Abstract
Hyperandrogenemia and insulin resistance are heritable traits in sisters of women with polycystic ovary syndrome (PCOS). Hyperandrogenemia also appears to be the male reproductive phenotype, however, it is less clear whether male relatives are at risk for the metabolic disorders associated with PCOS. In this study, we tested the hypothesis that brothers of women with PCOS have defects in insulin action and/or secretion. Twenty-three Non-Hispanic White brothers of women with PCOS and 23 Non-Hispanic White control men of comparable age matched for BMI underwent a modified frequently sampled intravenous glucose tolerance test. Parameters of insulin sensitivity and secretion were determined using minimal model Bergman protocol. Disposition index (DI) was significantly decreased (2540 [1080, 3172] vs. 2901 [2096, 4487], P=0.009), independent of a family history of diabetes mellitus (DM) and glucose effectiveness (SG) was significantly increased (2.4 [1.9, 2.7] vs. 2.0 [1.8, 2.2], P=0.02) in brothers compared to control men. We conclude that brothers of women with PCOS have evidence for pancreatic β-cell dysfunction and may be at increased risk for type 2 DM.
Key Terms: Insulin sensitivity, insulin secretion, disposition index, glucose effectiveness, frequently sampled intravenous glucose tolerance test
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common disorder of premenopausal women, affecting ~7% of this population (1). The reproductive phenotype is characterized by hyperandrogenemia, disordered gonadotropin secretion and polycystic ovaries (2). Women with PCOS frequently have profound insulin resistance (3), pancreatic β-cell dysfunction (4, 5), dyslipidemia (6), obesity and other features of the metabolic syndrome (7). Indeed, PCOS is likely the leading risk factor for type 2 diabetes mellitus (DM) in adolescent girls and young adult women (8, 9).
Familial aggregation of PCOS has been well established consistent with a genetic susceptibility to the disorder and up to ~40% of premenopausal sisters have the reproductive phenotype of hyperandrogenemia (10–12). Sisters with this reproductive phenotype also have the characteristic metabolic defects of PCOS (13, 14). These observations have led to a search for male phenotypes in PCOS families and we have found that hyperandrogenemia is the reproductive phenotype in the brothers of women with PCOS (15). Several studies have suggested that male relatives may also have metabolic abnormalities, such as dyslipidemia (16), glucose intolerance (17) and insulin resistance (12, 17, 18). However, the underlying defects in glucose homeostasis have not been defined in male relatives compared to control men. We performed this study to test the hypothesis that male first-degree relatives of women with PCOS have defects in insulin action and/or secretion.
METHODS
Subjects
We studied 23 brothers of the 19 Non-Hispanic White women with PCOS. The diagnosis of PCOS was made in the probands by an elevation of circulating testosterone (T) and/or non-sex hormone binding globulin bound (unbound [u]) T levels associated with chronic oligomenorrhea (≤six menses per year) (10, 13, 15). Women with non-classical 21-hydroxylase deficiency, hyperprolactinemia and androgen-secreting tumors were excluded by appropriate tests (10, 13, 15). One family had three brothers, two families had two brothers and 16 families had a single brother. The clinical and biochemical features on the probands have been reported as part of previous studies (10, 13, 15). Twenty-three unrelated Non-Hispanic White control men were matched to each brother for BMI. All subjects were ages 16–48 years, in good health and had no history of hypertension or dyslipidemia. Subjects were not taking any medications known to alter sex hormones, or insulin sensitivity or secretion for at least one month prior to study. None of the control men had a history of DM personally or in a first degree relative. None of the brothers had a personal history of DM, however, five families had a first-degree relative with DM. Of these families, one had three brothers, one had two brothers and three had a single brother. The Institutional Review Boards of the Pennsylvania State University College of Medicine, Brigham and Women’s Hospital and Northwestern University’s Feinberg School of Medicine approved this study, and all subjects gave written informed consent.
Height, weight and blood pressure were obtained, and body mass index (BMI) was determined in all subjects. A 75-g oral glucose tolerance test with glucose and insulin levels obtained at 0 and 2 h post-challenge was performed after a 300 g carbohydrate preparatory diet and an overnight fast in 17 brothers and all control subjects; the control subjects were required to have normal glucose tolerance as a selection criterion (19). A baseline blood sample was obtained for T, uT and dehydroepiandrosterone sulfate (DHEAS) measurements.
A frequently sampled intravenous glucose tolerance test (FSIGT) was performed in the morning after a 300 g carbohydrate preparatory diet and a standard overnight fasting period of 10 h as reported (5). All subjects had two intravenous catheters inserted, one in each arm, and were then allowed to rest for 30 min. At 0 min, glucose (0.3 g/kg) was injected over 1 min; at 20 min, 500 mg of tolbutamide was injected over 20 s. Blood samples were drawn at −15, −10, −5, −1, 0, 2, 3, 4, 5, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 90 and 100 min and every 20 min thereafter until 180 min. The insulin sensitivity index (SI) and glucose effectiveness (SG) were calculated by application of the minimal model of glucose kinetics (MINMOD computer program Millennium version, copyright R.N. Bergman) to the dynamics of plasma glucose and insulin during the FSIGT (20, 21). The acute insulin response to glucose (AIRg) was calculated as the increment area under curve from basal of insulin values measured at 2–10 min and disposition index (DI; β-cell compensation index) as product of SI and AIRg (21–23). Glucose clearance (KG), a parameter of glucose tolerance, was calculated from the FSIGT as the slope of the least square regression line to the natural log of the glucose concentration vs time from 10 min to 19 min after the glucose injection (5, 24).
Assays
Plasma glucose levels were determined by glucose oxidase method. Assays for insulin, T, uT and DHEAS were performed as previously reported (10, 13, 15).
Data Analyses
For analysis of the data, the family unit was the case; in families with multiple brothers, brothers’ and their matched controls’ data were averaged to yield one mean value per family for brothers and their matched controls (15). However, we repeated the analysis without adjusting for multiple brothers by using data from all of the individual brothers and their matched control subjects. All parameters were compared using Wilcoxon signed ranks test since data were not normally distributed. Analyses were repeated after exclusion of brothers with impaired glucose tolerance (IGT) (n=3) and after exclusion of brothers (n=8) with family history of DM. Analyses were performed using the 11.0 PC package of SPSS statistical software (SPSS, Inc., Chicago, IL). A P ≤ 0.05 was considered significant. Data are reported as the untransformed mean ± SD.
RESULTS
Clinical and Biochemical Features
The ages of PCOS brothers and control men were comparable and the BMIs were well-matched by design (Table 1). Blood pressure was similar in the two groups (Table 1). There were no differences between the two groups in T, uT and DHEAS levels (Table 1). There were no significant differences between fasting or 2 h post-glucose challenge glucose and insulin levels, however, three of 17 brothers (18%) had IGT (Table 1). All of the 6 brothers who did not have an OGTT had fasting glucose levels less than 100 mg/dl and these levels did not differ from those in the brothers with normal glucose tolerance by OGTT.
Table 1.
Clinical features and reproductive hormone levels in PCOS brothers and control men
| Variables | Brothers n=23 | Control n=23 | P |
|---|---|---|---|
| Age (yr) | 30 [21, 34] | 31 [25, 37] | 0.21 |
| BMI (kg/m2) | 28.1 [22.9, 32.3] | 26.7 [23.4, 30.3] | 0.81 |
| Systolic blood pressure (mm Hg) | 118 [110, 130] | 115 [110, 126] | 0.64 |
| Diastolic blood pressure (mm Hg) | 72 [62, 82] | 72 [70, 78] | 0.95 |
| Testosterone (ng/dl)a | 522 [419, 616] | 512 [433, 593] | 0.39 |
| unbound Testosterone (ng/dl)a | 241 [219, 302] | 255 [166, 310] | 0.64 |
| DHEAS (ng/ml)b | 2820 [1704, 3697] | 2827 [2405, 3090] | 0.47 |
| Fasting glucose (mg/dl)c | 87 [81, 97] | 88 [82, 92] | 0.88 |
| 2 hour post-challenge glucose (mg/dl)c | 115 [72, 123] | 92 [77, 111] | 0.26 |
| Fasting insulin (μU/ml)d | 13 [10, 17] | 11 [8, 19] | 0.40 |
| 2 hour post-challenge insulin (μU/ml)d | 40 [21, 79] | 28 [22, 47] | 0.51 |
Values are the median [25%, 75% interquartile range]; P-value by Wilcoxon signed ranks test;
to convert to nanomoles/l, multiply by 0.00347;
to convert to micromoles, multiply by 0.002714;
n=17, to convert to millimoles/l, multiply by 5.55;
n=17, to convert to picomoles/l, multiply by 7.175.
Parameters of Insulin Action and Secretion
There were no significant differences in SI or in AIRg in brothers compared to control men (Table 2). However, the DI, the product of SI and AIRg, was significantly decreased (P=0.009) in brothers compared to control men (Table 2, Figure 1). The differences in DI remained significant after exclusion of the three brothers with IGT (2309 [1010, 3120] brothers vs. 2729 [2033, 4005] control, P=0.03). These results were also not changed by the removal of the five PCOS families (8 brothers) who had history of type 2 DM in their first-degree relatives (DI 2513 [1056, 3194] brothers vs. DI 2958 [2075, 4630] control, P=0.03). The difference in DI remained significant when all individual brothers were compared to their matched control subjects. SG was higher in brothers than in control men (P=0.02) (Table 2). The glucose disappearance, KG, did not differ in the two groups (Table 2). Brothers who did not have an OGTT had a trend towards higher KG compared to those with normal glucose tolerance on OGTT (2.97 ± 0.97 min−1 brothers who did not undergo an OGTT vs. 2.06 ± 0.97 min−1 brothers with normal glucose tolerance on OGTT, P=0.06).
Table 2.
Measures of glucose regulation in PCOS brothers and control men
| Brothers (n=19a) | Control (n=19) | P-Value | |
|---|---|---|---|
| AIRg (μU/ml. Min)b | 468 [285, 1200] | 569 [319, 892] | 1.00 |
| SI [×10−4 min−1/(μU/ml)]c | 4.5 [1.7, 7.4] | 5.2 [4.0, 7.7] | 0.47 |
| DI | 2540 [1080, 3172] | 2901 [2096, 4487] | 0.009 |
| SG (10−2/min) | 2.4 [1.9, 2.7] | 2.0 [1.8, 2.2] | 0.02 |
| Peak insulin after glucose infusion (μU/ml)b | 89 [51, 225] | 116 [64, 171] | 0.83 |
| Peak insulin after tolbutamide (μU/ml)b | 153 [84, 296] | 161 [104, 297] | 0.95 |
| KG (min−1) | 2.16 [1.73, 3.01] | 2.16 [1.55, 3.01] | 0.72 |
Values are the median [25%, 75% interquartile range]; P-value by Wilcoxon signed ranks test;
Multiple brothers and control men for a single family are averaged to yield one mean value per family;
to convert to picomoles/l, multiply by 7.175;
to convert to 10−5 per minute per picomoles/l, multiply by 1.67.
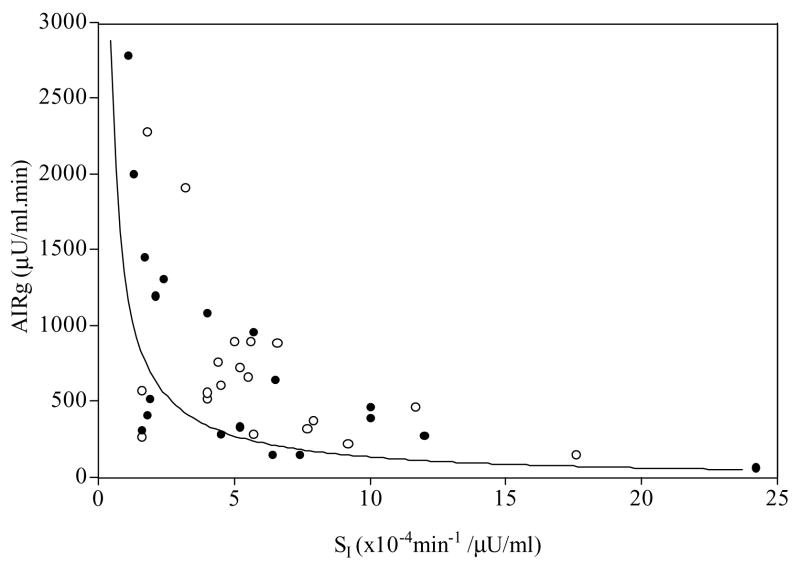
Figure 1.
The relationship between insulin secretion and insulin sensitivity in the brothers (●) and control men (○). The line depicts 50th percentile for this relationship from a large study (n=93) of normal men and women, where SI × AIRg = 0.02237 (48). Values below the 50th percentile are associated with an increased risk for type 2 DM (23).
DISCUSSION
We found that the brothers of women with PCOS have a significantly decreased DI compared to control men regardless of glucose tolerance. DI is the product of insulin sensitivity and insulin secretion (21–23). When pancreatic β–cell function is normal, DI is a constant hyperbolic relationship, i.e., if insulin sensitivity decreases, insulin secretion increases in a compensatory fashion and vice versa (23). The finding of a decreased DI suggests that insulin secretion was inappropriately low when corrected for peripheral insulin sensitivity (5, 21–23). Therefore, one male metabolic phenotype in PCOS families appears to be pancreatic β–cell dysfunction. SG was also significantly increased in brothers compared to control men (5, 21). This parameter has been suggested to be a measure of insulin-independent glucose-mediated glucose clearance known as glucose effectiveness (25), which is an important component of glucose tolerance (20, 21, 25, 26). There is concern about accuracy of minimal model estimates of this parameter (27). However, the finding of increased SG in first-degree relatives of patients with type 2 DM was confirmed by direct measurement (28).
First-degree relatives of individuals with type 2 DM also have insulin resistance (29) and decreased DI (28, 30); the latter predicts their risk for progression to type 2 DM (23, 31, 32). There appears to be an increased prevalence of type 2 DM in the first-degree relatives of women with PCOS (12, 17). Nevertheless, DI was significantly decreased, even in the PCOS brothers without a first-degree relative with type 2 DM. This finding suggests that abnormalities in insulin secretion in PCOS families were due to more than the coexistence of two common diseases: type 2 DM and PCOS. However, it remains possible that some of these first-degree relatives will develop type 2 DM. Furthermore, DI was still significantly decreased after exclusion of brothers with IGT. A limitation of this study is that we cannot exclude the presence of IGT in the brothers who did not undergo an OGTT. However, none of these brothers had impaired fasting glucose according to the new more stringent ADA criteria (33). Furthermore, mean KG tended to be higher in brothers who did not undergo an OGTT compared to those who had normal glucose tolerance based on OGTT. KG decreases as glucose tolerance declines; KG <1.5 min−1 indicate poor glucose tolerance (22) and KG <1 min−1 is usually observed in diabetes (21). Taken together, these findings suggest that the untested brothers had normal glucose tolerance. Another limitation of this study is that the groups were pair-matched for BMI but not for age. Nevertheless, the age range in both the brothers and control men was similar and the age difference was within two years for most pairs. In those pairs where age differed by more than 10 years (n=2), the control was older than the brother, which would favor the null hypothesis if insulin sensitivity or secretion decreased with age. Furthermore, there have been no changes in these metabolic parameters with age in previous studies using FSIGT (34) or euglycemic hyperinsulinemic clamp (35) techniques across a similar age range. Therefore, it is unlikely that differences in age between brothers and control men biased our findings. Increased glucose effectiveness (28) has also been reported in first-degree relatives of individuals with type 2 DM and may represent a compensatory mechanisms for abnormalities in insulin action (21, 26, 28). We have not found changes in SG in women with PCOS (5), while other investigators have reported decreases (36, 37).
Metabolic abnormalities, such as dyslipidemia (16) and hyperinsulinemia (38), have been reported anecdotally in the male first-degree relatives of women with PCOS. The prevalence of type 2 DM is increased in parents of women with PCOS (12, 17). Eighteen percent of the 17 brothers tested had newly diagnosed glucose intolerance, which appears to be increased compared to rates in the general population of Non-Hispanic White men of similar age (39). However, this finding is constrained by the small sample of brothers who had normal glucose tolerance testing. Further, there were no differences in glucose clearance, another parameter of glucose tolerance (24), in brothers and control men. Previous studies of the male metabolic phenotype have found evidence for insulin resistance in male first-degree relatives of women with PCOS or polycystic ovary morphology compared to control men by oral glucose tolerance tests (12, 18). To our knowledge, the mechanisms of such defects in glucose homeostasis have not been investigated. There is evidence to suggest that both insulin secretion and DI are heritable in the siblings of women with PCOS (12). However, that study contained sisters as well as brothers and did not compare the findings in the siblings to a normal control group to define a metabolic phenotype. DI has been found to be highly heritable in several populations (40–42). In addition, prospective studies have shown that DI is the best predictor of risk for progression to diabetes (23). Women with PCOS are at substantially increased risk for development of type 2 DM (8, 9) due to defects in insulin action and secretion. Such abnormalities precede the development of glucose intolerance in these women (5). The present study suggests that defects in insulin secretion are present in brothers of affected women and thus may confer an increased risk for type 2 DM in this population.
Although defects in insulin action and secretion are heritable in PCOS families (12, 13, 43), the precise pathogenesis of these abnormalities is unknown. Recent studies in adult male rhesus monkeys exposed to exogenous testosterone in utero have found both insulin resistance and impaired insulin secretion, analogous to changes in similarly exposed female monkeys (44). These observations suggest that intrauterine exposure to androgens may contribute to the putative defects in pancreatic β-cell function in brothers of women with PCOS. Our studies in the sisters of women with PCOS provide strong evidence that hyperandrogenemia secondary to variation in a gene regulating ovarian and adrenal steroidogenesis is the major reproductive phenotype (10, 45). Further, metabolic abnormalities track with hyperandrogenemia in affected sisters suggesting that these abnormalities have a common pathogenesis (13, 14). We have also found evidence for a similar defect in androgen biosynthesis in brothers of women with PCOS, who have significant elevations in DHEAS levels (15). In the present study, mean DHEAS did not differ in brothers compared to control men, which was likely the result of a type 2 error due to the relatively small sample size. Nevertheless, it remains plausible that there may also be increased androgen production by affected male as well as female fetuses in PCOS families that programs abnormalities in glucose homeostasis in the adult (2, 46, 47).
In summary, we have found evidence for impaired insulin secretion in brothers of women with PCOS. The increase in SG in brothers may be due to a compensatory increase in insulin-independent glucose clearance. Prospective studies will be needed to determine whether such defects are predictive of development of type 2 diabetes in brothers of women with PCOS.
Acknowledgments
The authors wish to thank the women with PCOS and their brothers for participating in this study, Barbara Scheetz and Jennifer Schlinder for coordinating the study and the staff of the General Clinical Research Centers at the Pennsylvania State University College of Medicine, Brigham and Women’s Hospital and Northwestern University’s Feinberg School of Medicine for their care of the study participants.
Grant Support: NIH grants U54 HD34449, DK40605, M01 RR00048, M01 RR10732, K12 RR017707, M01 RR02635 and C06 RR016499.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–370. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–527. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:942–947. doi: 10.1210/jcem.81.3.8772555. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 7.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 13.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sam S, Legro RS, Bentley-Lewis R, Dunaif A. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4797–4802. doi: 10.1210/jc.2004-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:2134–2138. doi: 10.1210/jcem.87.5.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givens JR. Familial polycystic ovarian disease. Endocrinol Metab Clin North Am. 1988;17:771–783. [PubMed] [Google Scholar]
- 17.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 18.Kaushal R, Parchure N, Bano G, Kaski JC, Nussey SS. Insulin resistance and endothelial dysfunction in the brothers of Indian subcontinent Asian women with polycystic ovaries. Clin Endocrinol (Oxf) 2004;60:322–328. doi: 10.1111/j.1365-2265.2004.01981.x. [DOI] [PubMed] [Google Scholar]
- 19.Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples Diabetes. 1989;38:1630–1635. doi: 10.2337/diab.38.12.1630. [DOI] [PubMed] [Google Scholar]
- 20.Bergman RN, Hope ID, Yang YJ, Watanabe RM, Meador MA, Youn JH, Ader M. Assessment of insulin sensitivity in vivo: a critical review. Diabetes Metab Rev. 1989;5:411–429. doi: 10.1002/dmr.5610050501. [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002;32 (Suppl 3):35–45. doi: 10.1046/j.1365-2362.32.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 24.Doi K, Taniguchi A, Nakai Y, Kawamura H, Higaki Y, Yokoi H, Tanaka H, Fujitani J, Suzuki M, Tokuyama K, Sakai M, Fukushima M. Decreased glucose effectiveness but not insulin resistance in glucose-tolerant offspring of Japanese non-insulin-dependent diabetic patients: a minimal-model analysis. Metabolism. 1997;46:880–883. doi: 10.1016/s0026-0495(97)90073-1. [DOI] [PubMed] [Google Scholar]
- 25.Ader M, Ni TC, Bergman RN. Glucose effectiveness assessed under dynamic and steady state conditions. Comparability of uptake versus production components. J Clin Invest. 1997;99:1187–1199. doi: 10.1172/JCI119275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994;43:587–592. doi: 10.2337/diab.43.4.587. [DOI] [PubMed] [Google Scholar]
- 27.McDonald C, Dunaif A, Finegood DT. Minimal-model estimates of insulin sensitivity are insensitive to errors in glucose effectiveness. J Clin Endocrinol Metab. 2000;85:2504–2508. doi: 10.1210/jcem.85.7.6681. [DOI] [PubMed] [Google Scholar]
- 28.Henriksen JE, Alford F, Handberg A, Vaag A, Ward GM, Kalfas A, Beck-Nielsen H. Increased glucose effectiveness in normoglycemic but insulin-resistant relatives of patients with non-insulin-dependent diabetes mellitus. A novel compensatory mechanism. J Clin Invest. 1994;94:1196–1204. doi: 10.1172/JCI117436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen JE, Levin K, Thye-Ronn P, Alford F, Hother-Nielsen O, Holst JJ, Beck-Nielsen H. Glucose-mediated glucose disposal in insulin-resistant normoglycemic relatives of type 2 diabetic patients. Diabetes. 2000;49:1209–1218. doi: 10.2337/diabetes.49.7.1209. [DOI] [PubMed] [Google Scholar]
- 31.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 32.Bergman RN, Watanabe R, Rebrin K, Ader M, Steil G. Toward an integrated phenotype in pre-NIDDM. Diabet Med. 1996;13:S67–77. [PubMed] [Google Scholar]
- 33.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 34.Pacini G, Valerio A, Beccaro F, Nosadini R, Cobelli C, Crepaldi G. Insulin sensitivity and beta-cell responsivity are not decreased in elderly subjects with normal OGTT. J Am Geriatr Soc. 1988;36:317–323. doi: 10.1111/j.1532-5415.1988.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 36.Falcone T, Little AB, Morris D. Impaired glucose effectiveness in patients with polycystic ovary syndrome. Hum Reprod. 1992;7:922–925. doi: 10.1093/oxfordjournals.humrep.a137771. [DOI] [PubMed] [Google Scholar]
- 37.Gennarelli G, Rovei V, Novi RF, Holte J, Bongioanni F, Revelli A, Pacini G, Cavallo-Perin P, Massobrio M. Preserved insulin sensitivity and {beta}-cell activity, but decreased glucose effectiveness in normal-weight women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3381–3386. doi: 10.1210/jc.2004-1973. [DOI] [PubMed] [Google Scholar]
- 38.Norman RJ, Masters S, Hague W. Hyperinsulinemia is common in family members of women with polycystic ovary syndrome. Fertil Steril. 1996;66:942–947. doi: 10.1016/s0015-0282(16)58687-7. [DOI] [PubMed] [Google Scholar]
- 39.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20–74 yr. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe RM, Valle T, Hauser ER, Ghosh S, Eriksson J, Kohtamaki K, Ehnholm C, Tuomilehto J, Collins FS, Bergman RN, Boehnke M. Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum Hered. 1999;49:159–168. doi: 10.1159/000022865. [DOI] [PubMed] [Google Scholar]
- 41.Hong Y, Weisnagel SJ, Rice T, Sun G, Mandel SA, Gu C, Rankinen T, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Bergman RN, Bouchard C, Rao DC. Familial resemblance for glucose and insulin metabolism indices derived from an intravenous glucose tolerance test in Blacks and Whites of the HERITAGE Family Study. Clin Genet. 2001;60:22–30. doi: 10.1034/j.1399-0004.2001.600104.x. [DOI] [PubMed] [Google Scholar]
- 42.Bergman RN, Zaccaro DJ, Watanabe RM, Haffner SM, Saad MF, Norris JM, Wagenknecht LE, Hokanson JE, Rotter JI, Rich SS. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;52:2168–2174. doi: 10.2337/diabetes.52.8.2168. [DOI] [PubMed] [Google Scholar]
- 43.Colilla S, Cox NJ, Ehrmann DA. Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab. 2001;86:2027–2031. doi: 10.1210/jcem.86.5.7518. [DOI] [PubMed] [Google Scholar]
- 44.Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–6223. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- 45.Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss JF, 3rd, Dunaif A, Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- 46.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 47.Gitau R, Adams D, Fisk NM, Glover V. Fetal plasma testosterone correlates positively with cortisol. Arch Dis Child Fetal Neonatal Ed. 2005;90:F166–169. doi: 10.1136/adc.2004.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta- cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]