Abstract
Although adaptor ADAP (FYB) and its binding to SLP-76 has been implicated in TcR-induced “inside-out” signaling for LFA-1 activation in T cells, little is known regarding its role in LFA-1-mediated “outside-in” signaling. In this study, we demonstrate that ADAP and SLP-76-ADAP binding are coupled to LFA-1 costimulation of IL-2 production, F-actin clustering, cell polarization, and T cell motility. LFA-1 enhancement of anti-CD3-induced IL-2 production was completely dependent on SLP-76-ADAP binding. Further, anti-CD3 was found to require CD11a ligation by antibody or ICAM1 to cause T cell polarization. ADAP augmented this polarization induced by anti-CD3/CD11a, but not by anti-CD3 alone. ADAP expression with LFA-1 ligation alone was sufficient to polarize T cells directly and to increase T cell motility whereas the loss of ADAP in ADAP−/− primary T cells reduced motility. A mutant lacking SLP-76-binding sites (M12) blocked LFA-1 costimulation of IL-2 production, polarization, and motility. LFA-1-ADAP polarization was also dependent on src kinases, Rho GTPases, phospholipase C, and phosphoinositol 3-kinase. Our findings provide evidence of an obligatory role for the SLP-76-ADAP module in LFA-1-mediated costimulation in T cells.
Keywords: outside-in signaling, T cell activation
Integrins are transmembrane receptors that mediate cell adhesion, motility, proliferation, and differentiation. T lymphocytes express at least 12 integrins with αLβ2, leukocyte function-associated antigen-1 (LFA-1) also termed CD11aCD18, binding to intracellular adhesion molecule (ICAM)-1, -2, and -3 (1–3). Whereas LFA-1 is in a low-affinity state on resting cells, ligation by the antigen-receptor complex (TCR/CD3) generates “inside-out” signals that increase the avidity of binding by clustering (2, 3). Mediators involved in this process include CD4/CD8-Lck, IL2-inducible T cell kinase (ITK), the guanine nucleotide exchange factor Vav-1, phosphatidylinositol 3-kinase (PI 3K), Rap1 and its binding partner RapL (regulator of cell adhesion and polarization enriched in lymphoid tissues) or Riam (Rap1-interacting adaptor molecule), and adaptors SLP-76 (76-kDa src homology 2 domain-containing leukocyte phosphoprotein), ADAP (adhesion and degranulation-promoting adaptor protein) [HUGO Gene Nomenclature: FYB-120/130 (Fyn-binding protein-120/130)], and SKAP1 [src kinase-associated phosphoprotein 1, also SKAP-55 (55-kDa src kinase-associated phosphoprotein)] (3, 4).
SLP-76 is an immune cell adaptor with key N-terminal tyrosine residues and a C-terminal SH2 domain that binds to ADAP (5, 6). ADAP is also an adaptor with a unique N-terminal region, a canonical and a noncanonical SH3 domain, a putative Ena/VASP homology 1 (EVH1)-binding domain, and nuclear localization motifs (7, 8). ADAP deficient T cells show defective LFA-1 clustering and adhesion (9, 10). SLP-76 SH2 domain binds to the 2 YDDV sites on ADAP (Y595/651DDV) (11–13). Mutation of the sites disrupts LFA-1 clustering and adhesion, localization at the periphery of the immunological synapse and T cell proliferation (11, 14). ADAP also regulates adhesion of platelets and a basophilic cell line (15, 16). Further, ADAP interacts with SKAP1 primarily via SKAP-1 SH3 domain binding to ADAP proline residues, as well as by ADAP SH3 domain binding to a noncanonical motif in SKAP1 (17–20). These interactions are needed for adhesion and T cell proliferation (21–23).
In addition to inside-out signaling, LFA-1 generates so-called “outside-in” signals that costimulate cell function (4, 24, 25). α4β1 integrin VLA-4 binding to its ligands fibronectin or VCAM-1 (vascular cell adhesion molecule-1) enhances T cell proliferation via FAK (focal adhesion kinase) (26) and the stabilization of microclusters (27). In neutrophils or macrophages, phosphorylation of ITAMs (immunoreceptor tyrosine-based activation motifs) in adaptor DAP12 or FcRγ is needed to recruit SYK for outside-in signaling (28). An analogous ITAM-dependent scenario in T cells has yet to be defined. Other players include SLP-76 and Vav-1 where SLP-76 deficient neutrophils fail to spread with reduced Vav-1 phosphorylation (29). Similarly, N-terminal SLP-76 tyrosine binding to Vav-1 (6) is needed for integrin signaling (30). Upon integrin ligation, Vav−/− macrophages show decreased phagocytosis with defective Rho and PYK2 (proline-rich tyrosine kinase-2) activity (31).
Despite these advances, the full range of mediators responsible for LFA-1 costimulatory effects in T cells is unclear. In this study, LFA-1 potentiation of anti-CD3-induced IL-2 production was completely dependent on SLP-76-ADAP binding. Anti-CD3 was found to require CD11a ligation by antibody or ICAM1 to cause T cell polarization, an effect potentiated by ADAP and blocked by the SLP-76 binding mutant (hereafter called M12). ADAP also cooperated with LFA-1 alone to polarize T cells directly and to increase T cell motility whereas expression of M12 and the loss of ADAP reduced motility.
Results
LFA-1-Induced Cosignaling Requires SLP-76-ADAP Binding.
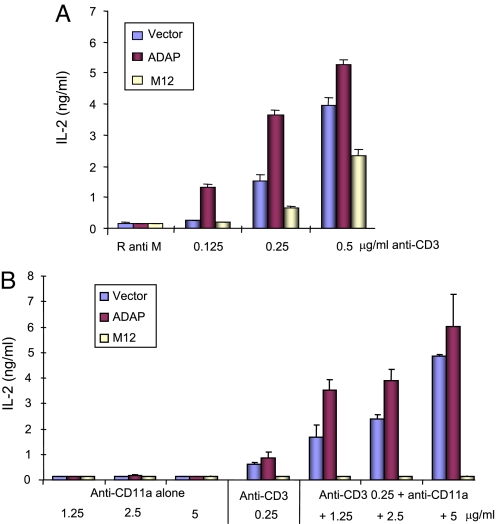
We showed that the SLP-76 SH2 domain interacts with 2 YDDV sites in ADAP (11–13), and mutation of these sites (termed M12) inhibits the TcR-driven inside-out activation of LFA-1 and IL-2 production (14). However, little is known regarding the role of the complex in LFA-1 driven outside-in signaling. To assess this, murine T8.1 T cells stably expressing GFP or ADAP/GFP or M12/GFP (termed GFP-T8.1, ADAP-T8.1, or M12-T8.1) were seeded on plates coated with anti-CD3 and/or anti-LFA-1 (i.e., anti-αL chain, also termed anti-CD11a) and assessed for IL-2 production (Fig. 1). Increasing anti-CD3 concentrations augmented the production of IL-2, an effect increased further by ADAP overexpression (Fig. 1A) (7). For example, at 0.25 μg/mL anti-CD3, ADAP-T8.1 cells showed 2.5-fold more IL-2 production than vector-transfected cells. By contrast, M12 inhibited IL-2 production by >50% (Fig. 1A). However, increasing anti-CD3 concentrations were able to partially reverse the inhibitory effect of M12 (Fig. 1A, yellow bars). These results indicate that the SLP-76-ADAP complex formation is needed by optimal anti-CD3 induced IL-2 production although the dependency could be partially overridden by increasing the strength of the anti-CD3 induced signal. A similar finding was made by us and others in ADAP- and SKAP1-deficient T cells (23, 32).
Fig. 1.
SLP-76-ADAP binding is crucial for LFA-1 cosignaling to amplify IL-2 production. (A) GFP-T8.1, ADAP-T8.1 and M12-T8.1 cells were cultured in various concentrations of anti-CD3 antibody coated 96-well plates for 40 h and IL-2 in the supernatant was measured by ELISA. (B) Titrated anti-CD11a antibody alone or together with anti-CD3 was used to stimulate GFP-T8.1, ADAP-T8.1, and M12-T8.1 cells for 40 h. The level of IL-2 was measured by ELISA. Data represent the means ± SD (n = 3; P < 0.01). The results are representative of 3 independent experiments.
Given this, we next assessed the contribution of SLP-76-ADAP to LFA-1 cosignaling. Suboptimal levels of anti-CD3 (i.e., 0.25 μg/mL) were used to measure the anti-CD11a costimulatory effects. Although anti-CD11a ligation alone failed to induce IL-2 production, it increased IL-2 production in a concentration dependent fashion with anti-CD3 (Fig. 1B, blue bars). ADAP transfection further amplified this costimulation (Fig. 1B, red bars) whereas M12 completely blocked the enhancement (Fig. 1B, yellow bars). Significantly, unlike in the case of anti-CD3, increasing concentrations of anti-CD11a (i.e., from 1.25 to 5 μg/mL) failed to overcome the M12 block. Unlike with anti-CD3, LFA-1 costimulation is therefore entirely dependent on the SLP-76-ADAP complex.
LFA-1-Induced T Cell Polarization Requires SLP-76-ADAP.
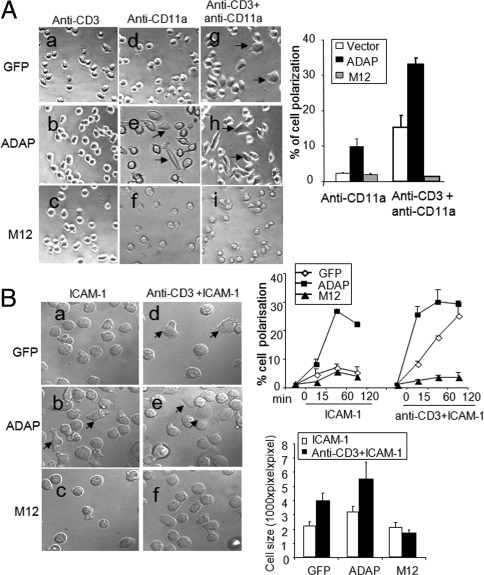
We next assessed a connection between LFA-1 and SLP-76-ADAP in T cell polarization. T8.1 cells expressing GFP, ADAP, or M12 were incubated with either immobilized antibodies (Fig. 2A) or natural ligand ICAM-1 (Fig. 2B) on plates and were visualized for various times by transmitted light microscopy. Over the short time course (i.e., 120 min), T8.1 cells showed no change in morphology with various anti-CD3 concentrations (i.e., titrated to 10 μg/mL, Fig. S1A). Further, ADAP or M12 overexpression did not promote polarization on cells stimulated with anti-CD3 (see examples at 60 min in Fig. 2A, b and c vs. a). In striking contrast, whereas anti-CD11a alone had no detectable effect on morphology in vector cells (Fig. 2Ad), coligation with anti-CD3 induced extensive spreading and polarization (Fig. 2Ag, right histogram). Fifteen percent versus 2% of control cells polarized with this antibody combination. The effect was specific and was not due to the use of higher concentrations of antibody since equivalent concentrations of anti-CD3 did not induce these effects. This observation indicates that LFA-1 provides signals that cooperate with anti-CD3 ligation to induce T cell morphology changes.
Fig. 2.
LFA-1 signaling induced T cell polarization requires SLP-76-ADAP interaction. (A and B) GFP-T8.1, ADAP-T8.1 and M12-T8.1 cells were seeded to antibodies or ICAM-1 coated plates and incubated at 37 °C for indicated time courses and cell polarization was assessed at each time points. The size of the cells was analyzed by Image J software at 60 min. Images are representative of at least 3 independent experiments. Data in the histogram represent the means ± SD (n = 3). No significant difference was seen when M12 transfected cells were compared with control cells in ICAM-1 coated plates (P > 0.05) at different time points whereas ADAP overexpression significantly increased T cell polarization (P < 0.01).
Significantly, a connection to ADAP was seen by the ability of overexpressed ADAP to potentiate anti-CD3/CD11a induced polarization as observed in 33% of cells (Fig. 2Ah, right histogram), and second, by the ability of M12 to completely block polarization (Fig. 2 Af and Ai, right histogram). Further, polarization could also be observed by expressing ADAP with anti-CD11a alone (Fig. 2Ae), where 10% of cells showed a polarized phenotype.
Significantly, ICAM-1 on plates showed the same effects (Fig. 2B). Whereas ICAM-1 alone failed to polarize vector cells (Fig. 2Ba), anti-CD3 and ICAM-1 induced extensive spreading and polarization in vector cells over the time course of 0–120 min (Fig. 2Bd, upper right histogram). Further, ADAP overexpression with ICAM-1 polarized 25–30% of cells (Fig. 2Bb, upper right histogram). ADAP also potentiated polarization induced by anti-CD3/ICAM-1 (Fig. 2Be, upper right histogram). In this case, the rate of polarization was more rapid than observed with ADAP and anti-CD11a alone (i.e., optimal spreading by 15 vs. 60 min). Further, M12 failed to support polarization induced by anti-ICAM-1 alone (Fig. 2Bc), and blocked anti-CD3/ICAM-1 induced polarization (Fig. 2Bf, upper right histogram). Image J software analysis of the perimeter of cells confirmed that ADAP increased the cell size relative to GFP control cells (Fig. 2B, lower right histogram) whereas M12 inhibited these changes (Fig. 2 A and B). In this context, SLP-76-deficient Jurkat cells (J14 cells) also failed to undergo polarization (Fig. S1B). Real-time analysis showed that ADAP T8.1 cells formed dynamic extensions with the appearance of multiple lamellipodia and filopodia whereas M12 cells failed to spread (Movie S1). These observations indicate that ADAP and SLP-76-ADAP binding can cooperate directly with anti-CD11a or ICAM-1 to induce T cell polarization.
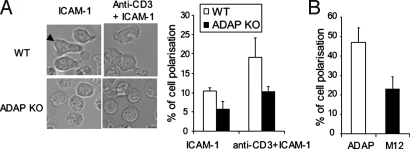
To confirm in primary T cells, WT, or ADAP-deficient T cells were stimulated with anti-CD3 and ICAM-1 and analyzed for morphology changes (Fig. 3A). Whereas 20% of WT cells polarized in response to anti-CD3/CD11a, only 10% of ADAP KO T cells became polarized. With immobilized ICAM-1, ADAP KO cells showed a 2-fold reduction in polarization relative to WT cells (i.e., 11% vs. 5%). Purified primary T cells were infected by retroviral gene transfer to overexpress ADAP/GFP or M12/GFP (Fig. 3B, left and right histogram). Whereas 48% of ADAP/GFP+ transduced CD4+ T cells spread, only 21% of M12/GFP+ transduced CD4+ T cells polarized (Fig. 3B). These observations confirm that in primary cells ADAP and its binding to SLP-76 plays a central role in outside-in costimulation.
Fig. 3.
ADAP and SLP-76-ADAP interaction regulates LFA-1 signaling induced primary T cell polarization. WT and ADAP deficient T cells (A) or mouse CD4+ T cells overexpressing ADAP or M12 (B) were loaded to ICAM-1 or anti-CD3 and ICAM-1 coated plates. Images were taken after 60 min and are representative of 2 independent experiments. Data in the histogram represent the means ± SD (P < 0.01).
ADAP Regulates T cell Motility.
Given that polarization is associated with movement, we next investigated the role of LFA-1/ADAP on T cell motility (Fig. 4). Cell movement was recorded every 5 s for 200 cycles followed by Velocity software analysis. As seen in tracking plots, WT cells moved randomly on ICAM-1-coated plates (Fig. 4A Left Upper). Increased ADAP expression augmented the number and extent of T cell movement (i.e., average speed of 10.2 vs. 5.4 μM/min) (Fig. 4 A and B and Movie S2). By contrast, the expression of M12 completely blocked motility (Fig. 4 A and B). This observation provided evidence that ADAP binding to SLP-76 regulates T cell motility and is consistent with the increased polarization of ADAP-T8.1 cells.
Fig. 4.
ADAP regulates T cell motility. Vector and ADAP-T8.1 cells (A and B) or WT and ADAP-deficient T cells (C) were loaded to ICAM-1 alone or anti-CD3 and ICAM-1-coated surfaces and cell movements were recorded by time lapse. Cell motility and tracks was analyzed by Velocity software. Data are presented as tracking diagrams and as plots.
We then assessed whether the LFA-1-ADAP axis could influence the stop-signal induced by anti-CD3 that might enable T cells to make contacts with APCs (33). Our previous data showed that the coreceptor CTLA-4 overrides the TcR stop-signal for motility (34, 35). Interestingly, anti-CD3 blocked T cell motility in both vector and ADAP-T8.1 cells (Fig. 4A Lower and B). There was no evidence that ADAP overexpression could override the anti-CD3 stop signal. Further, in a comparison of WT and ADAP−/− primary T cells, although WT cells moved more quickly than ADAP−/− cells in ICAM-1 plates, both were stopped by anti-CD3 ligation (Fig. 4C). ADAP is needed for optimal T cell motility in response to ICAM-1, but does not affect the TcR stop-signal.
The SLP-76-ADAP Module Regulates F-Actin Aggregation.
Given effects on polarization and motility, we assessed the distribution of F-actin (Fig. 5A). T cells were stimulated with anti-CD3/CD11a and stained with TRITC conjugated phalloidin. Discrete F-actin aggregates or bundles were shown in 60–70% of vector and ADAP-T8.1 cells (Fig. 5A). By contrast, resting cells showed an absence of these clusters, and only 17% of M12-T8.1 cells formed F-actin aggregates with smaller cell size (Fig. 5A, right histogram). The overall level of stained F-actin in ADAP and M12 cells remained constant as measured by the mean of immunofluorescence staining of TRITC-phalloidin (Fig. 5A, right histogram). This indicates that the M12 affects the remodeling rather than the overall level of F-actin expression. This effect might be related to binding sites on ADAP for members of the Ena/VASP family (36). Whether the SLP-76-ADAP complex regulates adhesion/spreading first that leads to actin remodeling or vice versa is not clear.
Fig. 5.
SLP-76-ADAP binding is needed for F-actin polymerization but not for SKAP1 stabilization and ADAP-SKAP1 interaction. (A) GFP-T8.1, ADAP-T8.1, or M12-T8.1 cells were seeded to anti-CD3 and anti-CD11a-coated coveslips for 60 min and stained with TRITC-phalloidin. Mean of fluorescence intensity (MFI) of F-actin staining was measured by Image J software. Images are representative of 3 independent experiments. Data in the histogram represent the means ± SD (n = 3). Compared ADAP to M12-transfected samples, P < 0.001 when F-actin clustering formation was measured whereas P = 0.4578 when MFI of F-actin was measured. (B) Cell lysates were prepared for immunoblotting with various antibodies (Left) or immunoprecipitation with anti-ADAP followed by blotting with anti-SKAP1 (Right). (C and D) Cells were either stimulated with anti-CD3 (C) or with Ttox peptide pulsed L625 cells (D) followed by immunoblotting with 4G10.
M12-T8.1 Cells Reduce ADAP Phosphorylation Without Affecting SKAP1 Expression.
To investigate the underlying mechanism further, we assessed whether M12 indirectly affected LFA-1 signaling by altering SKAP1 expression. ADAP regulates SKAP1 degradation where ADAP−/− cells reduced SKAP1 expression (20, 23, 37), However, SKAP1 expression was not reduced in M12 expressing cells. In fact, the expression of both ADAP and M12 increased SKAP1 expression (Fig. 5B Left, lanes 2 and 3 vs. 1). Levels of SLP-76 or VAV-1 were not affected. Further, M12 bound SKAP1 at the same level as ADAP and the interaction was not increased by anti-CD3 stimulation (Fig. 5B Right, lanes 3, 4, 7, and 8 vs. 2 and 6). SKAP1-EGFP-transfected cells were used as a positive control (Fig. 5B Right). These findings indicate that SLP-76 binding to ADAP is not needed for ADAP binding to SKAP1, and that M12 does not inhibit by affecting downstream SKAP1 expression.
Our previous work has shown that the phosphorylation of the YDDV residues on ADAP is needed for SLP-76 binding (11). Similarly, anti-CD3 induced phosphorylation of M12 on tyrosine residues was reduced relative to endogenous and transfected ADAP (Fig. 5C). We showed that LFA1-ICAM-1 binding plays a dominant role for conjugate formation between T8.1 cells and antigen-presenting cells (APCs) L625.7 cells that express HLA-DR β1*1102 to present Ttox peptide (14). M12 decreased tyrosine phosphorylation compared with ADAP after T-APC conjugates formed for 30 min in the absence (Fig. 5D Upper, lane 4 vs. 3) and presence of peptide (lanes 7 vs. 6). As a loading control, the same amount of β-actin protein was detected in each sample (Fig. 5D Lower). This was consistent with the finding that M12 also strongly blocked Ca2+ mobilization when Fura-2 prelabeled M12-T8.1 cells formed conjugates with peptide pulsed APCs (Fig. S1C). These observations are consistent with the notion that the 2 YDDV sites for SLP-76 binding serve as major phosphorylation sites in ADAP for TcR/LFA-1 stimulation.
Src Kinases, PI 3K, PLC, and RhoGTPase Are Needed for LFA-1-ADAP-Induced Polarization.
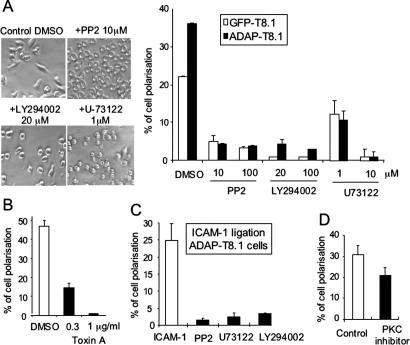
To evaluate the involvement of other mediators in SLP-76-ADAP modulation of T cell spreading, vector or ADAP-T8.1 cells were pretreated with inhibitors, then loaded to anti-CD3- and anti-CD11a-coated plates (Fig. 6). The concentrations of inhibitors in this study were reported and did not affect cell viability. Src kinase inhibitor PP2 was used at 10 μM or 100 μM to inhibit LCK and FYN, but not ZAP-70 (38). PP2 significantly reduced ADAP-T8.1 cell spreading (Fig. 6A). This fits with the findings that FYN phosphorylates ADAP (12, 13) and the M12 mutant, with lower levels of phosphorylation, blocks T cell polarization (Fig. 5 C and D). PI 3K inhibitor LY294002 also effectively blocked ADAP-T8.1 cell shape change, as did the phospholipase C (PLC) inhibitor U-73122 at 10 μM (Fig. 6A). The negative control U-73343 at 10 μM did not influence ADAP-T8.1 spreading. Toxin A that inhibits Rho, Rac, and Cdc42 activity also completely blocked cell polarization without any cell viability loss at 1 μg/mL (Fig. 6B). GTPases Rac and Cdc42 regulate lamellipodial and filopodial formation respectively whereas Rho GTPases regulate actin stress fibers formation for cell spreading. This inhibition by Toxin A is therefore consistent with the induction of lamellipodial and filopodia by anti-CD3/CD11a ligation. Significantly, inhibitors against Src kinases, PI 3K, and PLC also inhibited ADAP-T8.1 cell polarization after stimulation with ICAM-1 alone (Fig. 6C and Fig. S1D). PLCγ1 hydrolyzes PIP2 into inositol (3, 4, 5)-triphosphate (IP3) to mobilize Ca2+ and diacylglycerol (DAG) to activate PKC. Although PKC inhibitor 20–28 that specifically competes for DAG, had significant blocking effect on spreading (Fig. 6D) (P < 0.05), it inhibited polarization at a much less degree compared with the inhibitors against Src kinases, PI 3K, and PLC. This is consistent with previous reports that active PKC isotypes did not induce LFA-1 conformation changes (39).
Fig. 6.
Src kinases, PI 3K, PLC, and RhoGTPase is needed for ADAP-induced cell polarization. Src kinases inhibitor PP2, PI 3K inhibitor LY294002, PLC inhibitor U-73122, and the negative control U-73343 (A), Rho GTPase inhibitor Toxin A (B), or cell permeable PKC inhibitor 20–28 (D) was used to pretreat vector control cells or ADAP-T8.1 cells followed by incubation in anti-CD3 and anti-CD11a antibodies coated plates. Images are representative of at least 3 independent experiments. Data in the histogram represent the means ± SD (n = 3). (C) ADAP-T8.1 cells were pretreated with various inhibitors as described in A and loaded to ICAM-1-coated plates for 60 min.
Discussion
LFA-1 plays a central role in regulating T cell function and the development of autoimmune disease and inflammation (40). In addition to mediating ICAM-1 adhesion, it can generate outside-in signals that costimulate T cells (25, 41, 42). The nature of the outside-in pathway has been unclear, but may involve PYK-2 (proline-rich tyrosine kinase 2) and FAK (24, 25). ADAP and its binding to SLP-76 can regulate TcR mediated inside-out signaling for integrin activation (9, 10, 14). In this study, one central finding was that LFA-1 ligation by antibody, or ICAM-1 cooperated with anti-CD3 to provide a unique signal that induced T cell polarization (Figs. 2 and 3). Although a titration of various concentrations of anti-CD3 alone failed to affect morphology over the incubation period (i.e., 120 min), the simple coligation of LFA-1-induced polarization. This was not the result of increased affinity for ICAM1 because both anti-LFA-1 and ICAM1 had the same effect. Therefore, LFA-1 coligation provided a distinct, additional signal for polarization. ADAP augmented this polarization in conjunction with anti-CD3/CD11a, but not with anti-CD3 alone, whereas M12 blocked the phenotype. Further, ADAP overexpression in conjunction with LFA-1 ligation sufficed to polarize T cells (Fig. 2). The level of polarization was not as high as observed with anti-CD3/CD11a, but was nevertheless significant and rapid (Fig. 2, i.e., 10 vs. 30% within 60–120 min of ligation). From this, it is clear that LFA-1 signaling has a close connection to ADAP and requires the SLP-76-ADAP complex to generate signals for T cell polarization. Except for being a part of the LFA-1-mediated outside-in pathway per se, whether ADAP and SLP-76-ADAP can also provide a substitute signal that is normally initiated by anti-CD3 remains to be determined.
Our findings also implicate ADAP and ADAP-SLP-76 in T cell motility (Fig. 4). Motility requires alterations in the affinity of LFA-1 and signaling events that induce the contractile forces needed for cell movement. Actin and various myosins and other signaling events have been reported to induce T cell motility. Motility was measured as random movement on the surface of ICAM-1-coated plates (Fig. 4). Overexpression of ADAP in T8.1 cells caused a 2-fold increase in the random motility of T cells whereas M12 completely blocked cell movement (Fig. 4A). Similarly, ADAP−/− primary T cells showed a loss of motility, confirming that ADAP is needed for optimal T cell motility in the context of LFA-1 engagement. LFA-1 affinity and avidity changes are needed for T cell motility (43). The blockade of motility by M12 could be linked to reduced LFA-1 clustering on cells needed for movement but did not involve a loss of SKAP1 expression because both WT ADAP and M12 increase the expression of SKAP1. In either case, ADAP induced motility was not robust enough to overcome the ability of anti-CD3 to induce the TcR “stop signal” for motility arrest. Not surprisingly, this implies that the TCR engages additional signals that arrest motility aside from ADAP. Our findings represent a report implicating ADAP and SLP-76-ADAP in the promotion of random T cell motility. It also suggests that motility is influenced by LFA-1-induced outside-in signals that occur followed the initial up-regulation of LFA-1 activation on cells. Others have reported that ADAP is needed to increase chemokine SDF-1 induced directional motility in vitro (44), but is dispensable for naïve T cell trafficking to lymph nodes in vivo (32).
Our work showed that the ability of M12 to block costimulation was not due to a reduction in the expression of SKAP1, as observed in ADAP-deficient T cells (20, 23). In fact, as mentioned, both WT ADAP and M12 overexpression resulted in an increase in SKAP1 expression (Fig. 5B). This indicates that SLP-76 binding to ADAP is not needed for the ability of ADAP to protect SKAP1 from degradation. How the LFA-1-ADAP axis cooperates with other signaling proteins remains to be determined. One possibility is that M12 might bind and occupy SKAP1 to block endogenous ADAP binding to SKAP1. Alternatively, previous work has implicated kinases PYK-2 and FAK in LFA-1 outside-in signaling using affinity activating antibodies (24, 25). Our previous work showed that FYN phosphorylates ADAP (12, 13). However, it is possible that PYK-2 or FAK could phosphorylate it in response to LFA-1 ligation since FYN-deficient mice showed a reduced, but still some residual ADAP phosphorylation (45). In support of this, the Src kinase inhibitor did not completely block T cell spreading. Other mediators such as Rho GTP'ases, PLC, and PI 3K may operate in conjunction with the SLP-76-ADAP module or other effectors (Fig. 6). Rap1 and VAV-1 have also been implicated in outside-in signaling (24, 46). Cytoskeleton protein talin binds LFA-1 and controls LFA-1 activation (47), and our preliminary data also showed that M12 reduced the association of LFA-1-talin in resting states.
Last, our findings are consistent with a possible requirement for ITAMs in integrin signaling (28). In neutrophils or macrophages, phosphorylation of ITAMs in FcRγ and adaptor DAP12 can recruit SYK kinase for outside-in signaling (28). Although an analogous ITAM dependent system in T cells has yet to be defined, SLP-76 is phosphorylated by ZAP-70 (6), a kinase recruited by upstream ITAMs. By acting in response to both TcR and LFA-1 receptor ligation, the SLP-76-ADAP module is ideally placed to act as a node to integrate inside-out and outside-in signaling events. TCR would use the SLP-76-ADAP module to initially promote LFA-1 adhesion and clustering. The clustered LFA-1 increases its avidity for binding to the ligand ICAM-1. Followed by LFA-1-ICAM-1 binding, outside-in signals are generated that are dependent on the SLP-76-ADAP module again to promote cell polarization and IL-2 amplification. In this way, TcR ligation would increase LFA-1 adhesion that then acts in a feedback loop to promote LFA-1 outside-in signaling for other cell events such as increased polarization. Anti-CD11a engagement of SLP-76-ADAP might also operate together with other signals such as cytokine or chemokine to induce further ADAP-YDDV phosphorylation and SLP-76 recruitment.
The mechanism by which LFA-1 induces polarization could involve the presence of G-actin and VASP-binding sites on the adaptor to regulate the actin cytoskeleton (36). T cell polarization was accompanied by the appearance of actin aggregates, whereas M12 markedly reduced the number and size of aggregates. Whether this effect is a direct consequence of defective ADAP function is unclear. SLP-76 has been implicated in integrin αIIbβ3 triggered actin remodeling in platelets (48) whereas receptor-ADAP chimeras can induce the formation of actin “clouds” in T cells (49). Both sets of observations are consistent with a role for ADAP and SLP-76-ADAP binding in the regulation of the actin cytoskeleton. Future studies will be needed to determine the manner by which these various components intersect in the mediation of LFA-1 costimulation.
Materials and Methods
Antibodies, Reagents, and Cells.
Tritc-conjugated anti-phalloidin was purchased from Sigma–Aldrich. Anti-mouse CD3ε (2C11) and anti-CD11a (M17/4) and IL-2 ELISA kit were from BD PharMingen. Murine ICAM-1 Fc was purchased from R&D Systems. Src kinases inhibitor PP2, PI 3K inhibitor LY294002, phospholipase C inhibitor U-73122, and negative control U-73343, myristoylated protein kinase C inhibitor 20–28, and RhoGTPase inhibitor Toxin A were from Calbiochem. ADAP KO C57Black6 mice were kindly provided by Dr. Erik Peterson (University of Minnesota, Minneapolis). Murine hybridomas T8.1 cells were a gift from Dr. Oreste Acuto (University of Oxford, UK). T cells overexpressing ADAP/GFP or M12/GFP were generated and cultured as described in ref. 21.
Imaging Cell Morphology Change and Actin Clustering.
Anti-CD3 (0.5 μg/mL), anti-CD11a (5 μg/mL), and ICAM-1 (5 μg/mL) were used to coat plates at 4 °C overnight. GFP, ADAP, or M12-transduced primary mouse T cells or T8.1 cells were incubated in antibodies coated plates at 37 °C. Cell morphology changes were taken under bright light using Leica confocal microscopy at various time points. Cell movement was recorded every 5 s for 200 cycles and cell migration speed was measured by Velocity software. The contact area of the cell with plates was outlined to measure cell size using Image J software. For F-actin staining, cells were fixed/permeablized in Cytofix/Cytoperm (BD PharMingen) for 20 min, incubated in a blocking solution (5% FCS and 3% BSA in Perm/Wash buffer, BD PharMingen) for 1 h followed by incubation with TRITC conjugated phalloidin (Sigma–Aldrich) for 1 h at 4 °C.
For additional information see SI Text.
Supplementary Material
Acknowledgments.
This work was supported by a grant from the Wellcome Trust, London, United Kingdom (C.E.R. is the recipient of a Principal Research Fellow Award). We thank Dr. Helga Schneider for her helpful comments on reading the paper.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900510106/DCSupplemental.
References
- 1.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: More than just sticking points. J Cell Sci. 2003;116:4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myung PS, et al. Differential requirement for SLP-76 domains in T-cell development and function. Immunity. 2001;15:1011–1026. doi: 10.1016/s1074-7613(01)00253-9. [DOI] [PubMed] [Google Scholar]
- 6.Raab M, da Silva AJ, Findell PR, Rudd CE. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 7.da Silva AJ, et al. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musci MA, et al. Three domains of SLP-76 are required for its optimal function in a T cell line. J Biol Chem. 1997;272:11674–11677. [PubMed] [Google Scholar]
- 9.Griffiths EK, et al. Positive regulation of T-cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–2263. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 10.Peterson EJ, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 11.Geng L, Raab M, Rudd CE. Cutting edge: SLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J Immunol. 1999;163:5753–5757. [PubMed] [Google Scholar]
- 12.Raab M, Kang H, da Silva A, Zhu X, Rudd CE. FYN-T-FYB-SLP-76 interactions define a T-cell receptor zeta/CD3-mediated tyrosine phosphorylation pathway that up-regulates interleukin 2 transcription in T-cells. J Biol Chem. 1999;274:21170–21179. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- 13.Veale M, et al. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J Biol Chem. 1999;274:28427–28435. doi: 10.1074/jbc.274.40.28427. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, et al. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J Exp Med. 2004;200:1063–1074. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasirer-Friede A, et al. ADAP is required for normal alphaIIbbeta3 activation by VWF/GP Ib-IX-V and other agonists. Blood. 2007;109:1018–1025. doi: 10.1182/blood-2006-05-022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng L, Pfister S, Kraeft SK, Rudd CE. Adaptor FYB (Fyn-binding protein) regulates integrin-mediated adhesion and mediator release: Differential involvement of the FYB SH3 domain. Proc Natl Acad Sci USA. 2001;98:11527–11532. doi: 10.1073/pnas.191378198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, et al. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc Natl Acad Sci USA. 1998;95:8779–8784. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie-Cardine A, et al. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J Biol Chem. 1998;273:25789–25795. doi: 10.1074/jbc.273.40.25789. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, et al. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 2000;19:2889–2899. doi: 10.1093/emboj/19.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kliche S, et al. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, et al. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 22.Jo EK, Wang H, Rudd CE. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J Exp Med. 2005;201:1733–1739. doi: 10.1084/jem.20042577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, et al. Functional defects of SKAP-55 deficient T-cells identify a regulatory role for the adaptor in LFA-1 adhesion. Mol Cell Biol. 2007;27:6863–6875. doi: 10.1128/MCB.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Fernandez JL, et al. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Fernandez JL, et al. LFA-1 integrin and the microtubular cytoskeleton are involved in the Ca(2)(+)-mediated regulation of the activity of the tyrosine kinase PYK2 in T cells. J Leukoc Biol. 2002;71:520–530. [PubMed] [Google Scholar]
- 26.Maguire JE, Danahey KM, Burkly LC, van Seventer GA. T cell receptor- and beta 1 integrin-mediated signals synergize to induce tyrosine phosphorylation of focal adhesion kinase (pp125FAK) in human T cells. J Exp Med. 1995;182:2079–2090. doi: 10.1084/jem.182.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Mócsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newbrough SA, et al. SLP-76 regulates Fcgamma receptor and integrin signaling in neutrophils. Immunity. 2003;19:761–769. doi: 10.1016/s1074-7613(03)00305-4. [DOI] [PubMed] [Google Scholar]
- 30.Abtahian F, et al. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–6949. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AB, et al. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. Adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T-cell sensitivity to antigen and T-cell survival. J Immunol. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
- 33.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue DR. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 35.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 36.Krause M, et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T-cell receptor (TCR) signaling to the actin cytoskeleton. J Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, et al. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J Biol Chem. 2005;280:23576–23583. doi: 10.1074/jbc.M413201200. [DOI] [PubMed] [Google Scholar]
- 38.Hanke JH, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 39.Katagiri K, et al. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimaoka M, Springer TA. Therapeutic antagonists and the conformational regulation of the beta2 integrins. Curr Top Med Chem. 2004;4:1485–1495. doi: 10.2174/1568026043387575. [DOI] [PubMed] [Google Scholar]
- 41.Abraham C, Griffith J, Miller J. The dependence for leukocyte function-associated antigen-1/ICAM-1 interactions in T-cell activation cannot be overcome by expression of high density TCR ligand. J Immunol. 1999;162:4399–4405. [PubMed] [Google Scholar]
- 42.Chirathaworn C, et al. Stimulation through intercellular adhesion molecule-1 provides a second signal for T-cell activation. J Immunol. 2002;168:5530–5537. doi: 10.4049/jimmunol.168.11.5530. [DOI] [PubMed] [Google Scholar]
- 43.Smith A, et al. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter AJ, Ottoson N, Boerth N, Koretzky GA, Shimizu Y. Cutting edge: A novel function for the SLAP-130/FYB adapter protein in beta 1 integrin signaling and T lymphocyte migration. J Immunol. 2000;164:1143–1147. doi: 10.4049/jimmunol.164.3.1143. [DOI] [PubMed] [Google Scholar]
- 45.da Silva AJ, et al. Biochemical analysis of p120/130: A protein-tyrosine kinase substrate restricted to T and myeloid cells. J Immunol. 1997;158:2007–2016. [PubMed] [Google Scholar]
- 46.de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–29476. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 47.Tadokoro S, et al. Talin binding to integrin beta tails: A final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 48.Obergfell A, et al. The molecular adapter SLP-76 relays signals from platelet integrin alphaIIbbeta3 to the actin cytoskeleton. J Biol Chem. 2001;276:5916–5923. doi: 10.1074/jbc.M010639200. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki JI, Yamasaki S, Wu J, Koretzky GA, Saito T. Actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.