Abstract
How biological diversity is generated and maintained is a fundamental question in ecology. Ecologists have delineated many mechanisms that can, in principle, favor species coexistence and hence maintain biodiversity. Most such coexistence mechanisms require or imply tradeoffs between different aspects of species performance. However, it remains unknown whether simple functional tradeoffs underlie coexistence mechanisms in diverse natural systems. We show that functional tradeoffs explain species differences in long-term population dynamics that are associated with recovery from low density (and hence coexistence) for a community of winter annual plants in the Sonoran Desert. We develop a new general framework for quantifying the magnitude of coexistence via the storage effect and use this framework to assess the strength of the storage effect in the winter annual community. We then combine a 25-year record of vital rates with morphological and physiological measurements to identify functional differences between species in the growth and reproductive phase of the life cycle that promote storage-effect coexistence. Separation of species along a tradeoff between growth capacity and low-resource tolerance corresponds to differences in demographic responses to environmental variation across years. Growing season precipitation is one critical environmental variable underlying the demographic decoupling of species. These results demonstrate how partially decoupled population dynamics that promote local biodiversity are associated with physiological differences in resource uptake and allocation between species. These results for a relatively simple system demonstrate how long-term community dynamics relate to functional biology, a linkage scientists have long sought for more complex systems.
Keywords: biodiversity, coexistence mechanism, functional trait, population dynamics, specific leaf area
How competing species stably coexist is a long-standing ecological problem. All niche-based mechanisms for stable coexistence rely on ecological differences that enable each species to recover when perturbed to low density and thus remain in the community. Some of these coexistence mechanisms, such as differential exploitation of multiple limiting resources (1, 2) and frequency-dependent predation (3), operate independently of fluctuations in the environment. Other stable coexistence mechanisms depend critically upon environmental fluctuations that allow species to recover from low density (4). These include competition/colonization tradeoffs in a disturbance matrix (5, 6), relative nonlinearity of competition (7) and the storage effect (8, 9). The storage effect combines species-specific responses to the environment and population-dynamic buffering by persistent life history stages in a way that results in a positive average low-density growth rate for each species. It is perhaps the dominant fluctuation-dependent mechanism for organisms in variable environments. Its role has been explored for diverse groups ranging from freshwater zooplankton (10) and coral reef fishes (8) to desert annuals (11), prairie grasses (12) and tropical trees (13), but in no case is the mechanism underlying species-specific responses to the environment well-understood. Specifically, temporal environmental variation increases coexistence through the storage effect when (i) demographic decoupling of species arises from partially uncorrelated responses to environmental variation, (ii) the strength of competition covaries with environmental conditions, and (iii) certain life history traits, such as seed banks or long-lived adults, limit the impact of competition in unfavorable environments (4, 9). Here, we address the critical challenges of identifying the functional differences between species that create demographic decoupling and quantifying their relationship to species coexistence (14).
The winter annual species of the Sonoran Desert have played an important role in the development and testing of general concepts about fluctuation-dependent community dynamics (11, 15) because they form a mature, persistent community where unpredictable weather creates substantial demographic variability. Desert annual germination is controlled by temperature and rainfall, and winter and summer rains in the Sonoran Desert give rise to distinct winter and summer annual plant communities. Annuals comprise up to 50% of the desert flora and have been the subject of classic investigations on physiology and population dynamics (16, 17). Winter annuals complete their life cycles within a few weeks to months. Their short life cycles, small size and sessile habit permit the monitoring of many individuals throughout their entire life cycle and the observation of multiple generations during the course of a single long-term project. These qualities enable quantitative estimates of the probability distributions that are critical to fluctuation-dependent theories. Desert annuals meet a key requirement for storage-effect coexistence: long-lived seed banks buffer populations during unfavorable periods (18). Also, by definition, years that favor high germination directly result in greater density, creating positive covariance between an environmental parameter and competition. A similar environment-competition covariance arises from varying environmental factors affecting seedling survival and individual growth, because higher survival and larger survivors increase demand for resources (19). Although these effects reduce the ability of a high density species to take advantage of favorable environment conditions, it does not alter our ability to measure the relative responses of different species to the environmental conditions.
Coexistence is promoted if a set of species is buffered by persistent seed banks and if species' environment-competition covariances decline when their densities decline. These effects create low-density advantages, and occur when there is sufficient demographic decoupling between species driven by differences in their responses to temporally varying physical environmental conditions (15, 19). In desert annuals, such demographic responses can be suitably divided into germination and fecundity. Decoupling through germination is a well-known and well-studied scenario by which low-density advantages are created (9, 15, 20). Decoupling through reproduction, which is the focus of this article, reflects the combined effects of survival and growth (19), and provides strong low-density advantages, which we show here (SI Appendix). The decoupling of reproductive success between species that promotes species coexistence can be measured as the statistical interaction between species and time for per germinant fecundity.
In this article, we present a general framework for quantifying the magnitude of the storage effect, assess the strength of the storage effect in a community of Sonoran Desert winter annuals, and identify functional differences between species in the growth and reproductive phase of the life cycle that promote storage-effect coexistence. We have examined interspecific variation in traits associated with resource uptake and resource allocation for 9 coexisting Sonoran Desert winter annuals to test the hypothesis that fundamental tradeoffs in plant function create the differential demographic responses to environmental variation that help maintain diverse species assemblages in plant communities. Specifically, we look for a relationship between functional trait variation and the species-by-time interaction for reproduction that contributes quantitatively to recovery from low density. Elucidating such functional links in annual plants should provide insights relevant to less tractable systems.
Results and Discussion
Using a dataset collected annually from 1982 to 2007 from 72 permanently marked quadrats, we obtained estimates of germination, survival and fecundity, and used these data to estimate variance components by standard ANOVA methods. Sonoran Desert winter annual species exhibit striking demographic fluctuations (11) and respond in partially dissimilar ways to yearly variation, as evidenced by species-by-year interactions for ln-transformed per capita germination (F105,91 = 6.44, P < 0.0001) and per germinant fecundity (F165,2926 = 7.99, P < 0.0001) (Table S1). For our system, the low-density advantage due to germination variation alone increases r̄ by ≈0.052, while that due to reproductive variation alone adds 0.025 to r̄, where r̄ is the species average recovery rate from low density (the low-density long-term per capita population growth rate) (Table S2 and SI Appendix). An additional increase to r̄ of 0.027 comes from the covariance of germination fraction and reproductive variation, giving a total storage effect of 0.103 (Table S2). For a system as large as this one (millions of individuals of most species at one field station), recovery rates need only be positive for indefinite coexistence (8, 9). The observed storage effect is a substantial population growth rate for a species bouncing back from low density in competition with other species (equivalent to a doubling time of 7 years).
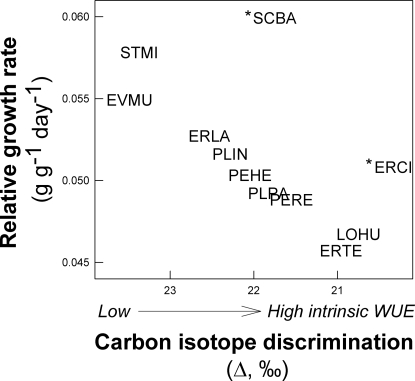
To understand how differences in functional traits create population dynamic decoupling, we examined interspecific variation in traits related to growth, allocation and low-resource tolerance and related these differences to the species-by-year interaction for per germinant fecundity. A fundamental tradeoff thought to be important in plants is that between the ability to photosynthesize and grow rapidly versus the ability to withstand the stresses inherent in low resource environments (21, 22). Such a tradeoff has been described across life forms, but we find the tradeoff within one functional guild (Fig. 1) (23). Sonoran Desert winter annuals are arrayed along a tradeoff between relative growth rate (RGR) and a measure of intrinsic water-use efficiency (WUE), carbon isotope discrimination [Δ, where lower Δ indicate higher intrinsic WUE (24)] (Fig. 1). Species with high RGR exhibit low WUE, whereas species with high WUE have low RGR. Our prior work has identified the key morphological and physiological traits that underlie growth capacity and low-water tolerance in these species (23, 25). Species that display high growth capacity allocate a large fraction of biomass to photosynthetic surfaces and have the ability to rapidly deploy large leaf area displays to maximize growth after infrequent, large rainfall events (23). Conversely, species that display high intrinsic WUE invest a large fraction of leaf nitrogen in the photosynthetic processes that become limiting at low temperatures characteristic of postrainfall periods, which optimizes carbon assimilation for the short windows of time after small but relatively frequent rain events (25). To capture this complexity, we conducted a principal components analysis of these traits that reflect physiological and morphological capacities underlying the growth/low-resource tolerance tradeoff: specific leaf area (SLA), leaf mass ratio (LMR), relative growth rate plasticity, the ratio of maximum electron transport to maximum carboxylation velocity (Jmax:VCmax), and leaf nitrogen content (Nleaf). Scores on the first principal component contrast species with high leaf mass allocation, leaf N, and electron transport capacity (which optimizes carbon assimilation at low temperatures after small rainfall events) versus species that attain high growth via high leaf area investment and morphological plasticity after large rainfall events (Table 1). Species' pairwise differences in PC I scores quantify composite differences in the key physiological traits that underlie the growth rate/low-resource tolerance tradeoff.
Fig. 1.
Interspecific tradeoff between growth capacity (relative growth rate, RGR, in g·g−1·day−1) and low-resource tolerance (intrinsic water-use efficiency, assayed by leaf carbon isotope discrimination, Δ, ‰). Species abbreviations are the first two letters of the genus and specific epithet given in Materials and Methods. Asterisks (*) denote 2 naturalized species.
Table 1.
Trait loadings, species scores, and percent variation explained by the first principal component of variation (PC I) in functional traits
| PC I results | Species or trait | Analysis 1 | Analysis 2 |
|---|---|---|---|
| Trait loading | LMR | −0.8293 | |
| Jmax:VCmax | −0.4611 | ||
| Nleaf | −0.5927 | ||
| SLA | 0.9128 | ||
| RGR plasticity | 0.7855 | ||
| Δ | 0.9914 | ||
| RGR | 0.9914 | ||
| Species score | ERCI | −0.7781 | −1.3221 |
| ERLA | 1.2518 | 0.6663 | |
| ERTE | −1.1007 | −1.7816 | |
| EVMU | 3.2855 | 1.7764 | |
| LOHU | −0.7294 | −1.7539 | |
| PEHE | −0.6152 | −0.1351 | |
| PERE | −2.1561 | −0.7760 | |
| PLIN | −0.7775 | 0.2810 | |
| PLPA | −0.4514 | −0.4976 | |
| SCBA | 0.8267 | 1.3805 | |
| STMI | 1.2930 | 2.2205 | |
| Variation explained | 54% | 98% |
Analysis 1: leaf mass ratio (LMR), maximum electron transport capacity (Jmax:VCmax), leaf nitrogen content (Nleaf), specific leaf area (SLA), and growth plasticity (RGR plasticity). Analysis 2: relative growth rate (RGR) and carbon isotope discrimination (Δ; inversely related to intrinsic water-use efficiency).
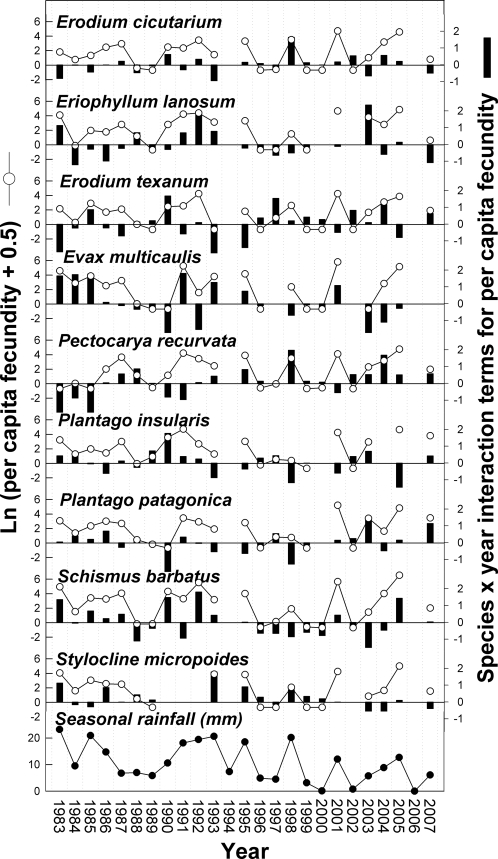
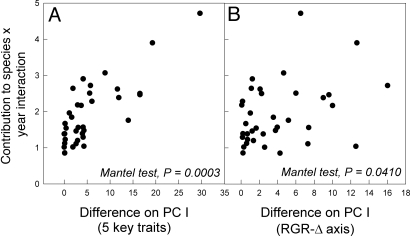
To calculate species' differences in response to year, we first decomposed the species-by-year interaction for per germinant fecundity into a residual effect for each species in each year after removing main effects and sampling error (Fig. 2). We then squared species' pairwise differences in residual interaction terms. The average over pairs of species and time of these squared pairwise differences estimates the species-by-year interaction component of variance that is used to calculate the magnitude of the storage effect due to fecundity (SI Appendix). The average squared differences between species in these interaction effects were placed in a 9 × 9 matrix that describes species' pairwise contributions to the species-by-year interaction term, and hence, the magnitude of their contributions to the demographic differences that promote coexistence (Table S3). Species that respond similarly to yearly variation will tend to have low differences, whereas species that respond dissimilarly to yearly variation will tend to have high differences. Using a matrix correlation approach, we find that the matrix of species differences in PC I scores (Table S4) is highly correlated with the matrix of contributions to the species-by-year interaction (Mantel test, P = 0.0003) (Fig. 3A). The relationship remains highly significant using a partial Mantel test with a third matrix of phylogenetic distances (Table S5). This finding provides a mechanistic explanation of how functional traits underlie species differences in population dynamic responses to the environment of different years. To see if this relationship can be captured with simpler integrative measures, we recalculated the matrix correlations using the PC I score based on the emergent growth rate/low-resource tolerance tradeoff alone. The matrix of species differences along the RGR-Δ tradeoff (Table S4) is also significantly related to the matrix of species contributions to the species-by-year interaction, but with a lower P value (Mantel test, P = 0.0410) (Fig. 3B). Thus, while a simple functional tradeoff is involved in the demographic decoupling that contributes to coexistence, explicit recognition of variation in the underlying parameters suggests a more intricate relationship between physiology and coexistence.
Fig. 2.
Annual per germinant fecundity (survivorship to reproduction, l, times fecundity, b) for each species (left axis, lines) and decomposition of the species-by-year interaction for per germinant fecundity into effects for each species and year after subtracting species and year main effects and sampling error (right axes, black bars). If the species-by-year interaction effects are positive, then lb was greater than expected based on the main effects of species and year alone. (Lower) Interannual variation in growing season precipitation (total precipitation from the first germination-inducing rain until the final reproductive census each year).
Fig. 3.
Species differences in response to yearly variation are correlated with differences in position along the first principal component of variation in 5 key functional traits that underlie a growth capacity/low-resource tolerance tradeoff (leaf mass ratio, maximum electron transport capacity, specific leaf area, relative growth plasticity and leaf nitrogen content) (A) and differences in position along the first principal component constructed using relative growth rate and intrinsic water-use efficiency alone (B). Each point represents the pairwise squared difference between 2 species. Significance was tested with Mantel permutation tests to account for non-independence of data points.
Temporal demographic decoupling captured by the species-by-year interaction reflects differences in demographic response to environmental variation. We investigated the relationship between demography, traits, and climate variables to determine how the environments of different years, operating through these functional traits, result in differential performance. Because precipitation controls the rate and timing of most biological processes in arid ecosystems (26), we hypothesized that differential response to precipitation is a major contributor to the species-by-year interaction. Species differ strongly in their demographic responses to precipitation (species by precipitation, F8,3104 = 11.77, P < 0.0001). We described each species' demographic sensitivity to precipitation as the slope of the relationship between per germinant fecundity and growing season precipitation. The matrix of species squared differences in demographic sensitivity to precipitation (Table S3) correlates with the matrix of contributions to the species-by-year interaction (Mantel test, P = 0.0075). Differences in demographic sensitivity to other climate parameters, such as growing season length and temperature, are not significantly related to the pairwise contributions to the species-by-year interaction (Mantel tests: season length, P = 0.30; average maximum temperature, P = 0.50; average minimum temperature, P = 0.72). Furthermore, the matrices of species differences in functional traits are significantly related to the matrix of differential demographic sensitivity to precipitation (Mantel tests: 5-trait matrix, P = 0.0068; RGR-Δ matrix, P = 0.0453). Thus, tradeoffs in key physiological processes that result in different utilization of soil moisture explain demographic decoupling that is driven by inter-annual variation in precipitation.
We have shown that the same fundamental tradeoff between growth capacity and low-resource tolerance that separates life forms (21, 22) is found within what is commonly considered to be 1 plant functional type. The degree of separation between species on this tradeoff axis is quantitatively related to the magnitude of their differential demographic response to environmental variation between years, specifically variation in the amount of growing season precipitation. Incorporation of lower-level functional traits that describe resource uptake and allocation behavior produces a more exact description of how physiological differences between species explain the temporal population dynamics that promote local biodiversity via the storage effect. These tests have relied on an extension of storage-effect theory to consider different degrees of decoupling between different pairs of species (SI Appendix). Although not required in theory for the storage effect (9), it stands to reason that more physiologically similar species are demographically more similar too. Recognition of such differences leads to the approaches for understanding the factors driving demographic decoupling that we have demonstrated here. The results from this investigation of short-lived plants in extreme environments should provide an important reference point for our emerging understanding of more complex communities.
Materials and Methods
From 1982 to 2007, permanent plots at the University of Arizona Desert Laboratory (Tucson, AZ) were censused after each rainfall to document germination, survivorship to reproduction (l), and fecundity (b) of all winter annual species (18). Species with at least 5 individuals in each of at least 15 years (76% of all seedlings in the dataset) were selected for analysis of lb: Pectocarya recurvata (Boraginaceae), Erodium cicutarium—naturalized, Erodium texanum (Geraniaceae), Eriophyllum lanosum, Evax multicaulis, Stylocline micropoides (Asteraceae), Plantago insularis, Plantago patagonica (Plantaginaceae) and Schismus barbatus—naturalized (Poaceae).
During 2004–2005, we measured functional traits of these species plus Lotus humistratus (Fabaceae) and Pectocarya heterocarpa, which were abundant that year. We determined relative growth rate (RGR), specific leaf area (SLA) and leaf mass ratio (LMR) by harvesting up to 30 plants per species biweekly throughout the season (see ref. 24). We calculated VCmax (maximum carboxylation rate by Rubisco) and Jmax (maximum light-saturated electron transport rate) from assimilation versus internal CO2 concentration curves on fully expanded leaves of 3 to 5 individuals per species (see ref. 26). Leaf nitrogen (Nleaf) and carbon isotope composition were analyzed at the University of Arizona Geosciences Stable Isotope Facility. Carbon isotope ratios were converted to discrimination values (Δ) (27).
We used ANOVA to assess the effects of species, year (or ln-seasonal precipitation) and their interaction on ln-transformed plot-level per germinant fecundity (lb + 0.5), weighted by the number of seedlings per species per plot (PROC GLM, SAS 9.1; SAS Institute). Significance of effects was tested with Type III sums of squares. We added 0.5 to lb because ln(0) is undefined, and lb occasionally equalled 0 if no seedlings of a given species germinated on a particular plot or if few seedlings of a given species germinated but all died before reproduction. Results were qualitatively identical when different constants were added to per germinant fecundity (e.g., lb + 0.17 or lb + 1). Results also were qualitatively identical when untransformed data were analyzed with generalized linear models (gamma distribution, log link function; PROC GLIMMIX). We decomposed the species-by-year interaction into an interaction effect for each species in each year based on the following equation: Interaction(Si,Yj) = LS(Si,Yj) − LS(Si) − LS(Yj) + LS(grand mean), where LS denotes the least-squares mean estimate for a particular species, Si, year, Yj, or Si,Yj combination. We used the “LSMEANS” statement of PROC GLM to obtain least-squares means for each Si,Yj and Si,Yj combination. The interaction(Si,Yj), when squared and summed over years, divided by the degrees of freedom, and corrected for sampling error, gives us the species x year interaction component of variance (σV,t×s2) for log per germinant fecundity, ln(lb), used to calculate the magnitude of the storage effect (Tables S1 and S2 and SI Appendix).
To see how this population dynamic interaction relates to species functional biology, we first expressed it as a species difference matrix. For each pair of species, we calculated the average of squared differences in their interaction effects by summing the squares of their differences in each year (excluding any years in which one of the species remained dormant) and then dividing by the number of years in which both species were observed in the vegetative phase (n = 19–23 years). These differences between species were placed in a 9 × 9 matrix describing species' pairwise contributions to the species-by-year interaction term (Table S3, upper diagonal). Species differences in demographic sensitivity to climate variables were estimated as squared differences in slopes of individual regressions of ln(lb + 0.5) versus growing season precipitation (Table S3, lower diagonal), season length, average maximum temperature or average minimum temperature. Daily precipitation was recorded at the University of Arizona Desert Laboratory. Daily temperature data were obtained from the University of Arizona weather station ≈5 km from the Desert Laboratory (National Climatic Data Center, National Oceanic & Atmospheric Administration, Asheville, NC)
To summarize interspecific variation in functional traits, we conducted principal component analysis on trait correlation matrices using SAS INSIGHT. The first analysis described species differences in 5 key traits that underlie the growth capacity/low-resource tolerance tradeoff: SLA, LMR, RGR plasticity, Jmax:VCmax, and Nleaf. The second analysis described species differences in position along the emergent tradeoff between RGR and Δ. We ran the principal component analysis using data from all native species and then manually calculated the 2 naturalized species' scores on the first principal component (PC I) by using the standardized regression coefficients relating each trait to PC I. However, our results do not change when all species are included in the principal component analysis. For each analysis, squared differences between species in PC I scores were placed in a 9 × 9 matrix of functional trait differences (Table S4).
Associations between trait and demographic difference matrices were examined using Mantel tests (28) [program supplied to D.L.V. by E. J. Dietz (Department of Mathematics and Computer Science, Meredith College, Raleigh, NC) and D. E. Cowley (Department of Fish, Wildlife and Conseration Ecology, New Mexico State University, Las Cruses, NM)]. Correlations of corresponding cells of each pair of matrices were calculated with Mantel's Z. Permutations preserving the dependencies between matrix elements were performed and the Z statistic was recalculated 4,000 times, generating a null distribution against which the observed statistic was tested.
We calculated phylogenetic distance matrices to assess the effects of phylogenetic history on our results. To estimate phylogenetic distances, we first used the online tool Phylomatic (29) to create a hypothesis of the relationships among species based on the conservative seed plant tree available at the Angiosperm Phylogeny Website (30). We created 2 trees, one with equal branch lengths and one with pseudo branch lengths based on ages given by Wikstrom et al. (2001) (31). We calculated matrices of pairwise phylogenetic distances using the phydist function in Phylocom (32). We then conducted partial Mantel tests to assess the relationship between the residuals of the demographic and trait difference matrices after removing phylogenetic distance from each. Partial Mantel tests were conducted, using the R platform (compilation 2.6.2) and using the package vegan. With all analyses, the results from partial Mantel tests controlling for phylogenetic distance were qualitatively identical to results from Mantel tests without phylogenetic matrices (Table S5).
Supplementary Material
Acknowledgments.
We thank S. Adondakis, G. Barron-Gafford, A. Bell, H. Bruce, T. Caprio, M. Clauss, B. Collins, C. Contreras, J. Cox, M. Davis, N. Douglas, J. Duke, C. Enquist, K. Gerst, K. Gilliam, C. Golightly, A. Halloran, A. Hazard, J. Horst, T. Hubbard, R. Janaway, A. Jaksha, G. Ketner, O. Kougot, S. Kunkel, H. Lawson, K. McCoy, C. McDonald, K. Moriuchi, C. Pake, M. Pantastico, C. Pearson, J. Pearson, S. Roberts, P. Sanchez, M. Schneider, S. Stebens, M. Stubbs, A. Tyler, M. Wagenheim, and B. Weeks for assistance with data collection; B. Igic, S. Kimball, S. Stark, and J. Tewksbury for providing valuable comments on the manuscript; and N. Swenson and A. Zanne provided advice on statistical analyses. This work was supported by the Philecology Foundation and National Science Foundation Grants BSR 9107324, DEB 9419905 (Long Term Research in Environmental Biology), DEB 0212782 (Long Term Research in Environmental Biology), DEB 0717466 (Long Term Research in Environmental Biology), DEB 0453781, DEB 0542991, and DEB 0717380.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904512106/DCSupplemental.
References
- 1.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 2.Tilman D. Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton: Princeton Univ Press; 1988. [Google Scholar]
- 3.Gendron RP. Models and mechanisms of frequency-dependent predation. Am Nat. 1987;130:603–623. [Google Scholar]
- 4.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 5.Levins R, Culver D. Regional coexistence of species and competition between rare species. Proc Natl Acad Sci USA. 1971;68:1246–1248. doi: 10.1073/pnas.68.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastings A. Disturbance, coexistence, history, and competition for space. Theor Popul Biol. 1980;18:363–373. [Google Scholar]
- 7.Armstrong RA, McGehee R. Competitive exclusion. Am Nat. 1980;115:151–170. [Google Scholar]
- 8.Chesson PL, Warner RR. Environmental variability promotes coexistence in lottery competitive systems. Am Nat. 1981;117:923–943. [Google Scholar]
- 9.Chesson P. Multispecies competition in variable environments. Theor Popul Biol. 1994;45:227–276. [Google Scholar]
- 10.Caceres CE. Temporal variation, dormancy, and coexistence: A field test of the storage effect. Proc Natl Acad Sci USA. 1997;94:9171–9175. doi: 10.1073/pnas.94.17.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pake CE, Venable DL. Is coexistence of Sonoran Desert annuals mediated by temporal variability in reproductive success? Ecology. 1995;76:246–261. [Google Scholar]
- 12.Adler PB, HilleRisLambers J, Kyriakidis PC, Guan QF, Levine JM. Climate variability has a stabilizing effect on the coexistence of prairie grasses. Proc Natl Acad Sci USA. 2006;103:12793–12798. doi: 10.1073/pnas.0600599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runkle JR. Synchrony of regeneration, gaps, and latitudinal differences in tree species diversity. Ecology. 1989;70:546–547. [Google Scholar]
- 14.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Chesson P, et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141:236–253. doi: 10.1007/s00442-004-1551-1. [DOI] [PubMed] [Google Scholar]
- 16.Forseth IN, Ehleringer JR, Werk KS, Cook CS. Field water relations of Sonoran Desert annuals. Ecology. 1984;65:1436–1444. [Google Scholar]
- 17.Tevis L., Jr A population of desert ephemerals germinated by less than one inch of rain. Ecology. 1958;39:688–695. [Google Scholar]
- 18.Venable DL. Bet hedging in a guild of desert annuals. Ecology. 2007;88:1086–1090. doi: 10.1890/06-1495. [DOI] [PubMed] [Google Scholar]
- 19.Chesson P, Donahue MJ, Melbourne BA, Sears ALW. In: Metacommunities: Spatial Dynamics and Ecological Communities. Holyoak M, Leibold MA, Holt RD, editors. Chicago: University of Chicago Press; 2005. pp. 279–306. [Google Scholar]
- 20.Facelli JM, Chesson P, Barnes N. Differences in seed biology of annual plants in arid lands: A key ingredient of the storage effect. Ecology. 2005;86:2998–3006. [Google Scholar]
- 21.Grime JP. Plant Strategies, Vegetation Processes, and Ecosystem Properties. 2nd Ed. Chichester, UK: Wiley; 2001. [Google Scholar]
- 22.Chapin FS, Autumn K, Pugnaire F. Evolution of suites of traits in response to environmental stress. Am Nat. 1993;142:S78–S92. [Google Scholar]
- 23.Angert AL, Huxman TE, Barron-Gafford GA, Gerst KL, Venable DL. Linking growth strategies to long-term population dynamics in a guild of desert annuals. J Ecol. 2007;95:321–331. [Google Scholar]
- 24.Seibt U, Rajabi A, Griffiths H, Berry JA. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia. 2008;155:441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- 25.Huxman TE, et al. Photosynthetic resource-use efficiency and demographic variability in desert annual plants. Ecology. 2008;89:1554–1563. doi: 10.1890/06-2080.1. [DOI] [PubMed] [Google Scholar]
- 26.Noy-Meir I. Desert ecosystems: Environment and producers. Annu Rev Ecol Syst. 1973;4:25–51. [Google Scholar]
- 27.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- 28.Dietz EJ. Permutation tests for association between two distance matrices. Syst Zool. 1983;32:21–26. [Google Scholar]
- 29.Webb CO, Donoghue MJ. Phylomatic: Tree assembly for applied phylogenetics. Mol Ecol Notes. 2005;5:181–183. Available at www.phylodiversity.net/phylomatic/phylomatic.html. [Google Scholar]
- 30.Stevens PF. Angiosperm Phylogeny web site. 2001 Version 9. Available at www.mobot.org/MOBOT/research/APweb.
- 31.Wikstrom N, Savolainen V, Chase MW. Evolution of angiosperms: Calibrating the family tree. Proc R Soc London Ser B. 2001;268:2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. Available at www.phylodiversity.net/phylocom. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.