Abstract
The assembly of the tegument of herpes simplex virus type 1 (HSV-1) is a complex process that involves a number of events at various sites within virus-infected cells. Our studies focused on determining whether tegument proteins, VP1/2 and UL37, are added to capsids located within the nucleus. Capsids were isolated from the nuclear fraction of HSV-1-infected cells and purified by rate-zonal centrifugation to separate B capsids (containing the scaffold proteins and no viral DNA) and C capsids (containing DNA and no scaffold proteins). Western blot analyses of these capsids indicated that VP1/2 associated primarily with C capsids and UL37 associated with B and C capsids. The above results demonstrate that at least two of the tegument proteins of HSV-1 are associated with capsids isolated from the nuclear fraction, and these capsid-tegument protein interactions may represent initial events of the tegumentation process.
Keywords: UL36, UL37, VP1/2, tegument, herpes simplex virus, virus assembly
INTRODUCTION
Virions of herpes simplex virus type 1 (HSV-1) have three morphologically distinct structures: an icosahedral capsid that encloses the genome, a proteinaceous tegument layer surrounding the capsid, and a host-derived lipid envelope containing viral glycoproteins and other membrane proteins (Roizman & A.E. Sears, 2001). Capsid assembly occurs in the nucleus yielding procapsids, as well as A, B, and C capsids, which can be separated in density gradients (Gibson & Roizman, 1972; Newcomb et al., 1996; Perdue et al., 1976). B capsids contain the major capsid protein VP5, triplex proteins VP19C and VP23, the minor capsid protein VP26, viral protease VP24, and the scaffold proteins VP22a and VP21 (Newcomb & Brown, 1991; Roizman & A.E. Sears, 2001). Scaffold proteins are released from the capsid as the viral genome enters through the UL6 portal complex to form the mature C capsid that becomes the nucleocapsid of the mature virion (Newcomb et al., 2001). A capsids are empty and contain no scaffold proteins or viral genome and are considered abortive packaging products (Perdue et al., 1976; Roizman & A.E. Sears, 2001).
Mature capsids exit the nucleus through an envelopment-deenvelopment process. They acquire a temporary envelope by budding into the inner nuclear membrane and subsequently lose this envelope upon fusion with the outer nuclear membrane and translocation into the cytoplasm (reviewed in Mettenleiter, 2002). The UL31 and UL34 gene products rearrange the nuclear lamina and are necessary for the efficient egress of capsids from the nucleus (Chang et al., 1997; Reynolds et al., 2004; Reynolds et al., 2001; Roller et al., 2000 and reviewed in Mettenleiter, 2002).
Although the events of capsid assembly and DNA packaging have been extensively studied, the molecular mechanisms associated with the assembly of the tegument have been described in limited detail. The addition of tegument onto capsids could occur within the nucleus, as the capsid traverses the inner and outer nuclear membranes, within the cytoplasm, and finally as the capsid acquires its envelope from vesicles derived from the trans-Golgi network (Mettenleiter, 2002; Roizman & A.E. Sears, 2001). Immunoelectron microscopy studies have shown the association of the tegument protein VP16 with primary enveloped virions located within the perinuclear space (Naldinho-Souto et al., 2006). Recent studies have suggested that HSV-1 envelopment of capsids may follow several diverse pathways (Leuzinger et al., 2005).
Studies described within this report were focused on determining whether specific tegument proteins are associated with capsids isolated from the nuclear fraction of HSV-1-infected cells. The two tegument proteins included within these studies are VP1/2 and UL37. VP1/2 is a 270 kDa protein encoded by the UL36 gene of HSV-1 (Heine et al., 1974; Honess & Roizman, 1973; Marsden et al., 1978; McNabb & Courtney, 1992a; Morse et al., 1978; Spear & Roizman, 1972). Previous studies have suggested that approximately 150 molecules of VP1/2 are present in each virion and that VP1/2 is tightly associated with capsids (Heine et al., 1974; McNabb & Courtney, 1992b; Roizman & D. Furlong, 1974). VP1/2 is localized to both the cytoplasm and nucleus of virus-infected cells (McNabb & Courtney, 1992b; Morrison et al., 1998). VP1/2 is an essential viral protein with apparent functions during early and late times after infection. The phenotype of a mutant virus (tsB7) containing a temperature-sensitive mutation in the UL36 gene suggests that VP1/2 is involved in the release of the viral genome from capsids during the initial events of virus infection (Batterson et al., 1983). Immunoelectron microscopy studies have suggested that VP1/2 and UL37 remain associated with the capsid until it docks at the nuclear pore (Granzow et al., 2005). Another mutant virus, K UL36, encoding only the amino terminal 361 amino acids residues of the VP1/2 protein, results in an accumulation of DNA-containing capsids in the cytoplasm that lack the major tegument proteins and an envelope (Desai, 2000). Furthermore, studies have shown that the pseudorabies virus VP1/2 homologue is necessary for microtubule-based capsid transport in the cytoplasm (Luxton et al., 2006). In addition, recent studies have shown that the cleaved amino terminal 500 amino acids of VP1/2 has deubiquitinating activity that is conserved among homologues of the herpesvirus family (Kattenhorn et al., 2005; Schlieker et al., 2005).
The 120 kDa protein encoded by the UL37 gene is phosphorylated (Albright & Jenkins, 1993; McLauchlan et al., 1994; Schmitz et al., 1995) and is found in both the cytoplasm and nucleus of virus-infected cells (Schmitz et al., 1995). A null mutant of UL37 (K UL37) results in an accumulation of unenveloped capsids forming aggregates along the inner nuclear membrane and in the cytoplasm suggesting that UL37 plays an essential role in the virion assembly process (Desai et al., 2001). The UL37, as well as the UL36 gene product, are conserved among all subfamilies of Herpesviridae (Mettenleiter, 2002). The pseudorabies virus homologues of the HSV-1 VP1/2 and UL37 proteins have been shown to physically interact (Klupp et al., 2002). Similar findings were recently reported for HSV-1 VP1/2 and UL37 (Vittone et al., 2005).
The accumulation of capsids within or near the nucleus for the UL37-null mutant KΔUL37 suggested the possibility that this tegument protein and its binding partner, VP1/2, are added to capsids in the nucleus. In addition, the strong association of VP1/2 with capsids suggested that VP1/2 may be one of the innermost tegument proteins of the virus. Using cell fractionation and density gradient analyses, we found that VP1/2 and UL37 are associated with capsids purified from the nucleus. Moreover, it appears that VP1/2 associates predominantly with C capsids as compared to B capsids. These results suggest that the addition of VP1/2 and UL37 may represent an initial tegumentation event in the assembly of HSV-1 virions.
RESULTS
Purity of isolated nuclear fractions
Throughout these studies, capsids from the nuclear fraction of virus-infected cells were used; therefore, it was essential to demonstrate that the nuclear fractions were free of components from the cytoplasmic fraction. The nuclear and cytoplasmic fractions from virus-infected cells were harvested at 15 h after infection and analyzed by Western blotting for two marker proteins of the cytoplasm and nucleus, calnexin and lamin B1, respectively (Fig. 1). The blot was probed simultaneously with antibodies to calnexin and lamin B1. Repeated analyses showed that nuclear fractions were free of detectable levels of the endoplasmic reticulum protein, calnexin, and the cytoplasmic fraction did not contain detectable levels of lamin B1.
FIG. 1.
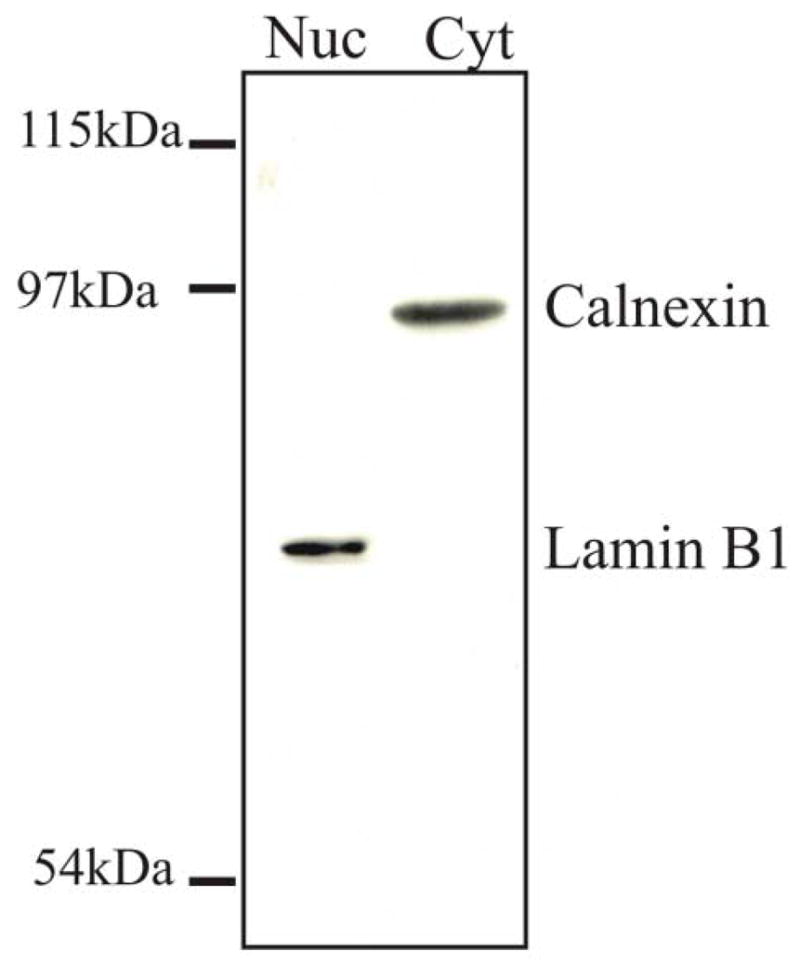
Analysis of the purity of the nuclear fraction obtained from HSV-1-infected cells. Approximately 2 x 106 Vero cells were infected with HSV-1 at a MOI of 10 pfu/cell. At 15 h after infection, the cells were harvested and separated into the cytoplasmic and nuclear fractions as described in the Methods. The proteins of the nuclear and cytoplasmic fractions were resolved by SDS-PAGE followed by Western blot analysis simultaneously using antibodies to the nuclear marker lamin B1 (70 kDa) and the endoplasmic reticulum marker calnexin (90 kDa) to assay the same nitrocellulose blot.
Purity of intranuclear capsids
Since the focus of this study is on capsids isolated from the nuclear fraction, it was important to demonstrate that this capsid preparation was free of capsids associated with the cytoplasmic fraction. Vero cells were infected with HSV-1 KOS, harvested at 15 hr pi and the cells were resuspended in 1.0% NP40 lysis buffer. This cell preparation was then mixed with the cytoplasmic fraction from HSV-1 K26GFP virus-infected cells harvested at 15 hr pi. All capsids within the K26GFP cytoplasmic fraction contained the VP26-GFP chimeric protein. Our goal was to determine if capsids obtained from the purified nuclear fraction of the KOS infected cells were cross-contaminated with the VP26-GFP capsids from the K26GFP cytoplasmic fraction. After a 30-minute incubation on ice in 1.0% NP40 lysis buffer, the nuclei were purified following the protocol described in the Methods. Capsids from the purified nuclear fraction were resolved by rate-zonal centrifugation as described in the Methods. Fractions from the density gradient were analyzed by SDS-PAGE and Western blotting with antisera to GFP and VP5 (Fig. 2). As shown in Fig. 2A, VP5 was detected in fractions 11–15 of the gradient, indicating the location of capsids within the gradient. However, no GFP-containing capsids were detected in the gradient fractions indicating that the cytoplasmic GFP-containing capsids were effectively removed during the purification of the nuclear fraction. As a positive control, the VP26-GFP capsids within the cytoplasmic fraction of K26GFP infected cells were also resolved on another gradient and GFP-containing capsids were readily detected in fractions 11–15 by Western blotting (Fig. 2B). The amount of the cytoplasmic fraction used to obtain the VP26-GFP capsids loaded onto the gradient in Fig. 2B was approximately 1/5 the amount of lysate that was mixed with the KOS infected cells prior to the purification of the nuclear fraction. In both preparations, the major capsid protein VP5 was detected in fractions 11–15. As will be presented below, it is within these fractions that most of the VP1/2 or UL37 is detected with capsids isolated from the nuclear fraction. The results from this experiment suggest that the nuclear isolation protocol used in this study yields a population of nuclear capsids that is not contaminated with capsids from the cytoplasmic fraction.
FIG. 2.
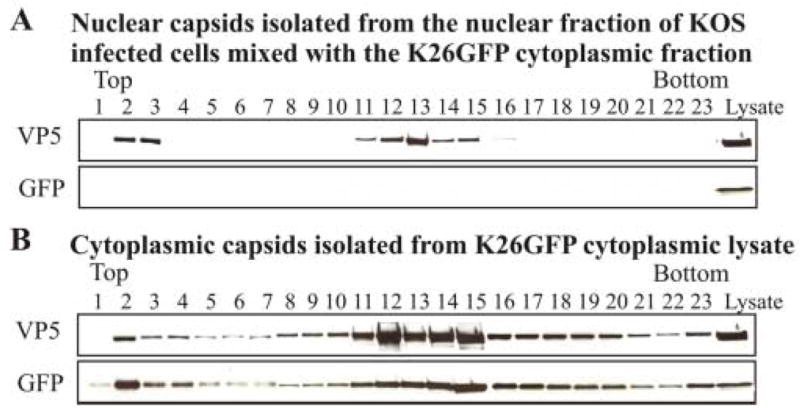
Analysis of the purity of the nuclear capsid preparation. (A) Approximately 1.5 x 108 HSV-1 KOS infected Vero cells were harvested at 15 h pi, resuspended in 1% NP40 lysis buffer and then mixed with the cytoplasmic fraction from approximately 1.3 x 108 Vero cells infected with HSV-1 K26GFP virus. This mixture was incubated on ice for 30 minutes and the nuclear fraction was then purified as described in the Methods. Capsids from the nuclear fraction were purified and separated on a 20–50% linear sucrose gradient following rate-zonal centrifugation. 0.5 ml fractions were collected from the bottom of the tube. The proteins within each fraction were precipitated with 10% TCA, separated by SDS-PAGE followed by Western blot analysis. The blot was probed sequentially with antibodies to GFP and the major capsid protein VP5. K26GFP lysate (far right) shown in all panels served as a positive control for the Western blots. (B) Western blot analysis of capsids isolated from the cytoplasmic fraction from approximately 2.6 x 107 K26GFP infected Vero cells purified and analyzed as described above. K26GFP lysate is shown in the far right lanes as a control.
Tegument proteins VP1/2 and UL37 associate with capsids isolated from the nuclear fraction
At 15 h after infection, HSV-1-infected Vero cells were fractionated into cytoplasmic and nuclear fractions. Capsids from the nuclear fraction were loaded onto a 20–50% (wt/wt) linear sucrose gradient and resolved by rate-zonal centrifugation. Fractions were collected, precipitated with 10% trichloroacetic acid, and analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting. Blots were reacted with five different antibodies (Fig. 3). To identify where capsids were located within the gradient, an antibody specific for the major capsid polypeptide, VP5, was used. Western blotting with anti-VP5 serum showed that most capsids were in fractions 11–15. Antibodies specific for VP1/2 and UL37 showed that these two tegument proteins were also present in the capsid-containing fractions. Scaffold proteins VP21 and VP22a are associated with B capsids and not with DNA-containing C capsids (Roizman & A.E. Sears, 2001). When the blot was reacted with an antibody specific for scaffold proteins, the major reactivity was seen in fraction 12 with fractions 11 and 13 also showing the presence of some scaffold protein. In contrast, most of the VP1/2 reactivity was detected in fraction 14 that contains high amounts of VP5 but little to no scaffold proteins. When the blot was reacted with a monoclonal antibody specific for the HSV-1 nonstructural DNA-binding protein UL42 (Gallo et al., 1988), no reactivity was detected in fractions containing VP5. The results presented above suggest that tegument proteins VP1/2 and UL37 are associated with capsids isolated from the nuclear fraction of virus-infected cells.
FIG. 3.
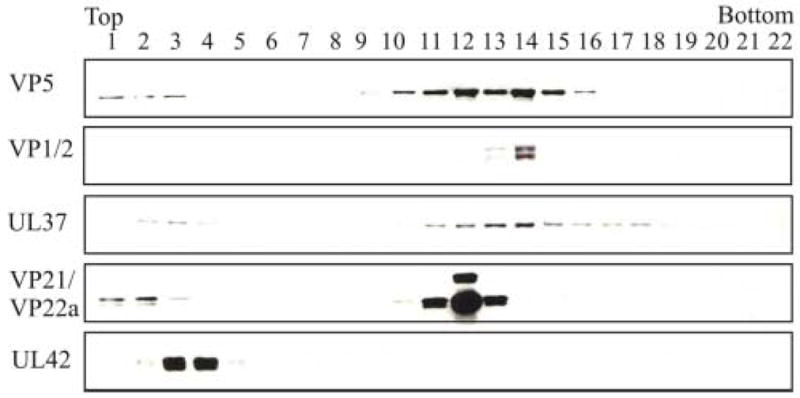
Rate-zonal centrifugation of HSV-1 capsids isolated from the nuclear fraction and analyzed for the presence of tegument proteins. Capsids were obtained from the nuclear fraction of HSV-1-infected Vero cells harvested at 15 h after infection and separated on a 20–50% linear sucrose gradient following rate-zonal centrifugation. 0.5 ml fractions were collected from the bottom of the tube. The proteins within each fraction were precipitated with 10% TCA, separated by SDS-PAGE followed by Western blot analysis using primary antibodies to specific tegument proteins followed by the addition of the secondary anti-rabbit or anti-mouse antibody conjugated to horseradish peroxidase. Western blot analysis was done using antibodies to capsid proteins VP5 (the major capsid polypeptide) and VP21/VP22a (scaffold proteins), tegument proteins VP1/2 and UL37, and the DNA binding protein UL42.
As a control for the specific binding of selected tegument proteins to capsids isolated from the nucleus, two additional tegument proteins were assayed, VP22 and UL11. VP22 is one of the most abundant tegument proteins of purified HSV-1 virions (Heine et al., 1974). Depending on the time after infection, VP22 is localized within the cytoplasm as well as the nucleus of virus-infected cells (Morrison et al., 1998; Pomeranz & Blaho, 1999). UL11 is a myristoylated tegument protein that is localized primarily to the Golgi region (Bowzard et al., 2000; MacLean et al., 1989). Capsids from the nuclear fraction were isolated, purified and assayed by Western blotting as described above. Antibody to VP5 was used to identify the location of the capsids resolved by rate-zonal centrifugation. In addition, antibody to the tegument protein UL37 was included as a positive control. As shown in Fig. 4, density gradient fractions demonstrating reactivity with antisera to VP5 and UL37 were similar to that shown previously in Fig. 3. However, when the blot was reacted with antisera specific for either VP22 or UL11, no reactivity was detected. Western blot analysis of purified extracellular virions showed strong reactivity with antisera to VP22 and UL11 (data not shown). These results further support the specificity of the detection of tegument proteins VP1/2 and UL37 with capsids isolated from the nuclear fraction of HSV-1-infected cells.
FIG. 4.
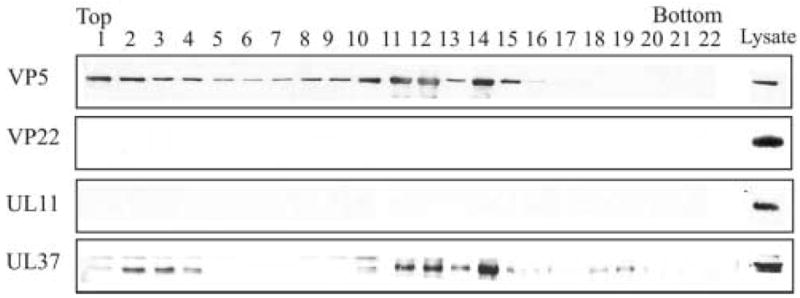
Rate-zonal centrifugation of HSV-1 capsids isolated from the nuclear fraction and analyzed for the presence of tegument proteins VP22 and UL11. At 15 h after infection the capsids from the nuclear fraction were obtained, separated by rate-zonal centrifugation as described in the legend to Fig. 2. The same blot was probed sequentially using antibodies specific for the major capsid protein VP5 and tegument proteins VP22, UL11, and UL37 (positive control).
The detection of VP1/2 and UL37 in density gradients is capsid dependent
To ascertain whether the co-sedimentation of VP1/2 and UL37 with VP5 was due to their association with capsids, the analysis was done in the absence of capsid formation. A mutant virus (K23Z) defective in the synthesis of the VP23 triplex protein was kindly provided by Dr. Prashant Desai. When this mutant infects Vero cells, no capsids form due to the absence of the UL18 gene, which encodes the VP23 triplex protein (Desai et al., 1993). Vero cells and the VP23 complementing cell line (C32) were infected with the K23Z virus and harvested at 15 h after infection. Prior to cell fractionation, a sample of the total cell lysate from K23Z virus-infected Vero and complementing cells (C32) was collected and assayed by SDS-PAGE and Western blotting for the presence of VP5, VP16 and UL37 (Fig. 5). In both cases, expression of VP5, VP16 and UL37 was readily detected by immunoblotting. These same cell lysates were fractionated, and capsids obtained from the nuclear fraction were resolved on a density gradient, precipitated with TCA, and analyzed by SDS-PAGE and Western blotting (Fig. 6). As expected, capsids from the complementing C32 cells exhibited VP5, VP1/2, and UL37 in similar fractions as seen in other gradients presented above. Most of the detectable VP1/2 was in fraction 13, the same fraction where there was a strong reactivity to anti-VP5. The detection of UL37 was also seen in similar fractions as described for other gradients. In contrast, neither VP5, VP1/2, nor UL37 was detectable in the region of the gradient where capsids normally sediment when the nuclear fraction from the nonpermissive Vero cells was analyzed. Although VP5, VP1/2 and UL37 are present in the nucleus in the absence of capsid formation, they are not readily detected in the linear sucrose gradient in the absence of capsid formation. When not associated with capsids, these proteins do not effectively pellet through the 35% (wt/wt) sucrose cushion purification step that precedes the linear sucrose gradient analysis. In some experiments, small but detectable amounts of UL37 were observed in fractions toward the bottom of the gradient suggesting the formation of non-capsid associated complexes. Overall, these results further support that VP1/2 and UL37 sediment in a capsid-dependent manner due to their association with intranuclear capsids.
FIG. 5.
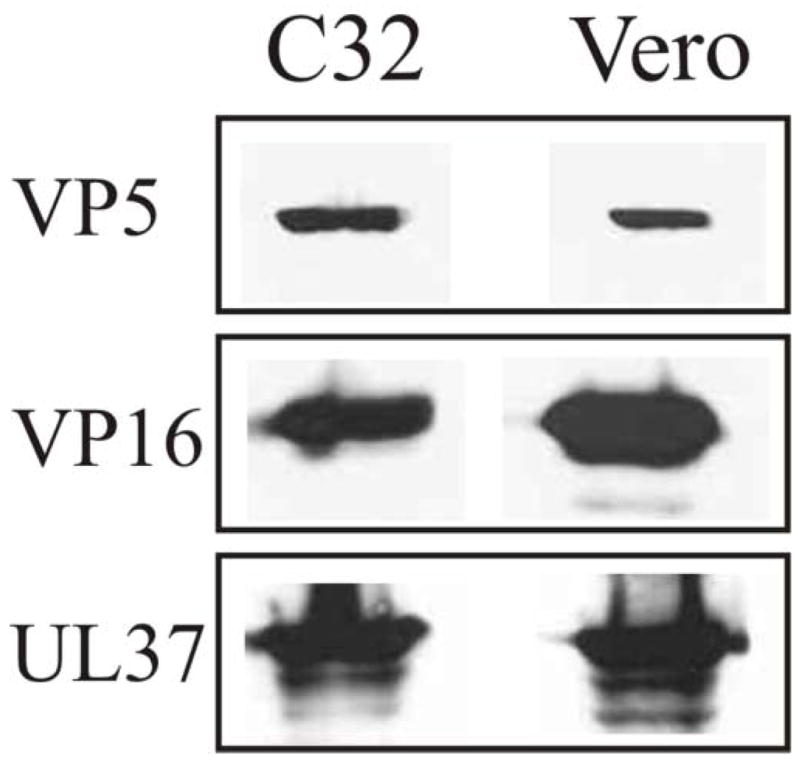
SDS-PAGE analysis of the cell lysates of Vero (VP23 non-complementing) and C32 (VP23 complementing) cells infected with the HSV-1 VP23-negative mutant virus, K23Z. The two cell lines were infected with the K23Z virus at a MOI of 10 pfu per cell and harvested at 15 h after infection. The whole cell lysates were resolved by SDS-PAGE followed by Western blot analysis. The same blot was probed sequentially with antibodies to VP5, VP16, and UL37.
FIG. 6.
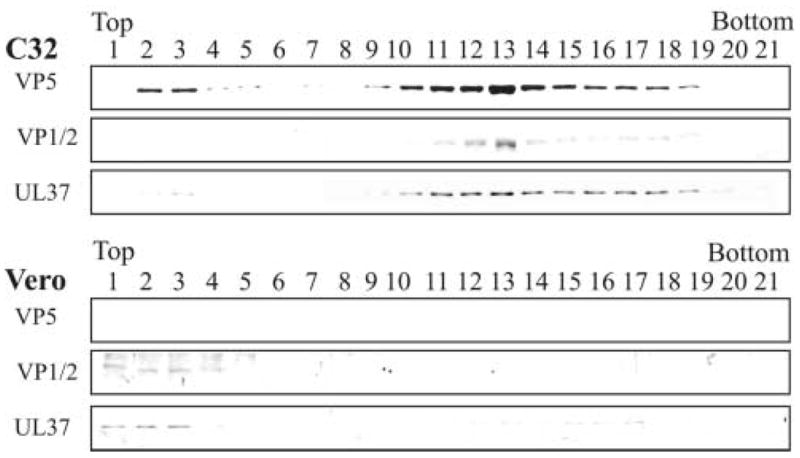
Rate-zonal centrifugation of capsids isolated from cells infected with the VP23-negative mutant (K23Z) and analysis for the presence of tegument proteins VP1/2 and UL37. Vero (non-complementing cell line) and C32 (complementing cell line expressing VP23) cells were infected with the VP23-negative mutant virus, K23Z, at a MOI of 10 pfu/cell. At 15 h after infection the capsids from the nuclear fraction were obtained, separated by rate-zonal centrifugation, analyzed by SDS-PAGE followed by Western blot analysis as described in the legend to Fig. 2. The same blot was probed sequentially for the presence of VP5 and tegument proteins VP1/2 and UL37.
VP1/2 associates primarily with C capsids
The results shown in Figure 3 suggest that the sucrose gradient resolved capsids into two populations. Based on the reactivity of the fractions with the anti-scaffold antibody, it appeared that the slower sedimenting capsid population contained a preponderance of B capsids. However, VP1/2 was predominantly found with the faster sedimenting capsid population, a fraction that showed no detectable reactivity with antiserum specific for scaffold proteins and presumably contains mostly C capsids. To confirm that the faster migrating band of capsids was indeed composed of DNA-containing C capsids, Vero cells were infected with wild-type HSV-1 and labeled with 3H-thymidine from 3–15 h after infection. These cells were harvested, fractionated into cytoplasmic and nuclear fractions, and capsids isolated from the nuclear fraction were resolved by rate-zonal centrifugation as described above. Each fraction was analyzed for 3H-thymidine, precipitated with TCA, and analyzed by SDS-PAGE and western blotting (Fig. 7). A peak of 3H-thymidine counts was detected in fraction 13 and immunoblotting showed the presence of strong reactivity for the anti-VP5 and anti-VP1/2 sera within this fraction. In contrast, when these same fractions were reacted with the anti-scaffold antibody, essentially all reactivity was detected in fraction 11. These data further confirm that density gradients were able to resolve populations of B capsids and C capsids, and that the majority of the detectable VP1/2 is associated with C capsids.
FIG. 7.
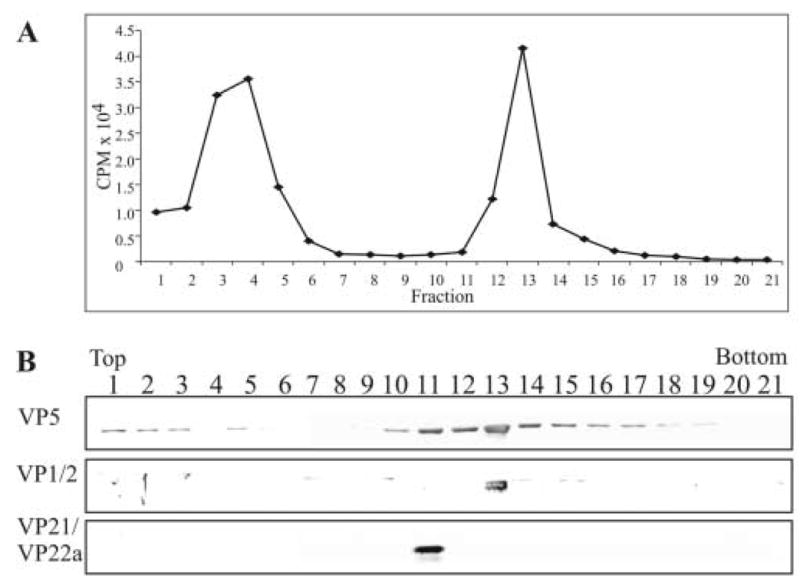
Rate-zonal centrifugation of 3H-thymidine-labeled capsids and analysis for the presence of the tegument protein VP1/2. Capsids were obtained from the nuclear fraction of HSV-1-infected Vero cells that were labeled with 10 μCi per ml of 3H-thymidine from 3 to 15 h after infection. The capsids were separated by rate-zonal centrifugation as described in the legend to Fig. 2. Prior to TCA precipitation of the proteins within each of the fractions, 50 μl was removed and 3H-thymidine cpm in each fraction were determined by liquid scintillation analysis (panel A). Following TCA precipitation, the same fractions were resolved by SDS-PAGE followed by Western blot analysis (panel B). The same blot was sequentially probed with antibodies to the tegument protein VP1/2 and capsid proteins VP5 and VP21/VP22a (scaffold proteins) as described in the legend to Fig. 2.
DISCUSSION
To our knowledge, the results within this report are the first to show that specific tegument proteins are added to capsids prior to their exit from the nucleus. This is consistent with the tight interaction of VP1/2 with virion capsids (McNabb & Courtney, 1992b; Roizman & D. Furlong, 1974). In fact, Zhou et al. suggested that VP1/2 may be an inner tegument protein associated with pentons of the capsid based on the interpretation of computer generated models from cryoelectron micrographs of HSV-1 virus particles (Zhou et al., 1999). Recent observations by Desai using a VP1/2 mutant virus, K UL36, showed that capsids synthesized in cells lacking the expression of VP1/2 were located within the cytoplasm; however, they lacked any of the major tegument proteins and did not progress to the envelopment stage of virus assembly (Desai, 2000). The rationale for analysis of the presence of UL37 on capsids within the nuclear fraction was supported by several observations. First, it was recently shown that the UL36 and UL37 gene products of pseudorabies virus, as well as HSV-1, physically interact (Klupp et al., 2002; Vittone et al., 2005). Second, other studies have shown that viruses not expressing UL37 were not enveloped and formed aggregates in the nucleus and cytoplasm (Desai et al., 2001). From the above observations, it appears that VP1/2 and UL37 may associate with capsids before subsequent tegumentation and envelopment events can occur. Thus it is possible that these two proteins are components of the “inner” tegument region and may serve as a platform for the addition of other tegument proteins to the capsid.
The UL36 and UL37 gene products are conserved among all members of the herpesvirus subfamilies (Mettenleiter, 2002), which may indicate their key role in the assembly process. In addition, the studies of Desai (Desai et al., 2001; Desai, 2000) suggest that these two proteins may be important for targeting the capsid to the proper maturation pathway for the addition of other tegument proteins and envelopment. Whether the addition of VP1/2 and UL37 to assembled capsids only occurs within the nucleus cannot be determined from the studies described within this report and is an aspect currently being addressed. Yeast two-hybrid analyses have shown the interaction of VP1/2 with the tegument proteins VP16 and UL37, and also demonstrated the self-association of UL37 (Vittone et al., 2005). Further understanding of these interactions may help to elucidate the composition of the “inner tegument” region. Interestingly, the Kaposi’s sarcoma-associated herpesvirus homologue of VP1/2, ORF64, is proposed to bind to the capsid at its N-terminus and attach to the viral envelope at its C-terminus (Zhu et al., 2005).
An unexpected finding from our studies was that VP1/2 is predominantly associated with C capsids as compared to B capsids. Although major differences in conformation and protein content of B vs. C capsids have not been suggested, subtle differences have been reported (Beard et al., 2004; Goshima et al., 2000; Ogasawara et al., 2001; Sheaffer et al., 2001; Thurlow et al., 2005; Zhou et al., 1999). For example, studies have shown that three- to four-fold greater amounts of UL25 are associated with C capsids as compared to B capsids, suggesting that UL25 may function to seal the capsid portal after DNA packaging (Ogasawara et al., 2001; Sheaffer et al., 2001). Cryoelectron microscopic observations suggest that the channel within pentons of B capsids appears open, in contrast the pentagonal channel in C capsids appears closed (Zhou et al., 1999). The selective binding of VP1/2 to C capsids suggests that either the conformation or the selective presence of other capsid or tegument proteins enhances the opportunity for the binding of VP1/2. Currently, our results do not demonstrate if the binding of UL37 is preferential to C or B capsids. Studies are currently in progress to determine if there is a sequential order for the binding of VP1/2 and UL37 to capsids.
MATERIALS AND METHODS
Cells and viruses
African green monkey kidney (Vero) cells (ATCC CCL-81) were grown in Dulbecco’s modified Eagles medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 2.25% sodium bicarbonate, 25mM HEPES buffer, glutamine (300 μg/ml), penicillin (100 μg/ml) and streptomycin (131 μg/ml) as described previously (O’regan et al., 2006). Cells were infected in the same medium except the serum concentration was reduced to 2.0 %. HSV-1 KOS strain was used as the wild-type virus (SMITH, 1964). An HSV-1 mutant virus (designated K23Z) with a defect in the synthesis of one of the capsid triplex proteins, VP23, and the complementing cell line (designated C32) that expresses this protein, were generously provided by Prashant Desai (Johns Hopkins University) (Desai et al., 1993; Person & Desai, 1998). The C32 complementing cell line was grown as described above, with the addition of 1.0 mg/ml G148 (Geneticin; Calbiochem, San Diego, CA) (Person & Desai, 1998).
Antibodies
Rabbit polyclonal antibodies to VP1/2 (McNabb & Courtney, 1992b), VP5 (McNabb, unpublished data), VP22 (Loomis et al., 2003) and UL11 (Loomis et al., 2003) have been described previously. Rabbit polyclonal antibody to GFP was generated against His-GFP antigen. Rabbit polyclonal antibody to VP16 was purchased from Clontech (Clontech, Mountain View, CA). Mouse monoclonal antibodies were used to detect the HSV-1 DNA-binding protein encoded by the UL42 gene (Martin et al., 1988) and the UL26 gene products (scaffold proteins) (MCA406; Serotec, Washington, DC) (Newcomb et al., 1996). A rabbit polyclonal antibody (designated 780) to the UL37 gene product was generously provided by Frank Jenkins (University of Pittsburgh) (Schmitz et al., 1995). Goat polyclonal antibodies to calnexin and lamin B1 (Santa Cruz Biotechnology, Santa Cruz, CA) were used to assess the purity of nuclear fractions.
Capsid isolation
Approximately 1.5 x 108 Vero cells grown in 18 100 mm plates were infected with HSV-1 at a multiplicity of infection (MOI) of 10 pfu/cell. At 15 h after infection, cells were harvested by scraping, pelleted using low speed centrifugation (225 x g, 10 minutes at 4°C) and washed in phosphate-buffered saline (PBS). Cells were disrupted in NP40 lysis buffer (approximately 9 x 106 cells/ml) [0.15 M NaCl, 0.01 M Tris-HCl (pH 7.2), 0.002 M MgCl2, 1.0% NP-40 (IGEPAL CA-630; Sigma, St. Louis, MO), 0.005 M DL-dithiothreitol, and 0.01% Protease Inhibitor Cocktail (Sigma)] (Gibson & Roizman, 1972; McNabb & Courtney, 1992c). The nuclei were pelleted using low speed centrifugation (225 x g, 7 minutes at 4°C), and the supernatant was considered the cytoplasmic fraction. The nuclear pellet was washed in NP40 lysis buffer and transferred to a clean conical tube to reduce contaminating cytoplasmic debris that might be stuck to the sides of the tubes. Capsids from the nuclear fraction were isolated as previously described with slight modifications (Sheaffer et al., 2000). The nuclear pellet was resuspended in NP40 lysis buffer, lysed by three cycles of freeze/thaw and then sonicated for three one-minute pulses. Cell debris was cleared by centrifugation at 8,000 rpm for 30 minutes at 4°C in an SW41 rotor. The supernatant was treated with 100 U DNase at 37°C for 10 minutes and the capsids were pelleted through a 35% (wt/wt) sucrose cushion [prepared in TNE (500 mM NaCl, 1 mM EDTA, 20 mM Tris pH 7.6)] in a SW41 rotor centrifuged at 24,500 rpm for 1 hour at 4°C. The pellet was resuspended in TNE and sonicated three times at 10 seconds each. The sample was layered onto a 20–50% (wt/wt) linear sucrose gradient (prepared in TNE) and centrifuged at 24,500 rpm for 1 h at 4°C in an SW41 rotor. After centrifugation, 0.5 ml fractions were collected from the bottom of the tube. Trichloroacetic acid (TCA) was added to each fraction for a final concentration of 10% TCA and incubated at 4°C for a minimum of one hour. Precipitated proteins were pelleted by centrifugation (14,000 x g for 15 minutes at room temperature), washed in 100% ethanol, dried by centrifugation in a heat-vac for 5 minutes, resuspended in 2X sample buffer [119 mM Tris-HCl pH 6.8, 19% glycerol, 0.05% bromophenol blue, 3.8% SDS, 9.5% β-ME, 0.5 M urea], boiled for 5 minutes, and electrophoretically separated on a SDS-polyacrylamide gel (PAGE). After transfer to nitrocellulose membranes, proteins were detected by Western blot analysis with primary antibodies to specific HSV-1 proteins, secondary antibodies conjugated to horseradish peroxidase (Sigma), ECL reagents (Amersham Biosciences, Piscataway, NJ), chemiluminescence autoradiography and Kodak BioMax XAR film. For sequential probing of the same nitrocellulose blot, membranes were stripped in 60 mM Tris-HCl pH 8.0, 2% SDS and 0.75% β-ME at 56°C for 37 minutes to remove bound antibodies.
Radiolabeling viral DNA
To radiolabel the viral DNA associated with C capsids, approximately 1.5 x 108 Vero cells grown in 18 100 mm plates were infected with HSV-1 at a MOI of 10 pfu/cell. At 3 h after infection, [methyl-3H]thymidine (specific activity of 79 Ci/mmol, 1.0 mCi/ml) (Perkin Elmer, Boston, MA) was added to 5 plates at a concentration of 10 μCi per ml. At 15 h after infection, infected cells were harvested and intranuclear capsids were purified on a linear sucrose gradient as described above. From each of the collected fractions, 50 μl was removed and assayed for 3H-thymidine activity by liquid scintillation analysis. The remainder of the 0.5 ml fractions was TCA precipitated, resolved on an SDS-PAGE gel and Western blotted for the presence of viral proteins.
Acknowledgments
We thank Frank Jenkins (University of Pittsburgh) for providing the antiserum to UL37 and Prashant Desai (Johns Hopkins University) for supplying the K23Z and K26GFP viruses and C32 cell line. We are appreciative of helpful discussions with Sandra Weller and April Burch (University of Connecticut) regarding the purification of capsids.
This work was supported by National Institutes of Health grant CA42460.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright AG, Jenkins FJ. The herpes simplex virus UL37 protein is phosphorylated in infected cells. J Virol. 1993;67 (8):4842–4847. doi: 10.1128/jvi.67.8.4842-4847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45 (1):397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard PM, Duffy C, Baines JD. Quantification of the DNA cleavage and packaging proteins U(L)15 and U(L)28 in A and B capsids of herpes simplex virus type 1. J Virol. 2004;78 (3):1367–1374. doi: 10.1128/JVI.78.3.1367-1374.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowzard JB, Visalli RJ, Wilson CB, Loomis JS, Callahan EM, Courtney RJ, Wills JW. Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera. J Virol. 2000;74 (18):8692–8699. doi: 10.1128/jvi.74.18.8692-8699.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YE, Van Sant C, Krug PW, Sears AE, Roizman B. The null mutant of the U(L)31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71 (11):8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, DeLuca NA, Glorioso JC, Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993;67 (3):1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, Sexton GL, McCaffery JM, Person S. A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation. J Virol. 2001;75 (21):10259–10271. doi: 10.1128/JVI.75.21.10259-10271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PJ. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J Virol. 2000;74 (24):11608–11618. doi: 10.1128/jvi.74.24.11608-11618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo ML, Jackwood DH, Murphy M, Marsden HS, Parris DS. Purification of the herpes simplex virus type 1 65-kilodalton DNA-binding protein: properties of the protein and evidence of its association with the virus-encoded DNA polymerase. J Virol. 1988;62 (8):2874–2883. doi: 10.1128/jvi.62.8.2874-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Roizman B. Proteins specified by herpes simplex virus. 8 Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10 (5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima F, Watanabe D, Takakuwa H, Wada K, Daikoku T, Yamada M, Nishiyama Y. Herpes simplex virus UL17 protein is associated with B capsids and colocalizes with ICP35 and VP5 in infected cells. Arch Virol. 2000;145 (2):417–426. doi: 10.1007/s007050050033. [DOI] [PubMed] [Google Scholar]
- Granzow H, Klupp BG, Mettenleiter TC. Entry of pseudorabies virus: an immunogold-labeling study. J Virol. 2005;79 (5):3200–3205. doi: 10.1128/JVI.79.5.3200-3205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine JW, Honess RW, Cassai E, Roizman B. Proteins specified by herpes simplex virus. XII The virion polypeptides of type 1 strains. J Virol. 1974;14 (3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Proteins specified by herpes simplex virus. XI Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973;12 (6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell. 2005;19(4):547–557. doi: 10.1016/j.molcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Klupp BG, Fuchs W, Granzow H, Nixdorf R, Mettenleiter TC. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J Virol. 2002;76 (6):3065–3071. doi: 10.1128/JVI.76.6.3065-3071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger H, Ziegler U, Schraner EM, Fraefel C, Glauser DL, Heid I, Ackermann M, Mueller M, Wild P. Herpes simplex virus 1 envelopment follows two diverse pathways. J Virol. 2005;79 (20):13047–13059. doi: 10.1128/JVI.79.20.13047-13059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis JS, Courtney RJ, Wills JW. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J Virol. 2003;77 (21):11417–11424. doi: 10.1128/JVI.77.21.11417-11424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton GW, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J Virol. 2006;80 (1):201–209. doi: 10.1128/JVI.80.1.201-209.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean CA, Clark B, McGeoch DJ. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J Gen Virol. 1989;70 (Pt 12):3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- Marsden HS, Stow ND, Preston VG, Timbury MC, Wilkie NM. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978;28 (2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Courtney RJ, Fowler G, Rouse BT. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988;62 (7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J, Liefkens K, Stow ND. The herpes simplex virus type 1 UL37 gene product is a component of virus particles. J Gen Virol. 1994;75 (Pt 8):2047–2052. doi: 10.1099/0022-1317-75-8-2047. [DOI] [PubMed] [Google Scholar]
- McNabb DS, Courtney RJ. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J Virol. 1992a;66 (12):7581–7584. doi: 10.1128/jvi.66.12.7581-7584.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb DS, Courtney RJ. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology. 1992b;190 (1):221–232. doi: 10.1016/0042-6822(92)91208-c. [DOI] [PubMed] [Google Scholar]
- McNabb DS, Courtney RJ. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992c;66 (5):2653–2663. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter TC. Herpesvirus assembly and egress. J Virol. 2002;76 (4):1537–1547. doi: 10.1128/JVI.76.4.1537-1547.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Stevenson AJ, Wang YF, Meredith DM. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J Gen Virol. 1998;79 (Pt 10):2517–2528. doi: 10.1099/0022-1317-79-10-2517. [DOI] [PubMed] [Google Scholar]
- Morse LS, Pereira L, Roizman B, Schaffer PA. Anatomy of herpes simplex virus (HSV) DNA. X Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978;26 (2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldinho-Souto R, Browne H, Minson T. Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J Virol. 2006;80 (5):2582–2584. doi: 10.1128/JVI.80.5.2582-2584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Brown JC. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991;65 (2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Thomsen DR, Booy FP, Trus BL, Steven AC, Spencer JV, Brown JC. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263 (3):432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J Virol. 2001;75 (22):10923–10932. doi: 10.1128/JVI.75.22.10923-10932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’regan KJ, Bucks MA, Murphy MA, Wills JW, Courtney RJ. A conserved region of the herpes simplex virus type 1 tegument protein VP22 facilitates interaction with the cytoplasmic tail of glycoprotein E (gE) Virology. 2006 doi: 10.1016/j.virol.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Suzutani T, Yoshida I, Azuma M. Role of the UL25 gene product in packaging DNA into the herpes simplex virus capsid: location of UL25 product in the capsid and demonstration that it binds DNA. J Virol. 2001;75 (3):1427–1436. doi: 10.1128/JVI.75.3.1427-1436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue ML, Cohen JC, Randall CC, O’Callaghan DJ. Biochemical studies of the maturation of herpesvirus nucleocapsid species. Virology. 1976;74(1) UNKNOWN. [PubMed] [Google Scholar]
- Person S, Desai P. Capsids are formed in a mutant virus blocked at the maturation site of the UL26 and UL26.5 open reading frames of herpes simplex virus type 1 but are not formed in a null mutant of UL38 (VP19C) Virology. 1998;242 (1):193–203. doi: 10.1006/viro.1997.9005. [DOI] [PubMed] [Google Scholar]
- Pomeranz LE, Blaho JA. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol. 1999;73 (8):6769–6781. doi: 10.1128/jvi.73.8.6769-6781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Liang L, Baines JD. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J Virol. 2004;78 (11):5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol. 2001;75 (18):8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Sears AE. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven; Philadelphia, Pa.: 2001. pp. 2399–2459. [Google Scholar]
- Roizman B, Furlong D. The replication of herpesviruses. In: Fraenkel-Conrat H, RWagner R, editors. Comprehensive Virology. Plenum Press; New York.: 1974. pp. 229–403. [Google Scholar]
- Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol. 2000;74 (1):117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieker C, Korbel GA, Kattenhorn LM, Ploegh HL. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J Virol. 2005;79 (24):15582–15585. doi: 10.1128/JVI.79.24.15582-15585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JB, Albright AG, Kinchington PR, Jenkins FJ. The UL37 protein of herpes simplex virus type 1 is associated with the tegument of purified virions. Virology. 1995;206 (2):1055–1065. doi: 10.1006/viro.1995.1028. [DOI] [PubMed] [Google Scholar]
- Sheaffer AK, Newcomb WW, Brown JC, Gao M, Weller SK, Tenney DJ. Evidence for controlled incorporation of herpes simplex virus type 1 UL26 protease into capsids. J Virol. 2000;74 (15):6838–6848. doi: 10.1128/jvi.74.15.6838-6848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer AK, Newcomb WW, Gao M, Yu D, Weller SK, Brown JC, Tenney DJ. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J Virol. 2001;75 (2):687–698. doi: 10.1128/JVI.75.2.687-698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH KO. Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med. 1964;115:814–816. doi: 10.3181/00379727-115-29045. 814-6. [DOI] [PubMed] [Google Scholar]
- Spear PG, Roizman B. Proteins specified by herpes simplex virus. V Purification and structural proteins of the herpesvirion. J Virol. 1972;9 (1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow JK, Rixon FJ, Murphy M, Targett-Adams P, Hughes M, Preston VG. The herpes simplex virus type 1 DNA packaging protein UL17 is a virion protein that is present in both the capsid and the tegument compartments. J Virol. 2005;79 (1):150–158. doi: 10.1128/JVI.79.1.150-158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ. Determination of interactions between tegument proteins of herpes simplex virus type 1. J Virol. 2005;79 (15):9566–9571. doi: 10.1128/JVI.79.15.9566-9571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Chen DH, Jakana J, Rixon FJ, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73 (4):3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79 (2):800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


