Abstract
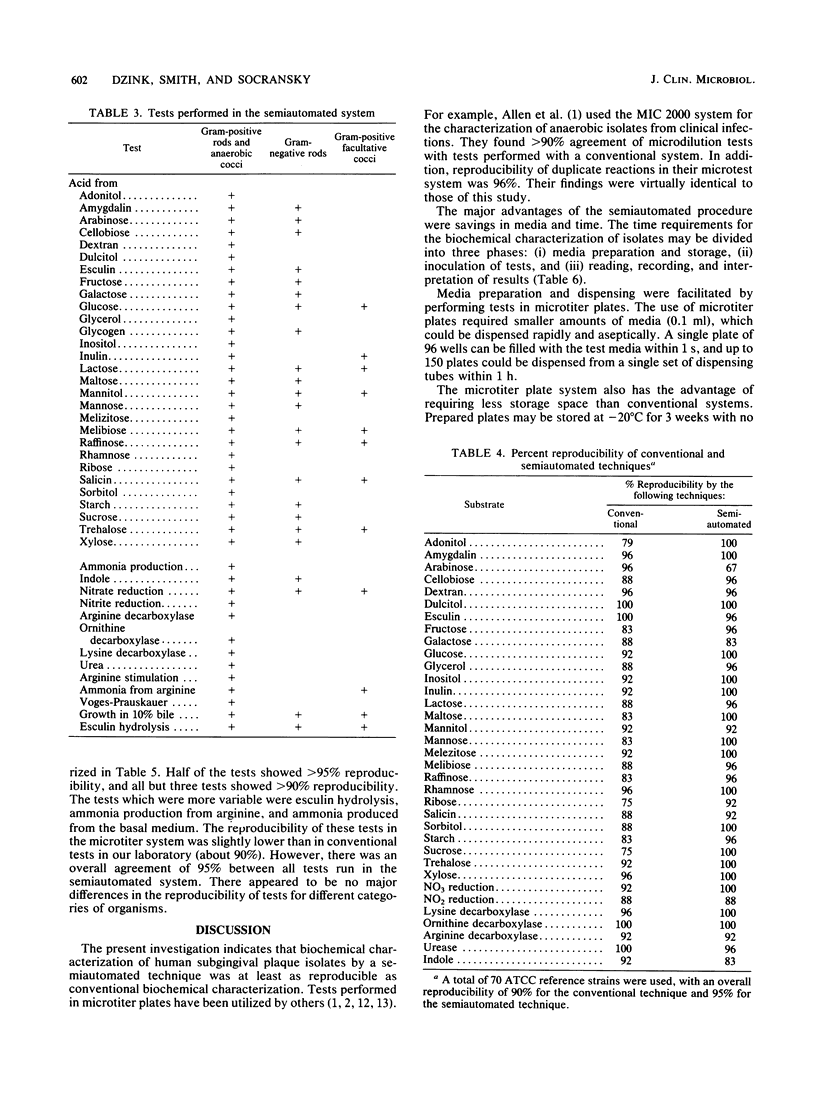
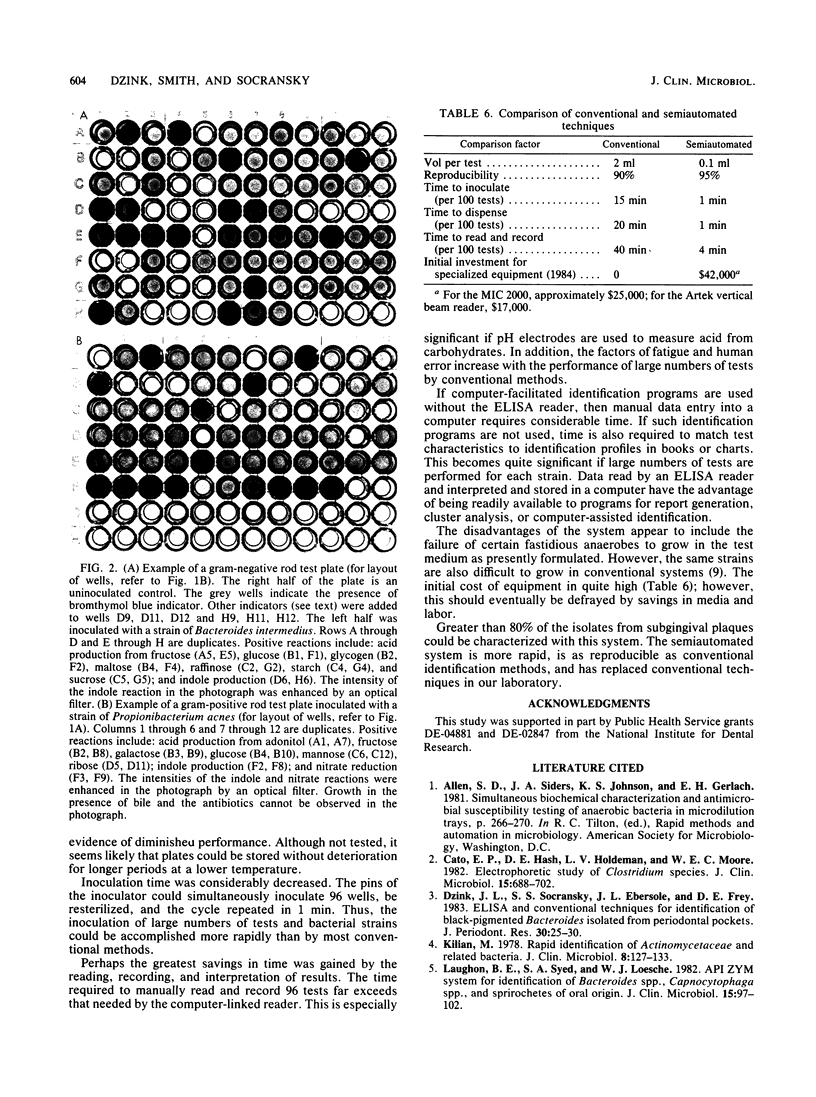
A semiautomated approach for the characterization of subgingival bacterial isolates which economizes in media preparation, inoculation, reading, recording, and interpretation of results was tested. Test ingredients were added to a basal medium consisting of Mycoplasma broth supplemented with 5 micrograms of hemin, 0.5 mg of NaHCO3, and 0.5 mg of L-cysteine per ml. Sterile test media were aseptically dispensed into wells of sterile microtiter plates with a MIC 2000 dispenser. Inocula were grown in broth or scraped from agar plates, dispersed, and inoculated with a MIC 2000 inoculator. After 2 to 4 days of incubation, the optical density of growth was determined with an Artek 210 vertical beam reader at 580 nm and stored on a floppy disk. Reagents were added to each well, and the changes in optical density were determined. Thresholds for positive reactions were determined after extensive preliminary studies for each test. The tests were run in duplicate on each plate and interpreted with an Artek vertical beam reader. Tests that were run in this system included: fermentation of carbohydrates, decarboxylase reactions, reduction of nitrate and nitrite, ammonia production, hydrolysis of esculin, growth in the presence of inhibitory or stimulatory substances, and indole production. Approximately 80% of all isolates from subgingival samples could be characterized by this technique. Comparisons were made between the semiautomated and conventional identification techniques. Overall reproducibility of 2,980 strains by the semiautomated and conventional techniques were 95 and 90%, respectively. There was an 86% similarity of results by the semiautomated and conventional methods. The semiautomated technique was more rapid, less expensive, and as reproducible as the conventional method of identification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cato E. P., Hash D. E., Holdeman L. V., Moore W. E. Electrophoretic study of Clostridium species. J Clin Microbiol. 1982 Apr;15(4):688–702. doi: 10.1128/jcm.15.4.688-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Rapid identification of Actinomycetaceae and related bacteria. J Clin Microbiol. 1978 Aug;8(2):127–133. doi: 10.1128/jcm.8.2.127-133.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Visconti R. A., Socransky S. S., Holt S. C. Classification and identification of Actinobacillus actinomycetemcomitans and haemophilus aphrophilus by cluster analysis and deoxyribonucleic acid hybridizations. J Periodontal Res. 1982 Nov;17(6):585–596. doi: 10.1111/j.1600-0765.1982.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Walker C. B. Development of a micromethod for identification of anaerobic bacteria. Appl Microbiol. 1975 Nov;30(5):825–830. doi: 10.1128/am.30.5.825-830.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Walker C. B., Moore W. E. Micromethod for identification of anaerobic bacteria: design and operation of apparatus. Appl Microbiol. 1975 Nov;30(5):831–837. doi: 10.1128/am.30.5.831-837.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



