surrounded by flat epithelial cells. Primary follicles were defined as having larger oocytes (>20 μm) surrounded by a single layer of cuboidal granulosa cells; secondary follicles were defined as larger oocytes surrounded by two or more layers of granulosa cells.
Immunohistochemistry
Immunohisochemistry was performed as previously described [10]. MSY2 and SCP3 rabbit immunoaffinity-purified antibody was kindly provided by Dr. Richard Schultz (University of Pennsylvania) and Dr. Peter B. Moens (York University, Toronto, ON, Canada), respectively. Anti-LHX8 guinea pig antibodies were generated by expressing a partial LHX8 protein corresponding to amino acids (1–127) in the pET-23b vector and immunization of guinea pigs at Cocalico Biologicals (Lancaster, PA). Antibodies were affinity purified over Affi-Gel-bound LHX8 protein columns (Bio-Rad, Hercules, CA). Preimmune serum was used in control sections, with little detectable background (data not shown).
RNA Isolation, RT-PCR, and Quantitative Real-Time PCR
Reverse transcriptase-polymerase chain reaction was performed as previously described [11]. Oligonucleotides corresponding to each gene were selected using PRIMER3 software and spanned introns. The sequences of these primers are available upon request. Polymerase chain reaction was carried out for 29 cycles on three independently collected pools of newborn ovaries. Quantitative real-time PCR (Q-PCR) was performed as previously described [9]. Mouse Gapdh was used for the endogenous control. Each sample was analyzed in duplicates from at least three independent wild-type (WT), Lhx8−/−, and Nobox−/− newborn ovary cDNA samples. The relative amount of transcripts was calculated by the ΔΔCT method. All of the data were normalized to the median. The average and standard errors were calculated for the triplicate measurements, and the relative amount of target gene expression for each sample was plotted. Significance was determined using the Student t-test.
Microarray Analysis
Total RNA isolated from WT, Lhx8−/−, and Nobox−/− ovaries was labeled and hybridized to mouse genome 430 2.0 Array (Affymetrix, CA) at the Baylor College of Medicine Microarray Core Facility according to the manufacturer. Affymetrix data from WT, Lhx8−/−, and Nobox−/− chips were analyzed with Genespring GX software (Silicon Graphics, Sunnyvale, CA). Gene lists were generated using the advanced filtering function. Genes with a raw signal greater than 200 and showing a 5-fold change between WT and null arrays were grouped into one list. These lists were then compared to the full gene list of the mouse 430 2.0 array using Genespring GX's Venn diagram function, and a gene list was extracted. Data sets have been deposited in GEO (GSE7774, GSE7775, and GSE 7776).
Meiotic Chromosome Analysis and Immunofluorescence
Ovaries were removed from Embryonic Day 15.5 (E15.5) embryos and incubated in trypsin-EDTA solution at 37°C for 13 min and washed briefly in PBS [12]. Trypsinized ovaries were pippetted repeatedly and then centrifuged, followed by resuspension in PBS. Cell suspensions were placed on poly-l-lysine-coated slides containing sucrose solution (40 mg/ml) and lysed with 0.05% Triton X-100. The slides were fixed in 2% paraformaldehyde and 0.02% SDS for 1 h at room temperature. The slides were washed six times in distilled water, air dried, and stored at −80°C until use. Immunostainings were prepared as described [12]. Slides were then incubated with a 1:500 dilution of rabbit anti-SCP3 antibody overnight at 4°C, washed with PBS, and incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:300 dilutions) for 1 h at room temperature. After washing with PBS, slides were mounted using VECTASHIELD medium with 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories).
RESULTS
Lhx8 Deficiency Affects Female but Not Male Gonadal Development
Lhx8 encodes a LIM-homeobox protein with homologs in humans and other mammals [13–15]. In females, Lhx8 expression commences as early as E13.5, when oocytes enter meiosis I during embryonic development [9]. To define the role of Lhx8 in early stages of folliculogenesis, we investigated mice with a targeted deletion of Lhx8 LIM and homeodomain, Lhx8tm1Lmgd [14]. We have previously shown that Lhx8−/− adult ovaries lack germ cells [9]; however, it is not known when Lhx8 homozygous deficiency affects ovarian development, and whether male gonadal development is disrupted.
Lhx8−/− males mated with WT females produced 8.4 ± 1.2 pups per litter (n = 8 breeding pairs) over a 6-mo period. Litter sizes were not statistically different from the WT average (8.7 ± 1.1 pups per litter; Fig. 1, K and L). Wild-type and Lhx8−/− testes were not different in morphology or histology (Fig. 1, A–E). Sohlh1 deficiency disrupts both male and female germ cell differentiation, and Lhx8 is highly downregulated in Sohlh1-deficient male and female gonads, Sohlh1tm1Rajk [9, 16]. Our results indicate that Lhx8 deficiency cannot account for male infertility observed in Sohlh1 knockout males.
FIG. 1.
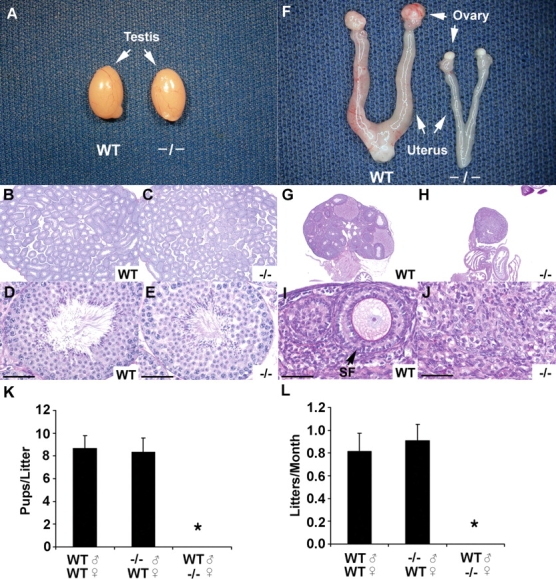
Lhx8 adult knockout anatomy and histology. A) Testes dissected from 10-wk-old WT and Lhx8−/− mice (−/−) show no appreciable difference in weight and morphology. B–E) Histology on 10-wk-old testes from WT and Lhx8-deficient mice, fixed in Bouin solution and stained with PAS. F) Female reproductive tracts dissected from 10-wk-old WT and Lhx8−/− mice. Note atrophic ovaries and uterine horns in Lhx8−/− mice. G–J) Histology of WT and Lhx8−/− ovaries. Lhx8−/− ovaries lack germ cells (J), whereas magnified view of the WT ovary shows well-developed secondary follicle (SF). K, L) Fertility assessment based on pups per litter (K) and litters per month (L). Male homozygous mating (n = 8) produced, on average, 8.4 ± 1.2 pups per litter (n = 10 breeding pairs) over a 6-mo period. Litter sizes were not statistically different from the WT average (8.7 ± 1.1 pups per litter). Data are represented as mean values, with error bars representing the SEM. Fisher exact t-test was used to calculate P values. *P < 0.01. Bars = 20 μm.
Lhx8+/− female mice and Lhx8+/− reproductive organs were grossly normal. Lhx8+/− females mated with either WT or Lhx8−/− males per litter over an 8-mo period showed no significant difference in fertility from WT females (data not shown). In contrast, all Lhx8−/− females were infertile, and their ovaries were atrophic at 10 wk of age (Fig. 1, F, H, and J) compared with WT (Fig. 1, F, G, and I). Adult Lhx8−/− ovaries contained no oocytes or follicles (Fig. 1, H and J).
Lhx8 Is Essential in Postnatal Oocyte Differentiation and Survival
We determined the onset of germ cell loss in Lhx8-deficient ovaries by examining newborn and 7-day-old knockout and WT mice. Newborn WT and Lhx8−/− ovaries did not show gross differences in morphology and histology (Fig. 2, A and B), and number of oocytes in the newborn Lhx8+/− and Lhx8−/− ovaries did not significantly differ (Fig. 2E), although the number of primordial follicles was significantly lower in the knockouts. At 7 days postpartum (Fig. 2, C and D), Lhx8−/− ovaries contained very few oocytes, and most follicles were degenerating (Fig. 2D). Histomorphometric analyses revealed a greater loss of oocytes in Lhx8−/− relative to the Lhx8+/− ovaries (Fig. 2F). These observations implicate Lhx8 as another critical transcriptional regulator of oocyte survival and differentiation.
FIG. 2.
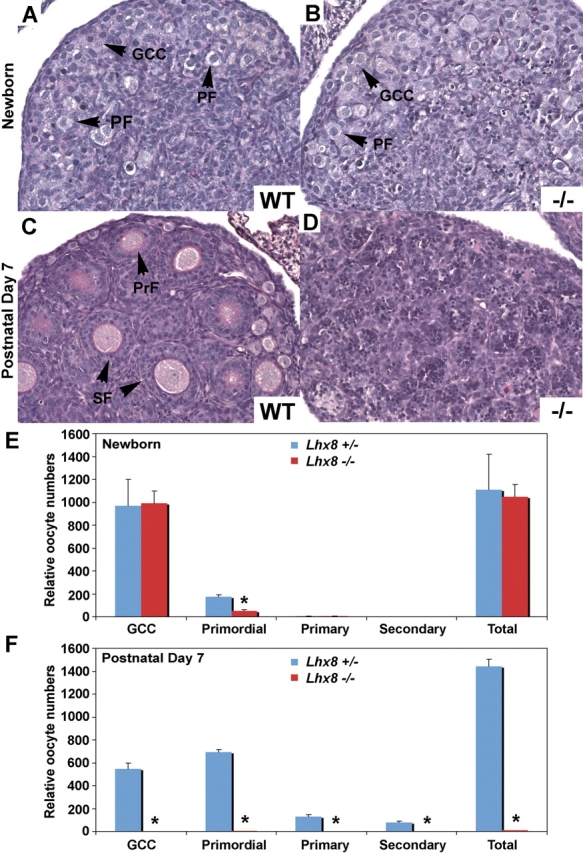
Histology and histomorphometric analysis of WT, Lhx8+/−, and Lhx8−/−ovaries at birth and Postnatal Day 7. A–D) Periodic acid-Schiff reagent staining of ovaries dissected from WT and Lhx8−/− mice. A, C) Newborn and Day 7 WT ovaries displaying germ cell clusters (GCCs), primordial follicles (PFs), primary follicles (PrFs), and secondary follicles (SFs). B, D) Lhx8−/− newborn ovaries contain GCCs and PFs, but PAS staining of 7-day ovaries shows very few oocytes. Original magnification x40 E, F) Five pairs of newborn and Postnatal Day 7 ovaries from Lhx8+/− (blue bar) and Lhx8−/− (red bar) mice were sectioned, and oocytes within the GCCs and PFs were counted. A significant decrease in PFs is noted in the newborn ovary, and few oocytes remain at 7 days. Data are represented as mean values, with error bars representing the SEM. Student t-test was used to calculate P values. *P < 0.01.
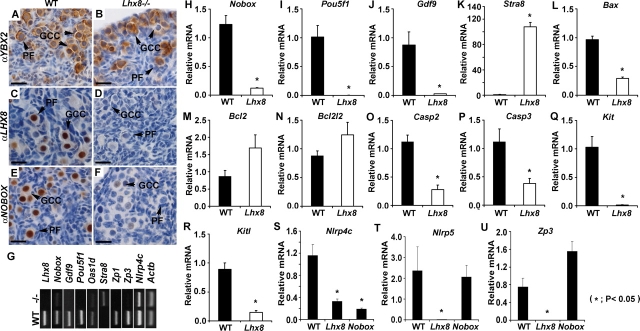
We also studied differential expression of the YBX2, Y Box protein 2 (also known as MSY2) in the Lhx8−/− ovaries (Fig. 3). YBX2 is a cytoplasmic marker expressed in oocytes that have entered the diplotene stage of meiosis I and beyond [17, 18]. YBX2 immunoreactivity is present in oocytes of germ cell clusters and primordial follicles of both newborn WT and Lhx8−/− ovaries (Fig. 3, A and B).
FIG. 3.
Molecular changes in the Lhx8−/− newborn ovaries. A, B) Newborn WT and Lhx8−/− ovaries both express germ cell-specific RNA-binding protein YBX2, as shown by brownish cytoplasmic staining of primordial follicles (PFs) and germ cell clusters (GCCs) with anti-YBX2 antibodies. C, D) LHX8 protein localizes within oocyte nuclei only in the WT newborn ovaries and as expected is not detected in the Lhx8−/− mice. E, F) Anti-NOBOX antibodies show that NOBOX protein is expressed significantly less in Lhx8−/− ovaries compared with WT. Bars = 20 μm. G) RNA expression of selected genes in WT and Lhx8−/− newborn ovaries with semiquantitative RT-PCR. H–U) Expression of oocyte-specific genes in newborn WT, Lhx8−/− (Lhx8), and Nobox−/− (Nobox) ovaries. Quantitative RT-PCR is shown for relative expression of Nobox (H), Pou5f1 (I), Gdf9 (J), Stra8 (K), Bax (L), Bcl2 (M), Bcl2l2 (N), Casp2 (O), Casp3 (P), Kit (Q), Kitl (R), Nlrp4c (S), Nlrp5 (T), and Zp3 (U). Data are normalized to Gapdh expression and presented as the mean relative quantity (compared with WT), with error bars representing the SEM. Student t-test was used to calculate P values. *P < 0.05.
Lhx8 Deficiency and Meiosis I
Rapid oocyte loss occurs when meiosis I components are perturbed, as exemplified by Msh5 [19], Rec8 [20], and Dmc1 [21, 22] knockout models. Lhx8−/− ovaries lose oocytes rapidly after birth, at the time when most oocytes have entered the diplotene stage of meiosis I. We studied whether gross perturbations in meiosis account for rapid oocyte loss in Lhx8−/− ovaries. Female gonads from WT and Lhx8−/− E15.5 littermates were immunostained with antibodies to SYCP3 (synaptonemal complex protein 3), a critical component of the synaptonemal complex [23–25]. Sycp3 transcripts were not significantly different between the WT and Lhx8−/− ovaries (Fig. 4K). The localization of SYCP3 in zygotene, pachytene, and diplotene stages was compared between WT and Lhx8−/− gonads (Fig. 4, A–F). SYCP3 was detected on the chromatin in both WT and Lhx8−/− oocytes in zygotene, pachytene, and diplotene stages, with no gross abnormalities in the chromatin structure and organization (Fig. 4, A–F).
FIG. 4.
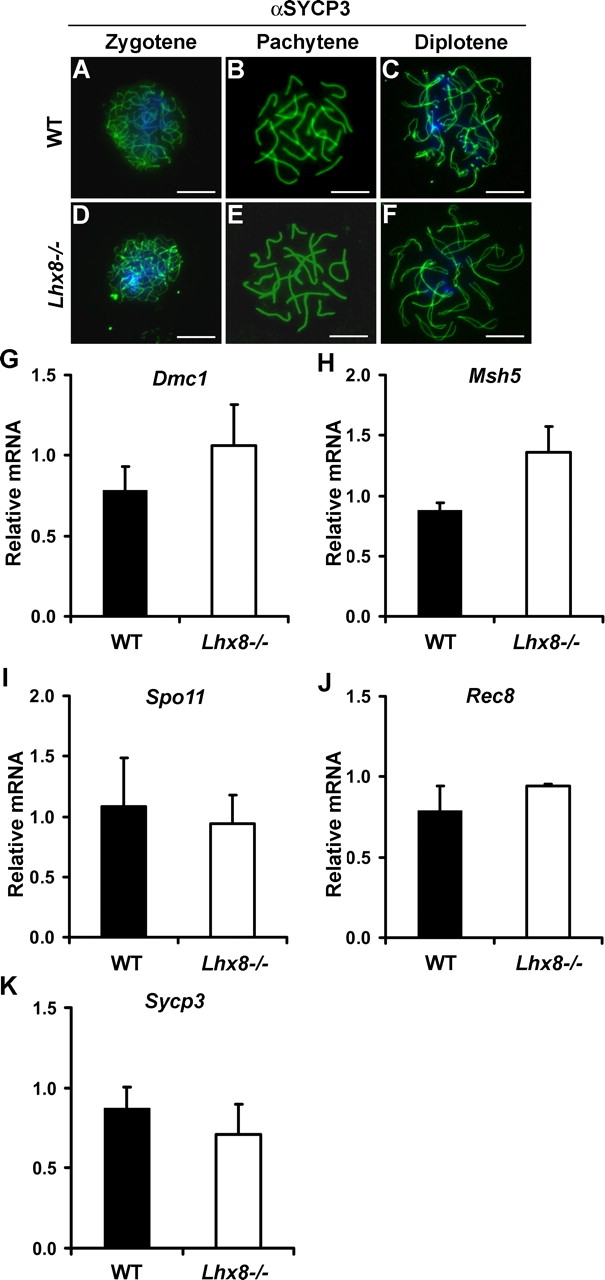
Meiotic markers in Lhx8-deficient ovaries. A–F) SYCP3 protein expression in germ cells from WT and Lhx8-deficient ovaries at E15.5 using immunofluorescent staining. The localization of SCP3 in zygotene (A, D), pachytene (B, E), and diplotene (C, F) stages of meiosis. No appreciable differences are noted between WT and Lhx8−/− ovaries. Bars = 10 μm. G–K) Quantitative PCR analysis of Dmc1 (G), Msh5 (H), Spo11 (I), Rec8 (J), and Sycp3 (K) transcripts shows no significant differences in the expression of WT and Lhx8-deficient ovaries.
In addition to Sycp3, we studied expressions of genes critical in meiosis: Dmc1 [21, 22, 26], Msh5 [19], Spo11 [27, 28], and Rec8 [20, 29, 30]. Deficiency of Dmc1, Msh5, Spo11, and Rec8 accelerates germ cell loss in the gonads. The relative levels of Dmc1, Msh5, Spo11, or Rec8 transcripts were not significantly different between the WT and Lhx8−/− ovaries (Fig. 4, G–J). These results indicate that meiotic components currently known to disrupt early oogenesis are not affected by Lhx8 deficiency and suggest that oocyte differentiation can be governed by factors independent of meiosis.
Gene Expression in Lhx8−/− Ovaries
Because Lhx8 is preferentially expressed in germ cells, we examined whether oocyte-specific gene expression is perturbed in the Lhx8−/− ovaries (Fig. 3). Nobox, Gdf9, Pou5f1, Oas1d, Zp1, Zp3, and Nlrp4c transcript levels were drastically reduced or undetectable in Lhx8−/− ovaries compared with the WT, semiquantitative and quantitative PCR (Fig. 3, G–K, S). Nobox transcript levels in the Lhx8−/− ovaries are reduced approximately 10-fold (Fig. 3H), and immunohistochemical staining shows significant reduction in the amount of NOBOX protein detected in Lhx8−/− ovaries compared with the WT (Fig. 3, E and F). Pou5f1 and Gdf9 mRNAs are undetectable in Lhx8−/− ovaries (Fig. 3, I and J). We previously described that Pou5f1 and Gdf9 lie downstream of Nobox [5], and NOBOX binds Pou5f1 and Gdf9 promoters [8]. These results indicate that Nobox expression is dependent, in part, on LHX8, and that genes downregulated by NOBOX are also downregulated in Lhx8−/− mice. However, not all genes that are downregulated in Lhx8−/− are also downregulated in Nobox−/− ovaries (Fig. 3, T and U). Although Nlrp5 and Zp3 transcript levels are not significantly affected by Nobox deficiency, these transcripts are significantly downregulated in Lhx8−/− ovaries. Lhx8, therefore, does not act solely via the Nobox pathway.
We also examined expression of genes that have been implicated in germ cell apoptosis and survival. Bax, Casp2, and Casp3 are all downregulated in Lhx8−/− ovaries (Fig. 3, L, O, and P), whereas Bcl2 and Bcl2l2 are not misexpressed (Fig. 3, M and N). Moreover, Kit and its ligand, Kitl, are drastically downregulated in Lhx8−/− ovaries (Fig. 3, Q and R). In contrast, Nobox−/− ovaries do not misexpress Kit and Kitl or known apoptosis genes [5], which argues that other pathways also play a significant role in germ cell survival and apoptosis.
Among few upregulated genes in Lhx8−/− newborn ovaries, and preferentially expressed in germ cells, is stimulated by retinoic acid gene 8 (Stra8; Fig. 3G). Stra8 is required for premeiotic DNA replication, meiotic chromosome condensation, cohesion, synapsis, and recombination [12], and its expression declines drastically by the time mice are born [31]. In contrast, Lhx8−/− newborn ovaries express significant levels of Stra8 transcripts (Fig. 3K). Stra8 is also upregulated in Nobox−/− newborn ovaries [32]. Our results suggest that Lhx8 and Nobox directly or indirectly repress Stra8 expression.
In order to better understand effects of Lhx8 deficiency on the global newborn ovary transcriptome, we compared WT and Lhx8−/− newborn ovaries because their grossly similar histology and oocyte numbers may allow us to detect molecular changes that antedate gross morphologic and histologic changes. Analysis of three independent experiments comparing Lhx8−/− and WT newborn ovaries revealed that a total of 326 genes were misexpressed greater than 5-fold. Of these, 180 genes were downregulated (Supplemental Table S1 available online at www.biolreprod.org ) and 146 were upregulated in Lhx8-deficient ovaries (Supplemental Table S2 available online at www.biolreprod.org ). Unigene EST database and GNF Sym Atlas resources were used to determine which genes are preferentially expressed in germ cells or oocytes [33]. A total of 80 (44%) of 180 downregulated genes showed preferential expression in germ cells and oocytes (Supplemental Table S1 available online at www.biolreprod.org ), whereas only 3 (2%) of 146 genes that were upregulated more than 5-fold showed germ cell specific (Supplemental Table S2 available online at www.biolreprod.org ). We identified transcripts that are commonly misexpressed in Nobox- and Lhx8-deficient ovaries by analyzing Lhx8−/− and Nobox−/− newborn ovary arrays. A total of 34 genes were differentially regulated 5-fold or more in both Lhx8−/− and Nobox−/− ovaries (Supplemental Table S3 available online at www.biolreprod.org ). A total of 20 (69%) of 29 genes that were downregulated more than 5-fold showed preferential expression in germ cells and oocytes and include Pou5f1, Gdf9, Oas1c (2′,5′-oligoadenylate synthase), Oas1d, Oas1e, Oas1h, Nlrp14, Nlrp4c, and Nlrp4f (Supplemental Table S3 available online at www.biolreprod.org ). Two of five genes, Stra8 and Dmrt1 (doublesex and mab-3-related transcription factor 1), were upregulated more than 5-fold, and showed preferential expression in germ cells (Supplemental Table S3 available online at www.biolreprod.org ).
The Nlrp family members, such as Nlrp14, Nlrp4c (Fig. 3), and Nlrp4f, are also drastically downregulated in both Lhx8−/− and Nobox−/− newborn ovaries (Supplemental Table S1 available online at www.biolreprod.org ). Nlrp14 is preferentially expressed in oocytes and spermatogonia, and mutations in NLRP14 occur in men with spermatogenic failure [34, 35]. Little is known about the regulatory mechanism of Nlrp14, Nlrp4f, and Nlrp4c, but Lhx8 and Nobox might be involved either directly or indirectly in upregulation of this Nlrp family of genes in oocytes.
Interestingly, not all of the genes misexpressed in Nobox−/− ovaries are also misexpressed in Lhx8−/− newborn ovaries. A total of 40 genes were differentially regulated only in Nobox−/− newborn ovaries but not in Lhx8−/− newborn ovaries (Supplemental Table S4 available online at www.biolreprod.org ), and include astacinlike metalloendopeptidase (Astl), jagged 1 (Jag1), oocyte-secreted protein 1 (Oosp1), and regulating synaptic membrane exocytosis 1 (Rims1). Astl, a member of the astacin family of metalloproteinases, is preferentially expressed in human and mouse oocytes [36], although its function is unknown. Oosp1 is exclusively expressed in oocytes [37], is predicted to be secreted, and may play a role in folliculogenesis.
In addition, 292 of 326 regulated genes are misexpressed only in Lhx8−/− newborn ovaries but not in Nobox−/− ovaries and include, among other genes, Nlrp5 and Zp3 (Supplemental Table S5 available online at www.biolreprod.org ). Nlrp5 and Zp3 are significantly downregulated in Lhx8−/− newborn ovaries, but not in Nobox−/− newborn ovaries, as confirmed by Q-PCR (Fig. 3, T and U). Nlrp5 is a maternal effect gene that is required for early embryonic development beyond the two-cell stage in mice [38], whereas Zp3 encodes a glycoprotein which is part of the zona pellucida family of genes [39]. Zp3-deficient mice lack zona pellucida with resulting infertility in female mice [40, 41]. It is not known whether Nlrp5 and Zp3 are directly regulated by LHX8; however, Nlrp5 and Zp3 null mice have normal ovarian folliculogenesis, and as such cannot account for the phenotype observed in Lhx8−/− mice.
DISCUSSION
Lhx8 gene encodes a highly conserved LIM homeodomain (LIM-HD) that shares 93% identity between mouse and humans. LIM homeodomain genes play an important function in tissue patterning and differentiation, especially in the neural tissues [13–15]. The LIM domain consists of two specialized zinc fingers that are located N-terminally of the homeodomain and enable LIM-HD proteins to interact with other transcriptional regulators in a homomeric or heteromeric fashion. Although their functions have been mostly studied in neuronal specification, Lhx9 was the first LIM-homeodomain gene shown to be essential for gonadal development [42]. Lhx9 is expressed in the somatic component of the gonad, and Lhx9-deficient mice do not form a discrete gonad, because somatic cells of the genital ridge fail to proliferate, despite normal migration of germ cells [42]. Unlike Lhx9, Lhx8 expression is confined to the germ cell component of the embryonic ovary. Lhx8 was initially implicated in the development of cholinergic neurons and telencephalon, as well as palate [13–15]; however, it was not until recently that Lhx8 preferential expression within oocytes of the gonad was discovered [9]. Lhx8 therefore represents the first member of the LIM homeodomain family to be expressed in germ cells.
Lhx8 lies downstream of the spermatogenesis- and oogenesis-specific basic helix-loop-helix transcription factor, Sohlh1 [9, 16]. Sohlh1 deficiency disrupts both spermatogonial and oocyte differentiation. However, Lhx8 deficiency does not affect male gonadal development, and therefore Lhx8 is not the effector of Sohlh1 action in the male gonads.
Large loss of oocytes occurs during fetal ovarian development as oocytes progress through meiotic prophase I. Mouse oogonia enter meiosis circa E13.5 to become oocytes, but not all oogonia enter meiosis at once, with some not entering meiosis until after birth [43]. Oocytes progress through the stages of prophase I of meiosis, and most arrest in the diplotene stage shortly after birth [44]. In the mouse, the oocyte loss stabilizes after birth, when germ cell clusters are breaking down and primordial follicles form [1, 45]. Causes for perinatal loss/elimination of oocytes include meiotic errors, apoptosis, self-sacrifice of surrounding oocytes, and yet-unknown pathways [1, 45]. Spo11, Dmc1, Atm, Msh4, Mlh1, and Msh5 are genes involved in recombination and mismatch repair, and female and male mice deficient in these genes are infertile, with early loss of germ cells [19, 21, 28, 46–48]. Lhx8−/− ovaries do not misexpress these genes. Moreover, immunostaining with antibodies against SCP3, a marker for axial elements and synaptonemal complex during meiotic prophase I, shows that Lhx8−/− germ cells have normal chromosome paring and synapsis (Fig. 4, A–F). These results argue that meiosis is not grossly perturbed in Lhx8-deficient ovaries, although we cannot rule out misexpression of novel synaptonemal components. This may be especially true in light of multiple expressed sequence tags (ESTs) with unknown functions discovered to be misexpressed in Lhx8−/− ovaries.
Apoptosis, or programmed cell death, has received a lot of attention as a mechanism responsible for large loss of oocytes. However, apoptosis accounts for only a fraction of oocyte elimination that occurs perinatally in mice [49]. The B-cell lymphoma/leukemia-2 (Bcl2) family of proteins regulates apoptosis in both reproductive and nonreproductive tissues. Bcl2-deficient mice are fertile, with reduced primordial oocyte numbers in adults [50]. Bcl2 is not significantly misexpressed in Lhx8−/− ovaries. Deficiency of Bax, a proapoptotic member of the Bcl-2 family, causes an increased number of oocytes per ovary [51]. Lhx8 ovaries express significantly fewer transcripts corresponding to Bax (Fig. 3), and lower Bax transcript levels, therefore, cannot account for the observed early oocyte loss. In addition to Bax, Bcl2l2 (Fig. 3) regulates germ cell survival and apoptosis, but Bcl2l2 transcripts are not misexpressed in Lhx8−/− ovaries [52]. Caspases are considered primary executioners of apoptosis, although Casp2- and Casp3-deficient mice and double knockouts have little effect on early folliculogenesis [53], and they are downregulated in Lhx8−/− ovaries. The phenotype observed in Lhx8 mice does not phenocopy any of the known deficiencies in programmed cell death genes.
The prosurvival factor, Kit, and its ligand, Kitl, are downregulated more than 7-fold in Lhx8−/− ovaries. KIT receptor is expressed by the oocytes, whereas its ligand, KITL, is produced by the surrounding somatic tissue, and mutations within Kit and Kitl affect germ cell proliferation and migration, as well as primordial to primary follicle transition [54–56]. It is therefore plausible that downregulation of Kit and Kitl contributes significantly to the accelerated loss of oocytes observed in Lhx8−/− mice. Interestingly, Kit and Kitl expression is not affected in Nobox−/− ovaries. The downregulation of Kit and Kitl in Lhx8−/− ovaries may account for the more severe phenotype observed in the Lhx8−/− ovaries. Lhx8−/− ovaries lose oocytes almost completely by Day 7, whereas Nobox−/− ovaries still contain a significant number of nongrowing oocytes by Day 14. Kit and Kitl misexpression may, therefore, account for the severity of the oocyte loss in Lhx8−/− ovaries. However, pathways other than Kit/Kitl must be involved in promoting oocyte survival, as seen in the Nobox−/− ovaries.
We have previously shown that NOBOX can bind Gdf9 and Pou5f1 promoters via the NOBOX DNA-binding element [8]. Both Gdf9 and Pou5f1 are downregulated in Lhx8−/− and Nobox−/− ovaries, and our results indicate that their regulation in Lhx8−/− ovaries may in part be due to the diminished expression of Nobox in Lhx8−/− ovaries. We cannot exclude the possibility that Lhx8 and Nobox interact together to directly regulate transcription of Gdf9 and Pou5f1. However, not all genes downregulated in the Nobox knockout ovaries are downregulated in the Lhx8 knockout ovaries (Supplemental Table S4 available online at www.biolreprod.org ). For example, genes that encode secretory proteins Oosp1 and Astl, novel olfactory receptor (Olfr976), and notch signaling pathway protein (Jag1) are significantly downregulated in Nobox but not in Lhx8 ovaries. One possible explanation is that Lhx8 represses the expression of these particular genes, whereas Nobox activates them. Multiple transcriptional regulators are required to promote initiation of transcription, and it is likely that different stochiometries will have distinct effects on transcription. It is also possible that within Lhx8-deficient oocytes, remaining NOBOX protein occupies higher affinity promoter sites located in Oosp1, Astl, Olfr976, and Jag1 promoters. In such scenario, promoter sites with lower affinity for NOBOX will initiate transcription significantly less.
Among the genes upregulated in Lhx8−/− ovaries is Stra8 (Fig. 3). Stra8 transcripts are detectable between E12.5 and E16.5, and they decline thereafter, with no detectable postnatal expression [31]. Stra8 plays an important role in the initiation of meiosis, as shown by the Stra8 knockout whose germ cells fail to undergo premeiotic DNA replication, meiotic chromosome condensation, cohesion, synapsis, and recombination [12]. However, the true role of Stra8 is still unclear, and besides retinoic acid, no other regulators of Stra8 expression are known. The re-expression of Stra8 in Lhx8−/− ovaries argues that Lhx8 may play a role in suppressing Stra8 expression in the embryonic gonad. The significance of Stra8 downregulation is unknown, but it may be necessary for proper progression of meiosis and/or ovarian differentiation. These results raise a question of whether suppression of Stra8 is necessary for oocyte differentiation. Since meiosis does not appear to be grossly perturbed, and genes known to be necessary for female meiosis are not misexpressed, our experiments argue that postmeiotic oocyte differentiation, governed by transcriptional regulators such as Lhx8 and Nobox, is independent of meiosis.
Microarray analyses show that numerous genes are affected only by Lhx8 deficiency but are not affected by Nobox deficiency (Supplemental Table S5 available online at www.biolreprod.org ). Among genes only regulated by LHX8, but not NOBOX, are Nlrp5, Zp1, Zp2, and Zp3. Although transcription of Nlrp5 and Zp genes commences in small oocytes, knockouts show that their function is beyond early folliculogenesis. Nlrp5 is essential for embryonic development past the two-cell stage in mice [38]. Zp1, Zp2, and Zp3 genes are essential components of the zona pellucida that surrounds the oocyte [39]. Knockouts of Zp genes suffer various defects in later stages of oocyte differentiation and, just like Nlrp5, are unlikely to explain Lhx8 phenotype. However, it reinforces the importance of early oocyte differentiation in transcribing genes necessary for the later stages of oocyte development and early embryogenesis. Many genes downregulated in Lhx8-deficient mice are functionally uncharacterized and include several that are preferentially expressed in germ cells, such as BQ175373, 9230115E21Rik, and Pramef12 [33]. Some of these genes may as well be critical components of oocyte differentiation and survival.
Deficiency of Figla, another oocyte-specific, basic helix-loop-helix transcription factor, was recently described to downregulate expression of oocyte-specific genes, such as Nlrp14, Nlrp4f, Nlrp4b, and Pou5f1, and to upregulate expression of several male germ cell-specific genes [57]. Figla deficiency disrupts primordial follicle formation, with ovarian pathology that is very similar to pathologies observed in Lhx8- and Nobox-deficient ovaries [7]. Although Figla, Lhx8, and Nobox transcripts are expressed in the embryonic gonad as early as E15.5, ovarian pathology occurs postnatally. It is unclear whether birth, with associated declines in estrogen and progesterone, accelerates loss of oocytes in these mutants. Future studies aimed at understanding the role of external factors and their interaction with the mutations in Lhx8, Nobox, and Figla may give us further insight into the formation of the primordial follicle pool.
Like Nobox, Figla expression is significantly downregulated in Lhx8-deficient ovaries (Supplemental Table S1 available online at www.biolreprod.org ). Nobox, Figla, and Lhx8, unlike Sohlh1, do not affect male germline development, and therefore represent an important set of female-specific transcriptional regulators. Nobox, Figla, and Lhx8 transcriptional regulators share common genetic pathways, and future studies will be important to identify female-specific regulators of Figla, Nobox, and Lhx8, which ultimately may lead us to the identification of elusive regulators of early female sex determination pathways and help us answer how germline regulates gonadal differentiation.
Supplementary Material
Acknowledgments
We thank Heiner Westphal and Yangu Zhao for providing us with Lhx8 null mice.
Footnotes
Supported by National Institutes of Health grant HD44858 (A.R.) and March of Dimes Basil O'Connor Award 5-FY02-266 (A.R.).
REFERENCES
- Pepling ME, Spradling AC.Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234: 339–351. [DOI] [PubMed] [Google Scholar]
- Pepling ME.From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis 2006; 44: 622–632. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK.Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 2003; 144: 3329–3337. [DOI] [PubMed] [Google Scholar]
- Dean J.Oocyte-specific genes regulate follicle formation, fertility and early mouse development. J Reprod Immunol 2002; 53: 171–180. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM.NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305: 1157–1159. [DOI] [PubMed] [Google Scholar]
- Liang L, Soyal SM, Dean J.FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development 1997; 124: 4939–4947. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J.FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000; 127: 4645–4654. [DOI] [PubMed] [Google Scholar]
- Choi Y, Rajkovic A.Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. J Biol Chem 2006; 281: 35747–35756. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A.Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A 2006; 103: 8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Rademaker AW, Fishman DA, Woodruff TK.Localization of the activin signal transduction components in normal human ovarian follicles: implications for autocrine and paracrine signaling in the ovary. J Clin Endocrinol Metab 2002; 87: 2644–2657. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Yan MSC, Klysik M, Matzuk M.Discovery of germ cell-specific transcripts by expressed sequence tag database analysis. Fertil Steril 2001; 76: 550–554. [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC.In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430–1434. [DOI] [PubMed] [Google Scholar]
- Mori T, Yuxing Z, Takaki H, Takeuchi M, Iseki K, Hagino S, Kitanaka J, Takemura M, Misawa H, Ikawa M, Okabe M, Wanaka A.The LIM homeobox gene, L3/Lhx8, is necessary for proper development of basal forebrain cholinergic neurons. Eur J Neurosci 2004; 19: 3129–3141. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Guo YJ, Tomac AC, Taylor NR, Grinberg A, Lee EJ, Huang S, Westphal H.Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proc Natl Acad Sci U S A 1999; 96: 15002–15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H.The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A 2003; 100: 9005–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A.Sohlh1 is essential for spermatogonial differentiation. Dev Biol 2006; 294: 161–167. [DOI] [PubMed] [Google Scholar]
- Gu W, Tekur S, Reinbold R, Eppig JJ, Choi YC, Zheng JZ, Murray MT, Hecht NB.Mammalian male and female germ cells express a germ cell-specific Y-Box protein, MSY2. Biol Reprod 1998; 59: 1266–1274. [DOI] [PubMed] [Google Scholar]
- Yu J, Hecht NB, Schultz RM.Expression of MSY2 in mouse oocytes and preimplantation embryos. Biol Reprod 2001; 65: 1260–1270. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R.Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet 1999; 21: 123–127. [DOI] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ.Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005; 8: 949–961. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T.The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1998; 1: 707–718. [DOI] [PubMed] [Google Scholar]
- Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC.Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1998; 1: 697–705. [DOI] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB.Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci 1994; 107: 2749–2760. [DOI] [PubMed] [Google Scholar]
- Lammers JH, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, Heyting C.The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol 1994; 14: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Heddle JA, Spyropoulos B, Heng HH.Identical megabase transgenes on mouse chromosomes 3 and 4 do not promote ectopic pairing or synapsis at meiosis. Genome 1997; 40: 770–773. [DOI] [PubMed] [Google Scholar]
- Tarsounas M, Morita T, Pearlman RE, Moens PB.RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol 1999; 147: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD.The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 2000; 6: 975–987. [DOI] [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M.Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A 2005; 102: 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Iwai T, Yokota T, Yamashita M.Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 2003; 116: 2781–2790. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C.Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol 2003; 160: 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC.Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 2003; 262: 303–312. [DOI] [PubMed] [Google Scholar]
- Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A.Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod 2007; 77: 312–319. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A 2002; 99: 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa M, Kirkman NJ, Mayo KE, Mulders SM, Zhou J, Bondy CA, Hsu SY, King GJ, Adashi EY.The mouse germ-cell-specific leucine-rich repeat protein NALP14: a member of the NACHT nucleoside triphosphatase family. Biol Reprod 2005; 72: 879–889. [DOI] [PubMed] [Google Scholar]
- Westerveld GH, Korver CM, van Pelt AM, Leschot NJ, van der Veen F, Repping S, Lombardi MP.Mutations in the testis-specific NALP14 gene in men suffering from spermatogenic failure. Hum Reprod 2006; 21: 3178–3184. [DOI] [PubMed] [Google Scholar]
- Quesada V, Sanchez LM, Alvarez J, Lopez-Otin C.Identification and characterization of human and mouse ovastacin: a novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. J Biol Chem 2004; 279: 26627–26634. [DOI] [PubMed] [Google Scholar]
- Yan C, Pendola FL, Jacob R, Lau AL, Eppig JJ, Matzuk MM.Oosp1 encodes a novel mouse oocyte-secreted protein. Genesis 2001; 31: 105–110. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM.Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 2000; 26: 267–268. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM.Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc Natl Acad Sci U S A 1980; 77: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM.Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci U S A 1996; 93: 5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H, Dean J.Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 1996; 122: 2903–2910. [DOI] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H.The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 2000; 403: 909–913. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN.Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 1992; 47: 466–472. [DOI] [PubMed] [Google Scholar]
- Borum K.Oogenesis in the mouse. A study of the origin of the mature ova. Exp Cell Res 1967; 45: 39–47. [DOI] [PubMed] [Google Scholar]
- McClellan KA, Gosden R, Taketo T.Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol 2003; 258: 334–348. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI.Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol 1997; 184: 122–137. [DOI] [PubMed] [Google Scholar]
- Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, Wynshaw-Boris A.Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 1998; 125: 4007–4017. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell 1996; 85: 1125–1134. [DOI] [PubMed] [Google Scholar]
- Wartenberg H, Ihmer A, Schwarz S, Miething A, Viebahn C.Mitotic arrest of female germ cells during prenatal oogenesis. A colcemid-like, non-apoptotic cell death. Anat Embryol (Berl) 2001; 204: 421–435. [DOI] [PubMed] [Google Scholar]
- Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL.Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 1995; 136: 3665–3668. [DOI] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL.Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 1999; 21: 200–203. [DOI] [PubMed] [Google Scholar]
- Rucker EB, 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L.Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 2000; 14: 1038–1052. [DOI] [PubMed] [Google Scholar]
- Takai Y, Canning J, Perez GI, Pru JK, Schlezinger JJ, Sherr DH, Kolesnick RN, Yuan J, Flavell RA, Korsmeyer SJ, Tilly JL.Bax, caspase-2, and caspase-3 are required for ovarian follicle loss caused by 4-vinylcyclohexene diepoxide exposure of female mice in vivo. Endocrinology 2003; 144: 69–74. [DOI] [PubMed] [Google Scholar]
- Dolci S, Williams DE, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, Boswell HS, Donovan PJ.Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991; 352: 809–811. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo KM, Hogan BL.Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 1990; 347: 667–669. [DOI] [PubMed] [Google Scholar]
- Sakata S, Sakamaki K, Watanabe K, Nakamura N, Toyokuni S, Nishimune Y, Mori C, Yonehara S.Involvement of death receptor Fas in germ cell degeneration in gonads of Kit-deficient Wv/Wv mutant mice. Cell Death Differ 2003; 10: 676–686. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davies H, Sims LP, Levy SE, Dean J.Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol 2007; 7: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.