Abstract
Serine/threonine kinase Akt/PKB is a downstream effector molecule of phosphoinositide 3-kinase and is thought to mediate many biological actions toward anti-apoptotic responses. We found that Akt formed a complex with a 90-kDa heat-shock protein (Hsp90) in vivo. By constructing deletion mutants, we identified that amino acid residues 229–309 of Akt were involved in the binding to Hsp90 and amino acid residues 327–340 of Hsp90β were involved in the binding to Akt. Inhibition of Akt-Hsp90 binding led to the dephosphorylation and inactivation of Akt, which increased sensitivity of the cells to apoptosis-inducing stimulus. The dephosphorylation of Akt was caused by an increase in protein phosphatase 2A (PP2A)-mediated dephosphorylation and not by a decrease in 3-phosphoinositide-dependent protein kinase-1-mediated phosphorylation. These results indicate that Hsp90 plays an important role in maintaining Akt kinase activity by preventing PP2A-mediated dephosphorylation.
Keywords: PDK1, PP2A, PI(3)K
The characterization of survival signal transduction pathways stimulated by growth factors has revealed that phosphoinositide 3-kinase [PI(3)K] is involved in protecting cells from apoptosis (1–3). After growth factor stimulation, PI(3)K is activated and generates phosphatidylinositol 3,4,5-triphosphate [PtdIns(3,4,5)P3], which raises a diverse set of cellular responses. One target of PI(3)K is the pleckstrin homology (PH) domain-containing serine/threonine kinase Akt (4, 5). By stimulation with growth factors and cytokines, Akt is recruited from the cytosol to the plasma membrane and is phosphorylated at two key regulatory sites, Thr308 and Ser473, by 3-phosphoinositide-dependent protein kinase-1 (PDK1) (6–11). It has recently been reported that activated Akt can phosphorylate Bad (12, 13), caspase-9 (14), and FKHRL1 (15), leading to their inactivation and to cell survival. Akt has also been shown to phosphorylate IκB kinase and up-regulate its kinase activity, which results in promotion of NF-κB-mediated inhibition of apoptosis (16, 17).
Cells encounter environmental stress through the coordinated synthesis of heat-shock proteins (18). Many heat-shock proteins are expressed at high levels, even in unstressed conditions, and are involved in many cellular processes (19). The 90-kDa heat-shock protein (Hsp90) is a highly conserved protein and is essential for viability in yeast (20). Hsp90 plays an important role in refolding certain denatured proteins under stress conditions. Unlike the more general Hsp70 and Hsp60 chaperones, Hsp90 appears to have substrate-specific folding activity. Hsp90 has an additional role in the conformational regulation of certain signal transduction molecules (19).
To clarify the regulation of Akt kinase activity, we screened the Akt binding proteins and found that Hsp90 could bind to Akt in vivo. The blockade of the binding to Hsp90 inactivated Akt and increased sensitivity of the cells to apoptosis-inducing stimuli. We found that Hsp90 binding to Akt protected Akt from protein phosphatase 2A (PP2A)-mediated dephosphorylation. Thus, interaction with Hsp90 might play an important role in regulating Akt kinase activity.
Materials and Methods
Cell Culture Conditions.
All of the cells were cultured in DMEM supplemented with 10% FBS. In some experiments, VP-16 (etoposide; kindly provided by Bristol–Meyers Squibb), Z-Asp-CH2-DCB (Funakoshi, Tokyo), LY294002 (Sigma), or Okadaic acid (Wako, Tokyo) were added to the culture medium.
Expression Vector Construction.
Human full-length akt1 and murine bcl-2 cDNAs were generated by reverse transcription–PCR (21–23). The human full-length hsp90α, hsp90β, and PDK1 cDNAs in a pcDNA3.1/GS vector were purchased from Invitrogen. Several akt and hsp90β fragments were generated by PCR. Converting both Thr308 and Ser473 codons to Asp codons in wild-type (WT)-akt generated an active form of akt cDNA, D2-akt (T308D/S473D-akt). The kinase-dead form of akt cDNA (KD–akt) was produced by converting Lys179 codon to Met codon. These cDNAs were subcloned into a pFLAG-CMV-2 vector (Kodak), a pc5FLAG vector (21), or a pHM6 vector (Roche Molecular Biochemicals). The active mouse akt1 cDNA in a pUSEamp vector was purchased from Upstate Biotechnology (Lake Placid, NY).
Transfection, Immunoprecipitation, and Western Blot Analysis.
The appropriate plasmids were transiently transfected by using Superfect transfection reagent (Qiagen, Chatsworth, CA). In some experiments, cells were cultured in serum-free medium for 24 h following incubation at 45°C for 20 min (heat shock). Then, cells were lysed in lysis buffer (20 mM Tris⋅HCl, pH 7.5/0.2% Nonidet P-40/10% glycerol/1 mM EDTA/1.5 mM MgCl2/137 mM NaCl/50 mM NaF/1 mM NaVO3/12 mM β-glycerophosphate/1 mM PMSF/1 mM aprotinin). The cell lysates were incubated with the protein G agarose conjugated with a control sheep IgG, a sheep anti-Akt polyclonal antibody (pAb), a sheep anti-PDK1 pAb (Upstate Biotechnology), or an anti-(His)6 mAb (CLONTECH), or with the agarose conjugated with an anti-FLAG M2 mAb (anti-FLAG M2 agarose; Sigma).
Western blot analysis was performed, as described previously (24). The pAbs used were to Hsp90 (StressGen Biotechnologies, Victoria, Canada), Akt, phospho-Akt (Ser473) (New England Biolabs), phospho-Akt (Thr308) (Upstate Biotechnology), phospho-mitogen-activated protein kinase (MAPK) (pTEpY), poly(ADP-ribose) polymerase (PARP) p85 fragment (Promega), and MAPK (Santa Cruz Biotechnology). The mAbs used were to V5 tag (Invitrogen), FLAG tag M2 (Sigma), HA tag (Roche Molecular Biochemicals), p21Waf1/Cip1, and PDK1 (Transduction Laboratories, Lexington, KY).
Assay of Akt and PDK1 Kinase Activity.
The Akt or PDK1 kinase activity was estimated by using an Akt kinase assay kit or a PDK1 kinase assay kit according to the manufacturer's instructions (Upstate Biotechnology).
In Vitro PDK1 and PP2A Treatment.
The immunoprecipitated FLAG-tagged Akt was incubated with recombinant human active PDK1 (Upstate Biotechnology) in the presence or absence of lipid vesicles containing PtdIns(3,4,5)P3 or with purified human PP2A (Upstate Biotechnology) at 30°C for 30 min. In some experiments, 1 μM Okadaic acid was added into the reactions. The reactions were electrophoresed and were subjected to Western blot analysis.
Estimation of Apoptosis.
Staining with 4′,6-diamidino-2-phenylindole (DAPI) and the estimation of the caspase activity in the cell lysates were carried out, as described previously (24).
Results
Binding of Akt to Hsp90 in Vivo.
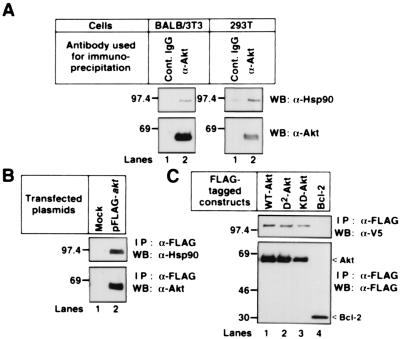
To find the molecules that interact with Akt, we performed immunoblot analysis after immunoprecipitation of Akt with an anti-Akt pAb. As shown in Fig. 1A, we found that endogenous Hsp90 interacted with endogenous Akt in both mouse fibroblast BALB/3T3 and human embryonic kidney 293T cells. The endogenous Hsp90 and the transfected V5-tagged Hsp90β formed a complex with WT-Akt, D2-Akt, KD-Akt, but not with Bcl-2 (Fig. 1 B and C). We could not detect the Akt binding to Hsp70 or to 14-3-3 protein (data not shown).
Figure 1.
Binding of Akt to Hsp90 in vivo. (A) BALB/3T3 and 293T cell lysates were incubated with protein G agarose conjugated with control sheep IgG or sheep anti-Akt pAb. (B) 293T cells were transfected with mock or the FLAG-tagged WT-akt. The cell lysates were incubated with an anti–FLAG M2 agarose. (C) 293T cells were cotransfected with the V5-tagged WT-hsp90β and the indicated FLAG-tagged plasmids. The cell lysates were incubated with an anti–FLAG M2 agarose. The immunoprecipitated proteins were immunoblotted with the indicated antibodies. Molecular size markers are indicated (in kDa).
Identification of the Domains Responsible for Akt–Hsp90 Interaction.
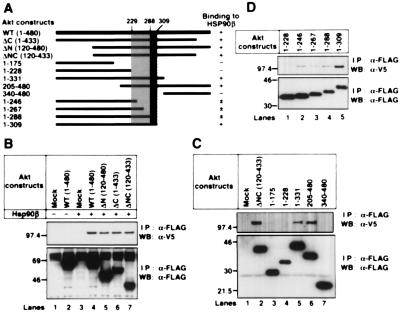
To identify the binding site in Akt, we prepared deletion mutants of Akt, as indicated in Fig. 2A. The V5-tagged Hsp90β was expressed in 293T cells with the FLAG-tagged WT- or deleted Akt (Fig. 2 B–D). Hsp90β could interact with WT-, ΔN-, ΔC-, and ΔNC-, 1–331, and 205–480 Akt (Fig. 2 B and C). Further analysis revealed that the binding of 1–246, 1–267, and 1–288 Akt to Hsp90β was diminished and was weaker than that of 1–309 Akt. No interaction was observed between 1–228 Akt and Hsp90β (Fig. 2D). These results indicate that amino acid residues 229–309 of Akt were involved in binding to Hsp90β and amino acid residues 289–309 of Akt were responsible for strong binding to Hsp90β (Fig. 2A, dotted and hatched areas, respectively).
Figure 2.
Binding properties of wild-type Akt and Akt deletion mutants to Hsp90β. (A) Structural domains of Akt and Akt deletion mutants used in these experiments are represented as black bars. The predicted sites that are involved in the strong and weak Hsp90β binding to Akt are shown schematically (hatched and dotted, respectively). (B–-D) Mock or the V5-tagged WT-hsp90β was transiently transfected into 293T cells together with mock or the indicated FLAG-tagged akt mutants. The FLAG-tagged proteins were immunoprecipitated and immunoblotted with the indicated antibodies. Molecular size markers are indicated (in kDa).
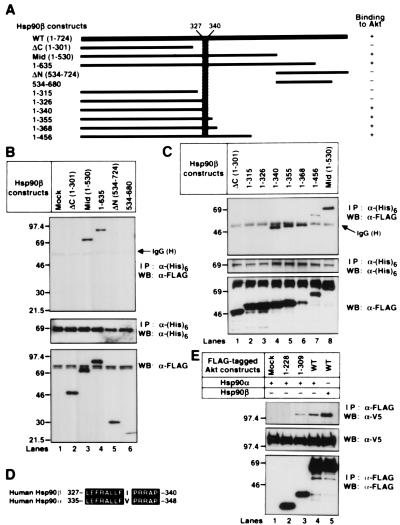
We then prepared deletion mutants of Hsp90β, as indicated in Fig. 3A. We found that Mid-, 1–635, 1–340, 1–355, 1–368, and 1–456 Hsp90β formed a complex with Akt (Fig. 3 B and C). We did the reciprocal immunoprecipitation-Western blot experiments and found that WT-Akt was coimmunoprecipitated with 1–340 and 1–635 Hsp90β but not with 1–326 Hsp90β (data not shown). These results indicate that amino acid residues 327–340 of Hsp90β are involved in binding to Akt (Fig. 3A, hatched areas). The identified binding domain of Hsp90β (L327EFRALLFIPRRAP340) was different from the corresponding Hsp90α sequence (L335EFRALLFVPRRAP348) by a single amino acid, Ile335 (Fig. 3D). Thus, we examined Akt binding to Hsp90α and found that Hsp90α could also bind to WT- and 1–309 Akt but not to 1–229 Akt (Fig. 3E).
Figure 3.
Identification of the Hsp90β domain responsible for binding to Akt. (A) Structural domains of Hsp90β and Hsp90β deletion mutants used in these experiments are represented as black bars. The predicted Akt binding site in Hsp90β is shown schematically (hatched). (B and C) The (His)6-tagged WT-murine akt was transiently transfected into 293T cells together with mock or the indicated FLAG-tagged hsp90β mutants. The (His)6-tagged proteins were immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with an anti–FLAG M2 mAb. Molecular size markers are indicated (in kDa). (D) Alignment of the amino acid sequence of the human Hsp90β with the equivalent region of human Hsp90α. Identical residues are denoted by white letters on black background. The GenBank accession nos. of human Hsp90β and human Hsp90α were M16660 and X15183, respectively. (E) The V5-tagged WT-hsp90α or WT-hsp90β was transiently transfected into 293T cells together with mock or the indicated FLAG-tagged akt mutants. The FLAG-tagged proteins were immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with an anti-V5 mAb. Molecular size markers are indicated (in kDa).
Interference with Akt Binding to Hsp90β Down-Regulates Akt Kinase Activity.
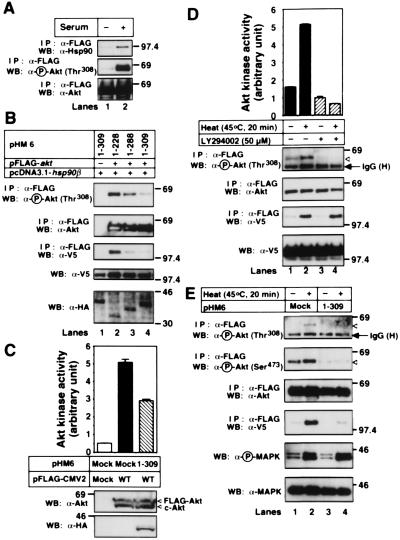
When 293T cells were serum starved, Akt was dephosphorylated. Under this condition, Akt was detached from Hsp90 (Fig. 4A). WT-Akt binding to Hsp90β and phosphorylation of WT-Akt were inhibited by overexpressing 1–288 and 1–309 Akt but not 1–228 Akt (Fig. 4B). Under this condition, Hsp90β bound to 1–288 or 1–309 Akt (data not shown). Inhibition of Akt-Hsp90 binding by expressing 1–309 Akt decreased Akt kinase activity to about 60% of the control (Fig. 4C). These results indicate that binding to Hsp90β plays an important role in maintaining Akt kinase activity.
Figure 4.
Dephosphorylation and inactivation of Akt after detachment from Hsp90. (A) 293T cells were transfected with the FLAG-tagged WT-akt. After transfection for 24 h, cells were cultured in the medium containing no serum (−) or 10% FBS (+) for 24 h. Then, the FLAG-tagged Akt was immunoprecipitated and immunoblotted with the indicated antibodies. (B) The HA-tagged akt mutants, the V5-tagged WT-hsp90β, and/or the FLAG-tagged WT-akt were cotransfected into 293T cells. The cells were serum-starved for 24 h, and then FLAG-tagged WT-Akt was immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with the indicated antibodies. (C) 293T cells were transfected with mock or the FLAG-tagged WT-akt together with mock or the HA-tagged 1–309 akt. The cells were serum-starved for 24 h and then harvested. The cell lysates were incubated with protein G agarose conjugated with a control IgG (lane 1) or an anti-Akt pAb (lanes 2 and 3). The Akt kinase activity of the immunoprecipitated Akt was estimated, as described in Materials and Methods. The vertical bars represent SD value of triplicate determinations. The immunoprecipitated proteins and the cell lysates were immunoblotted with the indicated antibodies. (D) HT1080 cells were transfected with the FLAG-tagged WT-akt together with V5-tagged WT-hsp90β. The cells were serum-starved for 24 h and then incubated at 37°C or 45°C for 20 min. In some experiments, cells were treated with 50 μM LY294002 for 10 min before heat shock. The Akt kinase activity of the immunoprecipitated FLAG-tagged Akt was estimated, as described in Materials and Methods. The vertical bars represent SD value of triplicate determinations. The cell lysates were also immunoblotted with the indicated antibodies. (E) HT1080 cells were transfected with the FLAG-tagged WT-akt, and the V5-tagged WT-hsp90β together with mock or the HA-tagged 1–309 akt. The cells were serum-starved for 24 h and then incubated at 37°C (−) or 45°C (+) for 20 min. The immunoprecipitated FLAG-tagged WT-Akt and the cell lysates were immunoblotted with the indicated antibodies. The expression of the HA-tagged 1–309 Akt was confirmed by immunoblot analysis with an anti-HA mAb (data not shown). Molecular size markers are indicated (in kDa).
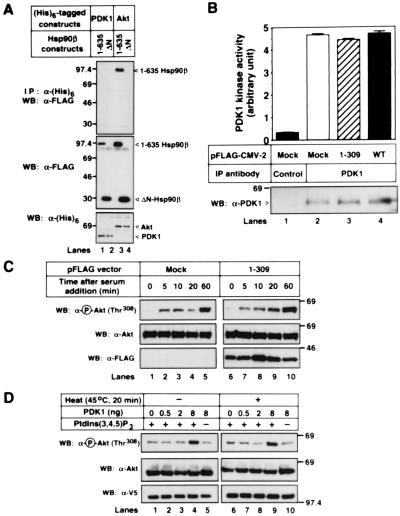
As previously reported (25), heat shock initiated Akt kinase activity and increased the amount of phospho-Akt in HT1080 cells (Fig. 4D). Under this condition, increased Akt binding to Hsp90β was observed. The heat shock-induced Akt phosphorylation and activation, but not Akt binding to Hsp90β, were prevented by pretreatment with the PI(3)K inhibitor LY294002. Therefore, phosphorylation of Akt at Thr308 was not required for Akt-Hsp90β binding. Overexpression of 1–309 Akt but not 1–228 Akt suppressed the heat shock-induced Akt binding to Hsp90β and Akt phosphorylation (Fig. 4E, and data not shown), indicating that NH2-terminal PH domain is not involved in Akt dephosphorylation. The heat shock-induced phosphorylation of MAPK was not affected by overexpression of 1–309 Akt (Fig. 4E). These results indicate that Akt binding to Hsp90β is essential for Akt phosphorylation.
Induction of Apoptosis by Inhibiting Akt Binding to Hsp90β.
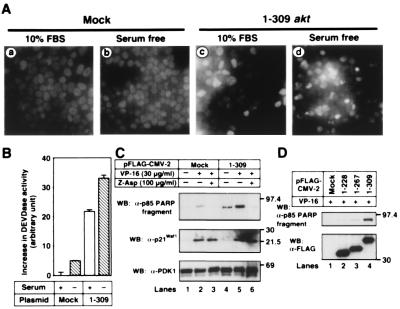
We next examined whether cells underwent apoptosis after inhibiting Akt-Hsp90β binding. Expression of 1–309 Akt alone induced apoptosis, to some extent, with nuclear condensation and fragmentation (Fig. 5A, c). During incubation of the cells in serum-free medium, many apoptotic cells were observed (Fig. 5A, d). Apoptotic cells were not observed in mock-transfected 293T cells (Fig. 5A, a), even when the cells were serum starved for 24 h (Fig. 5A, b). Overexpression of 1–309 Akt stimulated the activation of caspase-3-like protease, and the activity was promoted when cells were serum starved for 24 h (Fig. 5B). Almost no activity of caspase-3-like proteases was detected in the lysates of mock-transfected 293T cells.
Figure 5.
Induction of apoptosis by inhibiting Akt-Hsp90 binding. (A) 293T cells were transfected with mock or the FLAG-tagged 1–309 akt. After transfection for 24 h, the transfected cells were cultured for 24 h in serum-free medium or medium containing 10% FBS. Then the nuclei of the cells were stained with DAPI, as described in Materials and Methods. (B) 293T cells were treated as described in A. The increase in the activity of caspase-3-like proteases (DEVDase activity) was determined, as described in Materials and Methods. The vertical bars represent SD value of triplicate determinations. (C) 293T cells were transfected with mock or the FLAG-tagged 1–309 akt. After transfection for 24 h, the medium was replaced with serum-free medium containing vehicle (lanes 1 and 4), 30 μg/ml of VP-16 (lanes 2 and 5), or 30 μg/ml of VP-16 plus 100 μg/ml of Z-Asp-CH2-DCB (lanes 3 and 6). Cells were further incubated for 24 h and then harvested. The cell lysates were immunoblotted with the indicated antibodies. The expression of the FLAG-tagged 1–309 Akt was confirmed by immunoblot analysis with an anti–FLAG M2 mAb (data not shown). (D) 293T cells were transfected with mock or the indicated FLAG-tagged plasmids. After transfection for 24 h, the medium was replaced with serum-free medium containing 30 μg/ml of VP-16. Cells were further incubated for 24 h. The cell lysates were immunoblotted with the indicated antibodies. Molecular size markers are indicated (in kDa).
To estimate the effect of Akt-Hsp90 binding further, we treated cells with the chemotherapeutic drug VP-16 to induce apoptosis (Fig. 5C). PARP was one of the substrates of caspase-3 and was cleaved to produce the 85-kDa fragment (26). PARP was cleaved in 1–309 Akt-transfected 293T cells, but not in mock-transfected cells, cultured for 24 h in serum-free medium. Adding VP-16 promoted PARP cleavage. A caspase inhibitor Z-Asp-CH2-DCB suppressed the cleavage. We estimated the VP-16-mediated DNA damage by p21Waf1/Cip1 expression. It was equally induced in both mock- and 1–309 akt-transfected cells and was not inhibited by Z-Asp-CH2-DCB. No change was observed in the expression level of PDK1. Because 1–229 and 1–267 Akt had little effects on apoptosis induction (Fig. 5D), the apoptosis-inducing effect of 1–309 Akt might be the result of the detachment of endogenous Akt from Hsp90 but not of the dominant-negative effects of PH domain in 1–309 Akt. These results indicate that Hsp90 binding to Akt protects cells from undergoing apoptosis by preserving phospho-Akt level and Akt kinase activity.
Inhibition of Akt-Hsp90β Binding Increased Akt Dephosphorylation Catalyzed by PP2A.
The balance between phosphorylation and dephosphorylation might determine the amount of phospho-Akt. There are two possibilities for the mechanism by which inhibition of Akt binding to Hsp90β decreased the amount of phospho-Akt: first, Akt binding to Hsp90β promotes PDK1-mediated Akt phosphorylation; second, binding to Hsp90β protects Akt from PP2A-mediated dephosphorylation.
We first examined the Hsp90β-PDK1 binding. As shown in Fig. 6A, PDK1 could bind to neither 1–635 nor ΔN-Hsp90β, whereas Akt could bind to 1–635 Hsp90β. Thus, Hsp90 might not affect the kinase activity of PDK1. Furthermore, overexpression of WT- and 1–309 Akt did not affect the PDK1 kinase activity (Fig. 6B). This result was consistent with a previous report that PDK1's activity was unaffected by stimuli that strongly activate Akt through PI(3)K (27). We then examined the change of PDK1-mediated phosphorylation of Akt that either formed a complex with Hsp90 or did not. 293T cells were transfected with mock or 1–309 akt and were serum starved for 24 h, following incubation with serum-containing medium in the presence of 500 nM phosphatase inhibitor Okadaic acid. As shown in Fig. 6C, time-dependent Akt phosphorylation at Thr308 in vivo was equally observed whether Akt formed a complex with Hsp90β (by mock transfection) or did not (by 1–309 akt transfection). To confirm this result, we prepared Akt-Hsp90β complex by immunoprecipitating Akt from 293T cells that were incubated at 45°C or 37°C for 20 min. As shown in Fig. 6D, the in vitro sensitivity of PDK1-mediated phosphorylation of Akt at Thr308 was not affected whether the Akt formed a complex with Hsp90β (by incubation at 45°C) or not (by incubation at 37°C). Therefore, suppression of PDK1-mediated phosphorylation of Akt might not be the reason for Akt dephosphorylation after blocking the Akt-Hsp90β complex formation.
Figure 6.
Akt binding to Hsp90β does not promote the PDK1-mediated Akt phosphorylation. (A) The (His)6-tagged human PDK1 or WT-murine akt was transfected into 293T cells together with the FLAG-tagged 1–635 hsp90β () or 534–724 hsp90β (ΔN). The immunoprecipitated (His)6-tagged proteins and the cell lysates were immunoblotted with the indicated antibodies. (B) 293T cells were transfected with mock or the FLAG-tagged WT- or 1–309 akt. The cells were serum-starved for 24 h, and then the cell lysates were incubated with a protein G agarose conjugated with a control IgG (Control) or an anti-PDK1 pAb (PDK1). The PDK1 kinase activity was estimated, as described in Materials and Methods. The vertical bars represent SD value of triplicate determinations. The amount of the immunoprecipitated PDK1 proteins was estimated by performing Western blot analysis with an anti-PDK1 mAb. The expression of transfected FLAG-tagged WT- or 1–309 Akt was confirmed by immunoblot analysis with an anti-FLAG M2 mAb (data not shown). (C) Mock or the FLAG-tagged 1–309 akt was transfected into 293T cells together with the HA-tagged WT-akt. The cells were serum-starved for 24 h, and then the medium was replaced with medium containing 10% FBS and 500 nM Okadaic acid. At the indicated time points, cells were harvested and the cell lysates were immunoblotted with the indicated antibodies. (D) The FLAG-tagged WT-akt and the V5-tagged WT-hsp90β were cotransfected into 293T cells. The cells were serum-starved for 24 h and then incubated at 37°C or 45°C for 20 min. The FLAG-tagged WT-Akt was immunoprecipitated and incubated in vitro with the indicated amount of active human PDK1 in the presence (+) or absence (−) of lipid vesicles containing PtdIns(3,4,5)P3 at 30°C for 30 min. The reactions were electrophoresed and immunoblotted with the indicated antibodies. The expression of transfected WT-Akt and Hsp90β was confirmed by immunoblot of the cell lysates with an anti-FLAG M2 mAb and an anti-V5 mAb, respectively (data not shown). Molecular size markers are indicated (in kDa).
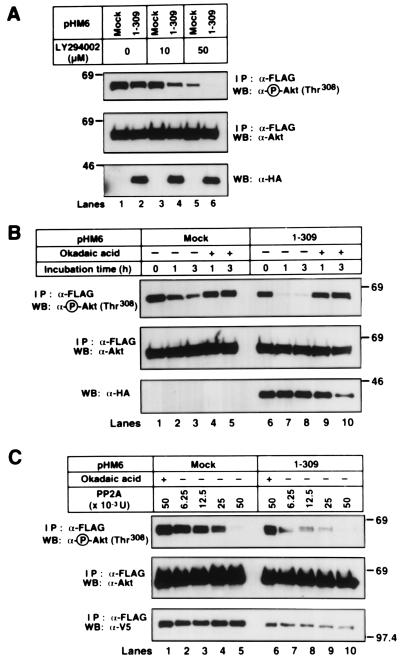
To examine the second possibility that the Akt binding to Hsp90β would affect PP2A-mediated Akt dephosphorylation, we examined the change of phospho-Akt level in 293T cells expressing 1–309 Akt after incubation with the PI(3)K inhibitor LY294002. The addition of 10 μM LY294002 decreased the phospho-Akt levels in 1–309 akt-transfected but not in mock-transfected 293T cells (Fig. 7A). Decrease in phospho-Akt levels was observed in both mock- and 1–309 akt-transfected 293T cells when cells were treated with 50 μM LY294002. When cells were serum starved, Akt was dephosphorylated in a time-dependent manner (Fig. 7B). Overexpression of 1–309 Akt resulted in the rapid dephosphorylation of Akt. The addition of 500 nM Okadaic acid rescued Akt from dephosphorylation. These results indicate that blocking the Akt-Hsp90β complex formation promotes PP2A-mediated Akt dephosphorylation.
Figure 7.
Inhibition of Akt-Hsp90β complex formation promotes PP2A-mediated Akt dephosphorylation. (A) The FLAG-tagged WT-akt was transfected into 293T cells together with mock or the HA-tagged 1–309 akt. After transfection for 24 h, cells were treated with 0, 10, or 50 μM LY294002 for 10 min. (B) Mock or the HA-tagged 1–309 akt was transfected into 293T cells together with the FLAG-tagged WT-akt. After transfection for 24 h, the medium was replaced with serum-free medium in the presence (+) or absence (−) of 500 nM Okadaic acid. (A and B) At the indicated time points, the FLAG-tagged WT-Akt was immunoprecipitated and immunoblotted with the indicated antibodies. The cell lysates were also immunoblotted with an anti-HA mAb. (C) Mock or the HA-tagged 1–309 akt was transfected into 293T cells together with V5-tagged WT-hsp90β and the FLAG-tagged WT-akt. The FLAG-tagged WT-Akt was immunoprecipitated and incubated in vitro with the indicated amount of purified human PP2A in the presence (+) or absence (−) of 1 μM Okadaic acid at 30°C for 30 min. The reactions were electrophoresed and immunoblotted with the indicated antibodies. Molecular size markers are indicated (in kDa).
We then try to confirm the increased sensitivity of freed Akt to PP2A-mediated dephosphorylation. Incubation with 6.25 and 12.5 mU purified human PP2A in vitro decreased the amount of phospho-Akt prepared from 1–309 akt-transfected but not from mock-transfected 293T cells (Fig. 7C). The PP2A-induced dephosphorylation was inhibited by adding 1 μM Okadaic acid. We, thus, concluded that detaching Akt from Hsp90β increased the sensitivity to PP2A-mediated dephosphorylation. These results indicate that Hsp90β binding to Akt may increase the amount of phospho-Akt and induce Akt activation by protecting it from PP2A-mediated dephosphorylation rather than by promoting PDK1-mediated phosphorylation.
Discussion
After stimulation with growth factors and cytokines, PI(3)K was activated, and the activated PI(3)K generated PtdIns(3,4,5)P3. Then Akt was recruited to the plasma membrane and was phosphorylated at Thr308 and Ser473 by PDK1 in a PtdIns(3,4,5)P3-dependent manner (7–11). The activity of serine/threonine-specific phosphatase PP2A has been reported to inhibit Akt kinase activity (28). Thus, the balance between PDK1-mediated phosphorylation and PP2A-mediated dephosphorylation might determine the Akt kinase activity in vivo. However, the mechanism that determines the balance remains unidentified.
Hsp90 is an abundant and highly conserved protein involved in a diverse array of cellular processes. Deletion studies in yeast have revealed that Hsp90 is essential for cell growth (20). In contrast to other heat-shock proteins, Hsp90 is not required for maturation or maintenance of most proteins in vivo. Most of the identified cellular targets are signaling proteins. Hsp90 acts as a chaperone for unstable signal transducers and keeps them poised for activation until they are stabilized by conformational changes associated with signal transduction. Our present results indicate that Akt is also a substrate of Hsp90 (Fig. 1); it formed a complex with Hsp90 (Fig. 3). Phosphorylation was not required for Akt binding to Hsp90 because the conversion of phosphorylation sites did not affect the binding to Hsp90 (Fig. 1C), and LY294002 did not affect the heat shock-induced Akt-Hsp90 complex formation (Fig. 4D).
The Akt binding domain of Hsp90 was different from the NH2-terminal domain responsible for binding to ATP, geldanamycin, unfolded proteins, and small peptides, or the COOH-terminal oligomerization domain (29–31). Our findings were supported by the fact that geldanamycin could not inhibit Akt binding to Hsp90 (data not shown). The connecting middle domain of Hsp90, where Akt could bind, was of variable length and was missing bacterial Hsp90s. Thus, the middle domain has been suggested to be involved in a function gain of eukaryotic Hsp90s (31). The middle domain of Hsp90 might regulate the cell growth and apoptosis by binding to Akt. This hypothesis was strengthened by the observation that Escherichia coli Hsp90 (which lack the middle domain) could not suppress the decrease in viability of hsp90(−/−) yeast strains (32).
Hsp90 was responsible for the stabilization of the phospho-Akt because inhibition of Akt-Hsp90 binding resulted in Akt dephosphorylation in vivo (Fig. 4 B and E), decrease in Akt kinase activity (Fig. 4C), and induction of apoptosis (Fig. 5). PDK1 might not be involved in the phospho-Akt level decrease in 1–309 akt-transfected cells (Fig. 6 B–D). These observations were supported by the fact that the PDK1's consensus sequence was T(F/L)CGT (in single-letter amino acid code) (27). PDK1 might phosphorylate Akt at Thr308 by recognizing the consensus sequence 308TFCGT312. Because Akt bound to Hsp90 through its amino acid residues 229–309, Hsp90 binding might not interfere with the recognition and phosphorylation capability of PDK1. Thus, detachment of Akt from Hsp90 might promote the PP2A-mediated dephosphorylation of Akt. Two lines of evidence support the assumption that PP2A was responsible for the dephosphorylation of Akt. First, suppression of PI(3)K-mediated phosphorylation by 10 μM LY294002 or by serum withdrawal led to the rapid dephosphorylation of Akt, which was detached from Hsp90 (Fig. 7 A and B); second, detachment of Akt from Hsp90 increased the sensitivity to PP2A-mediated dephosphorylation in vitro (Fig. 7C). Although the PP2A-recognition sequence has not been clarified yet, the site might overlap with the Hsp90 binding sequence (amino acid residues 229–309 in Akt).
When HT1080 cells were exposed to heat shock, Akt was activated (Fig. 4D). The heat shock-induced Akt activation was PI(3)K dependent (Fig. 4D), as reported previously (25). Thus, the interaction of PtdIns(3,4,5)P3, the product of the PI(3)K reaction, with the PH domain of Akt and the recruiting Akt to the plasma membrane might be a reason for the heat shock-induced Akt activation; the Hsp90 binding to Akt could be another reason. The fact that blocking the Akt-Hsp90 complex formation suppressed the heat shock-induced phosphorylation of Akt (Fig. 4E) indicates that inhibition of PP2A-mediated dephosphorylation by Hsp90 might be the most important reason for heat shock-induced Akt phosphorylation and activation.
In conclusion, we found that Akt could bind to Hsp90 in vivo. The formation of the Akt-Hsp90 complex stabilized the Akt kinase activity and protected cells from undergoing apoptosis by preventing phospho-Akt dephosphorylation that was catalyzed by PP2A. Because Akt is known to be overexpressed in certain human carcinomas, these results provide information that could aid in developing chemotherapeutic approaches by preventing anti-apoptotic Akt signaling pathway.
Acknowledgments
We thank Drs. Mikihiko Naito and Akihiro Tomida for helpful discussions. This study was supported by a special grant for Advanced Research on Cancer, a Grant-in-Aid for Cancer Research from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- MAPK
mitogen-activated protein kinase
- pAb
polyclonal antibody
- PARP
poly(ADP-ribose) polymerase
- PDK1
3-phosphoinositide-dependent protein kinase-1
- PH
pleckstrin homology
- PI(3)K
phosphoinositide 3-kinase
- PP2A
protein phosphatase 2A
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-triphosphate
- Hsp90
90-kDa heat shock protein
- WT
wild type
- KD-akt
kinase-dead form of akt cDNA
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170276797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170276797
References
- 1.Scheid M P, Lauener R W, Duronio V. Biochem J. 1995;312:159–162. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao R, Cooper G M. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 3.Yao R, Cooper G M. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- 4.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 5.Staal S P. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alessi D R, Andjelkovic M, Caudwell F B, Cron P, Morrice N, Cohen P, Hemmings B. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, et al. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 9.Stokoe D, Stephens L R, Copeland T, Gaffney P R J, Reese C B, Painter G F. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 10.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R J, Reese C B, McCormick F, Tempst P, et al. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 11.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes C P, Alessi D R. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 12.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 14.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T, Stanbridge E, Frisch S, Reed J C. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 15.Brunet A, Bonni A, Zigmond M J, Lin. M Z, Juo. P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 16.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. Nature (London) 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 17.Romashkova J A, Makarov S S. Nature (London) 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 18.Parsell D A, Lindquist S. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 19.Pratt W B. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- 20.Borkovich K A, Farrelly F W, Finkelstein D B, Taulien J, Lindquist S. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokudai S, Fujita N, Hashimoto Y, Tsuruo T. J Cell Physiol. 2000;182:290–296. doi: 10.1002/(SICI)1097-4652(200002)182:2<290::AID-JCP18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Fujita N, Tsuruo T. Biochem Biophys Res Commun. 1998;246:484–488. doi: 10.1006/bbrc.1998.8587. [DOI] [PubMed] [Google Scholar]
- 23.Hirotani M, Zhang Y, Fujita N, Naito M, Tsuruo T. J Biol Chem. 1999;274:20415–20420. doi: 10.1074/jbc.274.29.20415. [DOI] [PubMed] [Google Scholar]
- 24.Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- 25.Shaw M, Cohen P, Alessi D R. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 27.Belham C, Wu S, Avruch J. Curr Biol. 1999;9:R93–R96. doi: 10.1016/s0960-9822(99)80058-x. [DOI] [PubMed] [Google Scholar]
- 28.Andjelkovic M, Jakubowicz T, Cron P, Ming X F, Han J W, Hemmings A H. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prodromou C, Roe S M, O'Brien R, Ladbury J E, Piper P W, Pearl L H. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 30.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 31.Scheibel T, Siegmund H I, Jaenicke R, Ganz P, Lilie H, Buchner J. Proc Natl Acad Sci USA. 1999;96:1297–1302. doi: 10.1073/pnas.96.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer G, Louvion J F, Tibbetts R S, Engman D M, Picard D. Mol Biochem Parasitol. 1995;70:199–202. doi: 10.1016/0166-6851(95)00007-n. [DOI] [PubMed] [Google Scholar]