Acute lower respiratory tract infections are a persistent and pervasive public health problem. They cause a greater burden of disease worldwide than human immunodeficiency virus infection, malaria, cancer, or heart attacks.1 In the United States, they cause more disease and death than any other infection, and there has been little change in mortality due to respiratory tract infection for more than five decades.1,2 The outcome of an acute lower respiratory tract infection depends on the virulence of the organism and the inflammatory response in the lung. When small numbers of low-virulence microbes are deposited in the lungs, an effective defense can be mounted by resident innate immune defenses, such as the mucociliary escalator, antimicrobial proteins in airway surface liquid, and alveolar macrophages. In contrast, numerous or more virulent microbes elicit an inflammatory response. Although this response serves to reinforce innate immunity and is essential to rid the lungs of microbes, it contributes directly to lung injury and abnormal pulmonary function. This article reviews our current understanding of inflammatory responses in infected lungs, emphasizing recent advances and gaps in knowledge. Much of the information originates from animal experiments; studies with human volunteers and patient-derived data are included when appropriate and available.
INFLAMMATION AND INNATE IMMUNITY
Acute inflammation features the accumulation of neutrophils and a plasma exudate outside of blood vessels. In the pulmonary capillaries of uninfected lungs, these blood contents are normally separated from the alveolar air by less than 1 µm, the thinnest interface between the blood and the environment. The trapping of neutrophils in these capillaries, which is the result of geometric and biophysical constraints,3 increases their quantity per volume of blood by approximately 50 times as compared with other blood vessels, forming a marginated pool of neutrophils that is ready to respond when needed.
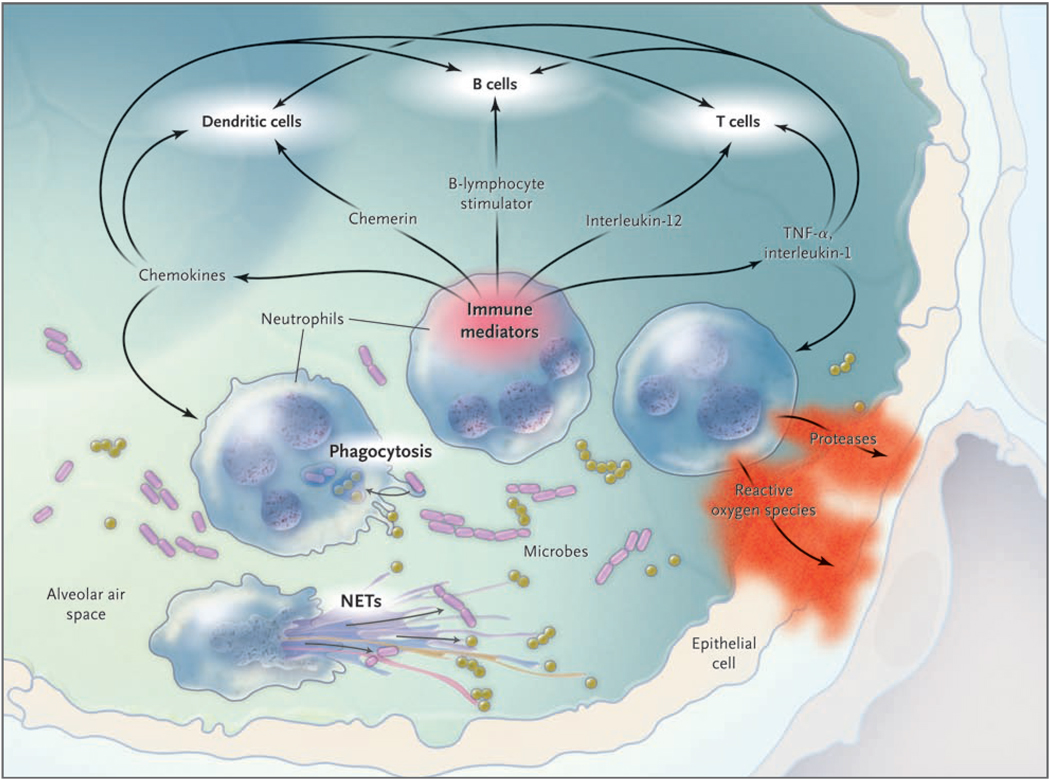
During pulmonary infection, neutrophils migrate out of the pulmonary capillaries and into the air spaces.4 Elie Metchnikoff, the discoverer of phagocytosis, considered neutrophils (or microphages, as he called them) to be “the defensive cells par excellence against microorganisms.”5 After phagocytosis, neutrophils kill ingested microbes with reactive oxygen species (e.g., hypochlorite), antimicrobial proteins (e.g., bactericidal permeability-inducing protein and lactoferrin), and degradative enzymes (e.g., elastase) (Fig. 1).6 An additional microbicidal pathway has also been identified — the neutrophil extracellular trap (NET). Neutrophils extrude NETs composed of a chromatin meshwork containing antimicrobial proteins, and these NETs ensnare and kill extracellular bacteria.7 It remains to be determined whether NETs are useful host defense mechanisms against motile microbes in the dynamic and unstructured liquid-filled air spaces of the infected lung.
Figure 1. Neutrophils and Lung Infection.
Neutrophils are effector cells of innate immunity, killing microbes using phagocytosis and neutrophil extracellular traps (NETs). Neutrophils also generate a variety of immune mediators to direct immune responses, influencing other cells of innate and adaptive immunity. Finally, neutrophils damage tissues, with products such as proteases and reactive oxygen species injuring cells and digesting matrix. TNF denotes tumor necrosis factor.
The content of plasma proteins in the interstitium and air spaces of infected lungs is determined by the combined actions of pericellular bulk flow and transcellular transport by endothelial and epithelial cells. Many plasma proteins, including natural antibodies, complement proteins, C-reactive protein (originally identified in serum from patients with pneumonia8), and pentraxin 3, are important for the defense against microbes in the lungs.9–13 They serve opsonic, bacteriostatic, and microbicidal functions during infection.
Deficits in neutrophil quantity (neutropenia) and defects in quality (e.g., chronic granulomatous disease) predispose patients to opportunistic lung infections, as do deficiencies of complement and immunoglobulins. Since neutrophils and plasma proteins mediate innate immune functions and are needed to prevent lung infection, acute inflammation can be considered an essential innate immune response in the lungs.
GENERATION OF ACUTE INFLAMMATION IN INFECTED LUNGS
MOLECULES THAT DETECT MICROBES
Microbes must be detected by host cells to initiate inflammation in infected lungs. The identification of microbial invaders relies on a set of diverse receptors called pattern-recognition receptors, which bind molecular moieties that are common to microbes. 14 Discoveries of new families of pattern-recognition receptors, including toll-like receptors, nucleotide-binding and oligomerization-domain proteins, and caspase-recruitment domain helicases, have fueled research in the biology of innate immunity. Table 1 lists some of the pattern-recognition receptors with direct relevance to innate immunity in the lungs or to respiratory infection.
Table 1.
Pattern-Recognition Receptors Implicated in Acute Lower Respiratory Tract Infections.
| Receptor | Microbial Ligands | References |
|---|---|---|
| Transmembrane | ||
| TLRs (toll-like receptors) | ||
| TLR2 | Peptidoglycans from bacteria | Knapp et al.15 |
| TLR3 | Respiratory syncytial virus | Rudd et al.16 |
| TLR4 | Lipopolysaccharides from gram-negative bacteria, fusion proteins from respiratory syncytial virus, pneumolysin from Streptococcus pneumoniae | Branger et al.,17 Kurt-Jones et al.,18 Malley et al.19 |
| TLR5 | Flagellin from bacteria | Feuillet et al.20 |
| TLR9 | CpG DNA from bacteria | Albiger et al.21 |
| MARCO (macrophage receptor with collagenous structure) | S. pneumoniae | Arredouani et al.22 |
| SRA-I and SRA-II (scavenger receptors AI and AII) | S. pneumoniae | Arredouani et al.23 |
| Dectin-1 | β-glucan of fungi, Pneumocystis carinii | Steele et al.24, 25 |
| DC-SIGN* | S. pneumoniae | Koppel et al.26 |
| FPR (formyl peptide receptor) | N-formylated peptides of bacteria | Fillion et al.27 |
| MR (mannose receptor) | P. carinii | Tachado et al.28 |
| NKp46 (natural killer cell p46) | Hemagglutinin from influenza viruses, hemagglutinin neuraminidase from parainfluenza viruses | Mandelboim et al.,29 Gazit et al.30 |
| Cytosolic | ||
| Naip5 (neuronal apoptosis-inhibiting protein 5) | Legionella pneumophila | Wright et al.31 |
| Ipaf† | L. pneumophila | Amer et al.32 |
| Nod1 and Nod2 (nucleotide oligomerization domains 1 and 2) | Peptidoglycan components from bacteria | Opitz et al.33 |
| RIG-I (retinoic acid–inducible gene I) | Influenza virus RNA | Le Goffic et al.34 |
| Extracellular | ||
| SP-A and SP-D (surfactant proteins A and D) | Influenza virus, respiratory syncytial virus, gramnegative bacteria, gram-positive bacteria | Wright35 |
| LBP (lipopolysaccharide-binding protein) | Lipopolysaccharides from gram-negative bacteria | Branger et al.36 |
| CD14 | Lipopolysaccharides from gram-negative bacteria | Frevert et al.37 |
| MD-2 | Lipopolysaccharides from gram-negative bacteria | Jia et al.38 |
| PTX3 (pentraxin 3) | Aspergillus fumigatus | Garlanda et al.10 |
| MBL (mannose-binding lectin) | Mannosylated moieties on microbes | Reading et al.39 |
| CRP (C-reactive protein) | Phosphocholine on S. pneumoniae | Thomas-Rudolph et al.40 |
| Complement | Microbial surfaces | Mold et al.11 |
DC-SIGN denotes dendritic-cell–specific ICAM-3–grabbing nonintegrim (ICAM denotes intercellular adhesion molecule).
Ipaf denotes ICE protease–activating factor (ICE denotes interleukin-1 beta–converting enzyme).
For any one microbe, there are a variety of molecules that can activate many different pattern-recognition receptors. Perhaps for this reason, deficiencies of individual pattern-recognition receptors result in more modest phenotypes during experimentally induced acute lower respiratory infections than deficiencies of downstream adapter proteins, which signal from multiple pattern-recognition receptors.41,42 The intracellular signaling pathways triggered by diverse pattern-recognition receptors converge on signaling hubs, such as transcription factors of the nuclear factor κB (NF-κB) and interferon regulatory factor families. These factors integrate signals from diverse stimuli (interacting with pattern-recognition receptors) and initiate responses. NF-κB mediates the transcription of adhesion molecules, chemokines, colony-stimulating factors, and other cytokines that are necessary for inflammatory responses.43 In mice with bacterial stimuli in the lungs, NF-κB RelA (also known as p65) is required for inducing the production of adhesion molecules and chemokines as well as for initiating neutrophil recruitment and host defense.44,45 Interferon regulatory factors mediate the expression of type I interferons and interferon-induced antiviral genes.46 Interferon regulatory factor 3 influences parainfluenza virus infection in mouse lungs,47 but the genes and immune functions that require it or other interferon regulatory factors during lung infection remain unknown.
SENTINEL CELLS IN THE LUNGS
Populations of myeloid cells with specialized functions as sentinels, the alveolar macrophages and dendritic cells, reside in the lungs. These cells are particularly well equipped with pattern-recognition receptors and are anatomically situated to encounter microbes in the air spaces.
Alveolar macrophages are mobile cells that patrol the luminal surfaces of the alveoli. They have been referred to as dust cells because of their abilities to remove and digest relatively inert inhaled materials. They are also sources of alarm signals when lungs are infected, but inhibition of these signals until the appropriate time is imperative. One possible inhibitory mechanism entails the globular heads of surfactant proteins A and D, which bind alveolar-macrophage receptors and suppress inflammatory activity in uninfected lungs. During infection, these globular heads bind pathogens, and the presentation of oligomerized collagenous tails (a result of the clustering of the surfactant proteins on pathogen surfaces) activates rather than quiets alveolar macrophages.48 It is plausible that the inflammatory activity of alveolar macrophages is constitutively suppressed by transforming growth factor β, which is presented to them by epithelial-cell integrins; microbial products initiate signaling that interferes with this suppression, thereby activating the inflammatory functions of alveolar macrophages.49
Dendritic cells are distributed throughout the respiratory tract. In the conducting airways, intraepithelial dendritic cells extend into the fluid within the airway lumen, where they ingest samples from the materials being swept by mucociliary transport from the alveoli toward the glottis.50 In response to the presence of microbes in the lungs, more dendritic cells migrate into the lungs, through the tissues, and also into the draining lymph nodes.50 Dendritic cells are antigen-presenting cells and are therefore central to adaptive immune responses. They also have important functions in innate immunity. Their pattern-recognition receptors render them especially suited to detecting viruses, and when stimulated they produce very high levels of type I interferons.51 The depletion of dendritic cells or the interruption of type I interferon signaling increases susceptibility to viruses in the lungs.52,53
Alveolar macrophages and dendritic cells have a limited ability to kill microbes, but they are particularly important for sensing microbes and passing this information along to other cells, such as epithelial cells and lymphocytes. These cells then recruit the effectors of innate immunity, neutrophils.
EFFECTORS OF INNATE IMMUNITY
Neutrophil recruitment is directed by lung cells. Adhesion molecules induced on lung cells provide traction and signaling information to neutrophils.54 Chemokines from lung cells stimulate chemotaxis and influence the directional motility of neutrophils.54 Colony-stimulating factors signal neutrophil production and release from hematopoietic tissues.55
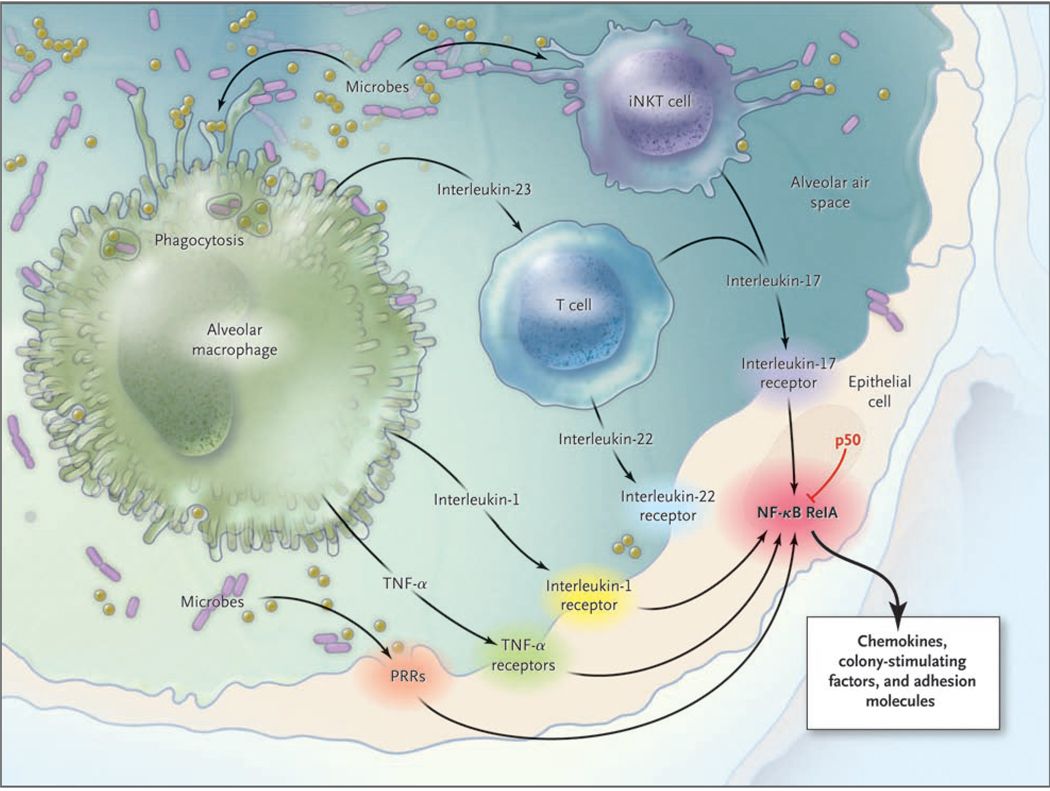
The epithelial barrier between the air-space content of the lungs (including microbes) and the rest of the body constitutes a critical interface for information transfer leading to neutrophil recruitment (Fig. 2). In transgenic mice in which an inhibitor of NF-κB activation is expressed exclusively in lung epithelial cells, the interruption of NF-κB activation decreases the expression of cytokines, including neutrophil chemokines.56,57 This defect in epithelial-cell gene expression compromises neutrophil recruitment and bacterial killing in the lungs.45,56,57
Figure 2. The Epithelial Interface and Lung Infection.
Activation of the epithelial cells forming an interface between the air spaces and the body induces the expression of molecules recruiting neutrophils as innate immunity reinforcements. Epithelial cells recognize some microbes directly through pattern-recognition receptors (PRRs). Alveolar macrophages recognizing microbes activate epithelial cells directly and through T-cell intermediates. Invariant natural killer T (iNKT) cells recognizing microbes can also activate epithelial cells. These diverse activation pathways converge on nuclear factor κB (NF-κB) transcription factors in the epithelial cell, with RelA responsible for inducing and p50 responsible for regulating the expression of proinflammatory mediators, including neutrophil chemokines, colony-stimulating factors, and adhesion molecules. TNF denotes tumor necrosis factor.
With the increasing recognition of the roles of epithelial cells in lung inflammatory responses,58 efforts are under way to illuminate the pathways of epithelial-cell activation in infected lungs. Lung epithelial cells can be activated directly by some microbes, such as Staphylococcus aureus and Pseudomonas aeruginosa.59,60 However, other microbes, such as pneumococci (the most common cause of community-acquired pneumonia), are less likely to be or cannot be recognized by epithelial cells.45 During pneumococcal pneumonia, alarm signals generated by sentinel myeloid cells, particularly tumor necrosis factor α (TNF-α) and interleukin-1 (1α and 1β), are essential for activation of the epithelium and downstream inflammatory responses.45,61 Blocking the signaling of either TNF-α or interleukin-1 produces modest effects as compared with blocking both pathways simultaneously,61–63 suggesting that these cytokines have overlapping functions during acute respiratory tract infection. Neutrophil-mediated host defense against pneumococci in the lungs requires such signaling.
Epithelial cells can also be activated by lymphocyte cytokines (Fig. 2). Interleukin-17 activates lung epithelial cells to express chemokines and colony-stimulating factors, and it is essential for neutrophil-mediated host defense during klebsiella pneumonia. 64 During such infection, interleukin-17 is produced by T cells, and its production is stimulated by another signal from macrophages — interleukin-23.65 A subpopulation of invariant natural killer T cells in the lungs can also generate interleukin-17 to stimulate epithelial cells and elicit neutrophil recruitment, and the interleukin-17 expressed by these cells may not depend on interleukin-23.66 Interleukin-17–secreting T cells also release interleukin-22, which functions like interleukin-17 in activating epithelial cells.67 If, when, and how interleukin-22 influences innate immune responses during lung infection are questions yet to be answered.
Neutrophils are not dead ends in these communication pathways but convey important information that directs immune responses (Fig. 1).6 They generate proinflammatory signals, including TNF-α, interleukin-1, and chemokines; chemerin, which recruits and activates dendritic cells; and B-lymphocyte stimulator, which promotes the selection, survival, and growth of B cells. Neutrophils are sources of the T-cell–activating cytokine interleukin-12 in the lungs,68 and interleukin-12 amplifies interferon-γ to enhance neutrophil-mediated host defense during pneumonia.69 Neutrophils constitutively express the extracellular pattern-recognition receptor pentraxin 3, and the compromised host defense of mice with pentraxin 3 deficiency can be improved by the administration of soluble pentraxin 3 or by transfer of neutrophils from wild-type but not pentraxin 3–deficient mice.10,70 Thus, acquired and innate immune responses against microbes in the lungs are shaped by signals derived from neutrophils.
INFLAMMATION AND ACUTE LUNG INJURY
Inflammation is critical for innate immunity and host defense, but it can injure the lungs. The accumulation of extravascular plasma fluids, as in noncardiogenic pulmonary edema, is a defining feature of acute lung injury. The neutrophil products generated to kill microbes, such as reactive oxygen species and proteases, also kill host cells and damage host tissues (Fig. 1). The risks of inflammation are starkly demonstrated in a transgenic mouse model in which the activation of NF-κB in lung epithelial cells is sufficient to cause neutrophil recruitment, pulmonary edema, arterial hypoxemia, and death in the absence of any infection or exogenous stimuli.71 Thus, the innate immune responses necessary for ridding the lungs of microbes can also cause injury and contribute to the pathophysiology of infection. Perhaps because of this, lung infection is a common underlying cause of the acute respiratory distress syndrome. 72
Inhibiting inflammatory signals can be protective during lung infections. For example, interrupting both TNF-α and interleukin-1 signaling (but neither alone) decreases the pulmonary edema and the loss of lung compliance that are often found in mice with Escherichia coli pneumonia.62,63 Triggering receptor expressed on myeloid cells 1 (TREM-1), which functions in a positive feedback loop to amplify TNF-α, interleukin-1, and inflammation, 73 is so strongly associated with pneumonia in patients that measurement of soluble TREM-1 in bronchoalveolar-lavage fluid has been proposed as a diagnostic test.74 Inhibition of TREM-1 diminishes TNF, interleukin-1, and pathophysiological features in rats with P. aeruginosa pneumonia.75 Corticosteroids can be effective albeit nonspecific inhibitors of inflammation. In a clinical trial of corticosteroid infusion in patients with severe community-acquired pneumonia, the 23 patients in the corticosteroid group had less lung injury and a higher rate of survival than the 23 patients in the placebo group.76 Results from this small study are provocative but must be viewed with caution until further studies are completed.77 Knowledge gaps remain substantial. It is not yet evident which patients with which infections may benefit from which antiinflammatory therapies at which times. Altogether, however, these studies suggest that inflammation-targeting therapies may be useful in treating certain severe lung infections, encouraging further research along these lines.
Highly pathogenic influenza viruses, such as avian influenza A (H5N1) virus and the virus causing the 1918 pandemic, induce strong inflammatory responses in humans and laboratory animals.78,79 The seemingly excessive responses bolster the idea that a so-called cytokine storm mediates pathophysiology during these infections, but direct evidence in support of this concept is scant. Neutrophil depletion increases viral growth and hastens death in mice infected with the H5N1 influenza A virus,80 suggesting that at least in this experimental infection, neutrophils do more good than harm. Interruptions of cytokine signaling in mice with H5N1 influenza A virus infections have modest or no effects,81 suggesting that the cytokines so far examined are not individually essential to the pathophysiology of H5N1 influenza virus infection. Further studies will be critical in determining whether and, if so, which inflammatory mediators influence the pathophysiology of highly pathogenic influenza virus infections, and whether the interruption of select cytokines or signaling pathways upstream or downstream from cytokines can protect the host from inflammatory injury during such infections.
REGULATION OF ACUTE INFLAMMATION IN INFECTED LUNGS
The body needs mechanisms to keep acute inflammation in check. Much less is known about these regulatory mechanisms than about the mechanisms initiating and amplifying inflammation. A few examples of how regulatory mechanisms influence the outcome of lung infections are presented here.
One such braking strategy is to limit NF-κB activity. The NF-κB protein p50 has multiple functions, which include curbing the transcription of genes with NF-κB–binding sites in their promoters. 43 During bacterial pneumonia in mice, a deficiency of p50 increases cytokine expression and exacerbates lung injury.82 Thus, p50 normally functions to prevent excess cytokines and inflammatory injury during pneumonia.
Another mechanism is interference with signaling from pattern-recognition receptors. The interleukin-1 receptor–associated kinase (IRAK)–like molecule (IRAK-M) inhibits IRAK-mediated signaling from the pattern-recognition and cytokine receptors that activate NF-κB. Sepsis induces IRAK-M in mouse alveolar macrophages, and this protein decreases cytokine expression and compromises pulmonary host defense.83 IRAK-M may therefore contribute to the susceptibility of patients with sepsis to nosocomial pneumonia. Other regulatory molecules inhibit pattern-recognition–receptor signaling indirectly. For example, carbon monoxide generated by heme oxygenase-1 inhibits signaling from transmembrane pattern-recognition receptors.84 A deficiency of heme oxygenase-1 increases, whereas its overexpression decreases, inflammation and injury induced by bacteria and influenza virus in mouse lungs.85–87 Prevention of injury is probably due to both the antiinflammatory and the tissue-protective activities of heme oxygenase-1.
The signal transducer and activator of transcription 3 (STAT3) also has antiinflammatory and tissue-protective effects. Mutations in STAT3 result in the hyper-IgE syndrome, which is characterized by recurrent and severe lung infections.88 This transcription factor is activated in macrophages and epithelial cells during acute pulmonary inflammation.89 Macrophage STAT3 mediates antiinflammatory responses induced by the cytokine interleukin-10,90 which compromises host defense but limits lung injury during pneumonia. 91–94 Epithelial-cell STAT3 is essential in preventing lung injury during infection.95 The signals that activate epithelial-cell STAT3 are uncertain, but are not likely to include interleukin-10. STAT3 activation in the lungs during E. coli infection depends partially on interleukin-6, which is essential for overcoming bacterial pneumonia.96
Prostaglandin I2 (prostacyclin) is generated during respiratory syncytial virus infection and has protective activities that may be mediated by antiinflammatory effects on dendritic cells.97,98 In addition to having antiinflammatory activities, other lipids, including lipoxins, resolvins, and protectins, help return inflamed tissues to health.99 During and after pneumonia, the return of the architecture of a lung lobe from complete consolidation to a seemingly normal state is remarkable. Unfortunately, few if any studies have reported the mechanisms underlying this process of resolution during lung infection, so the presumed role of lipids must at present be based on extrapolation.
RESPONSES OF MICROBES TO INFLAMMATION
Acute lower respiratory tract infections can be monomicrobial or polymicrobial, with organisms ranging in virulence from commensal to highly pathogenic.100–102 These microbes have mechanisms for counteracting many of the effector and signaling events described above. Microbial subversion of individual pathways may be a selective pressure driving mammalian hosts to have multiple, parallel, sometimes redundant-seeming pathways for innate immunity, as with epithelial activation (Fig. 2). A few microbial strategies specifically related to lung infections and the pathways of innate immunity described above are highlighted here as examples.
Counteracting effector mechanisms of innate immunity is of obvious advantage to a microbe. Although NETs were discovered only recently,7 microbial countermeasures are already recognized. For example, NETs extruded by neutrophils fail to contain and kill pneumococci.103 A pneumococcal DNase cleaves NETs and frees bacteria. During infection, this DNase is a virulence factor that gives the bacteria a competitive advantage against DNase-mutated strains in the lungs of mice, resulting in increased mortality from pneumonia in such mice.
Preventing the host from detecting pathogens is another strategy often used by microbes. For example, the retinoic acid–inducible gene I intracellular pattern-recognition receptor for viral RNA is bound by an influenza virus protein that prevents downstream signaling, activation of interferon regulatory factor, and expression of type I interferon. 104 Deleting this protein attenuates influenza virus infection, increasing type I interferon in the lungs and decreasing mortality.105 Many pathogens interrupt proinflammatory signaling pathways or mimic antiinflammatory signaling pathways.
Not only do lung pathogens interfere with host signaling, they also listen in on these immune conversations and use this information to guide their responses appropriately. For example, P. aeruginosa expresses a receptor that recognizes interferon-γ, and in the presence of interferon-γ, this receptor stimulates gene expression dictating biofilm formation.106 Since biofilms render bacteria more resistant to both innate immunity and antibiotics, this is probably an adaptive response during infection. In addition, P. aeruginosa and other bacteria respond to TNF-α and other cytokines with increased growth rates.107 In neutropenic mice, the ability of TNF-α to increase bacterial growth worsens lung infection.108 Thus, pathogens sense innate immune signaling and respond in ways that subvert host defense and facilitate infection.
GENETIC VARIATION IN INFLAMMATORY PATHWAYS
The mechanisms for generating and regulating acute inflammation, described above, determine the outcomes of experimentally induced lung infections in animals. Deficiencies and polymorphisms in human genes for the factors involved in these mechanisms have been associated with lung infection and its consequences, such as disseminated or invasive infection or acute lung injury (Table 2). Although the limitations of such genotype–phenotype associations warrant consideration, 129 these data indicate that knowledge of innate immunity and lung infection derived from experiments in animals can apply to humans. Genetic variations in innate-immunity mediators influence the outcome of inevitable exposures of the human lower respiratory tract to microbes.
Table 2.
Genetic Polymorphisms Associated with Lung Infection Outcomes.*
| Gene Product | Association | References |
|---|---|---|
| Initiating and amplifying mechanisms | ||
| TLRs (toll-like receptors) | ||
| TLR4 | Legionella pneumonia, severe respiratory syncytial virus infection | Tal et al.,109 Hawn et al.110 |
| TLR5 | Legionella pneumonia | Hawn et al.111 |
| CD14 | Respiratory syncytial virus bronchiolitis | Inoue et al.112 |
| IRAK-4 (interleukin-1 receptor–associated kinase 4) | Bacterial infections, particularly pneumococcal Infection | Ku et al.113 |
| NEMO — NF-κB activation | Recurrent invasive pneumococcal disease | Ku et al.114 |
| Mal (MyD88 adaptor-like protein) — toll-like receptor signaling | Invasive pneumococcal disease | Khor et al.115 |
| MBL (mannose-binding lectin) | Invasive pneumococcal disease, recurrent respiratory infections, acute lung injury | Roy et al.,116 Gomi et al.,117 Gong et al.118 |
| Complement C2 | Invasive pneumococcal disease and recurrent Pneumonias | Jönsson et al.119 |
| SP-A, SP-D (surfactant proteins A and D) | Severe respiratory syncytial virus infection | Lahti et al.,120 Löfgren et al.121 |
| Regulating mechanisms | ||
| NF-κB p50 | Acute lung injury | Adamzik et al.122 |
| IκB-α | Invasive pneumococcal disease, acute lung injury | Chapman et al.,123 Zhai et al.124 |
| Interleukins | ||
| Interleukin-6 | Invasive pneumococcal disease | Schaaf et al.125 |
| Interleukin-10 | Pneumonia outcomes and acute lung Injury | Wattanathum et al.,126 Gong et al.127 |
| HO-1 (heme oxygenase-1) | Pneumonia susceptibility | Yasuda et al.128 |
| STAT3 (signal transducer and activator of transcription 3) | Hyper-IgE syndrome — recurrent severe lung infections | Holland et al.88 |
NEMO denotes NF-κB essential modulator, and NF-κB nuclear factor κB.
Another reason why human genotype–phenotype studies are important is that they occur in natural instead of laboratory environments. Infections involve intersections of host and microbe within complex and dynamic ecosystems not mimicked in laboratory studies. For example, patients with a deficiency of IRAK-4 (which signals from multiple pattern-recognition receptors) are susceptible to a narrower spectrum of microbes, over a narrower age range, and with more variation across the population than in vitro experiments with human cells or in vivo experiments with mice would suggest.113 Patients with an immunodeficiency tend to present with select subgroups of infections (e.g., patients with chronic granulomatous disease are especially susceptible to five microbes130). Environmental and genomic variations result in a range of susceptibility among patients with similar immunodeficiencies. In the future, polygenic analyses may demonstrate that combined polymorphisms in multiple genes influence lung infections more dramatically than monogenic variation, since parallel paths and redundancies are common in innate immunity. An emerging theme is that genetic susceptibility to infection is more common than is now appreciated; susceptibility is probably polygenic, with incomplete penetrance restricted to narrowly defined clinical phenotypes.131
Conclusions
Innate immune responses to microbes in the lungs determine the outcome of infection; an insufficient response can result in life-threatening infection, but an excessive response can lead to life-threatening inflammatory injury. Further studies will help identify populations that are particularly susceptible to severe lung infection and will guide the development of prophylactic and therapeutic interventions.
Acknowledgments
Supported by grants from the National Institutes of Health (HL-68153 and HL-79392).
Dr. Mizgerd reports receiving consulting fees from Sirtris Pharmaceuticals and ImmunoGen and receiving pilot-study funding from Pulmatrix. No other potential conflict of interest relevant to this article was reported.
I thank Drs. Lester Kobzik and Mark Perrella, as well as members of the laboratory, for helpful critiques and suggestions concerning this article.
References
- 1.Mizgerd JP. Lung infection — a public health priority. PLoS Med. 2006;3(2):e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 4.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 5.Metchnikoff E, editor. Immunity in infective diseases. London: Cambridge University Press; 1905. The mechanism of natural immunity against micro-organisms; pp. 175–206. [Google Scholar]
- 6.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 10.Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 11.Mold C, Rodic-Polic B, Du Clos TW. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J Immunol. 2002;168:6375–6381. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 12.Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72:2899–2906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Knapp S, Wieland CW, van ’t Veer C, et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 16.Rudd BD, Smit JJ, Flavell RA, et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 17.Branger J, Knapp S, Weijer S, et al. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 19.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuillet V, Medjane S, Mondor I, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albiger B, Dahlberg S, Sandgren A, et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 22.Arredouani M, Yang Z, Ning Y, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol. 2006;35:474–478. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele C, Marrero L, Swain S, et al. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J Exp Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele C, Rapaka RR, Metz A, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1(4):e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppel EA, Wieland CW, van den Berg VC, et al. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur J Immunol. 2005;35:2962–2969. doi: 10.1002/eji.200526216. [DOI] [PubMed] [Google Scholar]
- 27.Fillion I, Ouellet N, Simard M, Bergeron Y, Sato S, Bergeron MG. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J Immunol. 2001;166:7353–7361. doi: 10.4049/jimmunol.166.12.7353. [DOI] [PubMed] [Google Scholar]
- 28.Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J Leukoc Biol. 2007;81:205–211. doi: 10.1189/jlb.1005580. [DOI] [PubMed] [Google Scholar]
- 29.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 30.Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 31.Wright EK, Goodart SA, Growney JD, et al. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- 32.Amer A, Franchi L, Kanneganti TD, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 33.Opitz B, Püschel A, Schmeck B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 34.Le Goffic R, Pothlichet J, Vitour D, et al. Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 35.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 36.Branger J, Florquin S, Knapp S, et al. LPS-binding protein-deficient mice have an impaired defense against Gram-negative but not Gram-positive pneumonia. Int Immunol. 2004;16:1605–1611. doi: 10.1093/intimm/dxh161. [DOI] [PubMed] [Google Scholar]
- 37.Frevert CW, Matute-Bello G, Skerrett SJ, et al. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J Immunol. 2000;164:5439–5445. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- 38.Jia HP, Kline JN, Penisten A, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 39.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas-Rudolph D, Du Clos TW, Snapper CM, Mold C. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc{gamma}R on dendritic cells. J Immunol. 2007;178:7283–7291. doi: 10.4049/jimmunol.178.11.7283. [DOI] [PubMed] [Google Scholar]
- 41.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- 42.Jeyaseelan S, Young SK, Yamamoto M, et al. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [Erratum, Nat Rev Immunol 2002;2:975.] [DOI] [PubMed] [Google Scholar]
- 44.Alcamo EA, Mizgerd JP, Horwitz BH, et al. Targeted mutation of TNF receptor 1 rescues the RelA-deficient mouse and reveals a critical role for NF-kB in leukocyte recruitment. J Immunol. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 45.Quinton LJ, Jones MR, Simms BT, et al. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [Erratum, Immunity 2006;25:849.] [DOI] [PubMed] [Google Scholar]
- 47.Kiyotani K, Sakaguchi T, Kato A, Nagai Y, Yoshida T. Paramyxovirus Sendai virus V protein counteracts innate virus clearance through IRF-3 activation, but not via interferon, in mice. Virology. 2007;359:82–91. doi: 10.1016/j.virol.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 48.Gardai SJ, Xiao YQ, Dickinson M, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 49.Takabayshi K, Corr M, Hayashi T, et al. Induction of a homeostatic circuit in lung tissue by microbial compounds. Immunity. 2006;24:475–487. doi: 10.1016/j.immuni.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Jahnsen FL, Strickland DH, Thomas JA, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–5867. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 51.Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007;19:24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of beta interferon in host defense against influenza A virus. J Virol. 2007;81:2025–2030. doi: 10.1128/JVI.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 55.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 57.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–L152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 58.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 59.Gomez MI, Lee A, Reddy B, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 60.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-{kappa}B activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I L1-1 receptor during Escherichia coli pneumonia in mice. J Immunol. 2001;166:4042–4048. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 63.Mizgerd JP, Lupa MM, Hjoberg J, et al. Roles for early response cytokines during Escherichia coli pneumonia revealed by mice with combined deficiencies of all signaling receptors for TNF and IL-1. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1302–L1310. doi: 10.1152/ajplung.00353.2003. [DOI] [PubMed] [Google Scholar]
- 64.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tateda K, Moore TA, Deng JC, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 69.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng DS, Han W, Chen SM, et al. Airway epithelium controls lung inflammation and injury through the NF-{kappa}B pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 72.Bauer TT, Ewig S, Rodloff AC, Müller EE. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis. 2006;43:748–756. doi: 10.1086/506430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 74.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert P-E. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 75.Gibot S, Alauzet C, Massin F, et al. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194:975–983. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 76.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 77.Rañó A, Agustí C, Sibila O, Torres A. Associated inflammatory response in pneumonia: role of adjunctive therapy with glucocorticoids. Curr Opin Infect Dis. 2006;19:179–184. doi: 10.1097/01.qco.0000216629.51563.f8. [DOI] [PubMed] [Google Scholar]
- 78.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 80.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szretter KJ, Gangappa S, Lu X, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-kB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med. 2003;168:810–817. doi: 10.1164/rccm.200303-412OC. [DOI] [PubMed] [Google Scholar]
- 83.Deng JC, Cheng G, Newstead MW, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakahira K, Kim HP, Geng XH, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashiba T, Suzuki M, Nagashima Y, et al. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8:1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 86.Tsuburai T, Kaneko T, Nagashima Y. Pseudomonas aeruginosa-induced neutrophilic lung inflammation is attenuated by adenovirus-mediated transfer of the heme oxygenase 1 cDNA in mice. Hum Gene Ther. 2004;15:273–285. doi: 10.1089/104303404322886129. [DOI] [PubMed] [Google Scholar]
- 87.Fredenburgh LE, Baron RM, Carvajal IM, et al. Absence of heme oxygenase-1 expression in the lung parenchyma exacerbates endotoxin-induced acute lung injury and decreases surfactant protein-B levels. Cell Mol Biol (Noisy-le-grand) 2005;51:513–520. [PubMed] [Google Scholar]
- 88.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 89.Severgnini M, Takahashi S, Rozo LM, et al. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1282–L1292. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- 90.Matsukawa A, Kudo S, Maeda T, et al. Stat3 in resident macrophages as a repressor protein of inflammatory response. J Immunol. 2005;175:3354–3359. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 91.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 92.van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 93.Kurahashi K, Kajikawa O, Sawa T, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang E, Bergeron Y, Bergeron MG. Ceftriaxone pharmacokinetics in interleukin-10-treated murine pneumococcal pneumonia. J Antimicrob Chemother. 2005;55:721–726. doi: 10.1093/jac/dki085. [DOI] [PubMed] [Google Scholar]
- 95.Matsuzaki Y, Xu Y, Ikegami Me, et al. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol. 2006;177:527–537. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 96.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis. 2006;193:360–369. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hashimoto K, Graham BS, Geraci MW, et al. Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness. J Virol. 2004;78:10303–10309. doi: 10.1128/JVI.78.19.10303-10309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou W, Hashimoto K, Goleniewska K, et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol. 2007;178:702–710. doi: 10.4049/jimmunol.178.2.702. [DOI] [PubMed] [Google Scholar]
- 99.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 100.de Roux A, Ewig S, García E, et al. Mixed community-acquired pneumonia in hospitalised patients. Eur Respir J. 2006;27:795–800. doi: 10.1183/09031936.06.00058605. [DOI] [PubMed] [Google Scholar]
- 101.Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:825–831. doi: 10.1086/518460. [DOI] [PubMed] [Google Scholar]
- 102.Webster RG, Govorkova EA. H5N1 influenza — continuing evolution and spread. N Engl J Med. 2006;355:2174–2147. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 103.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 104.Pichlmair A, Schulz O, Tan CP, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 105.Kochs G, Koerner I, Thiel L, et al. Properties of H7N7 influenza A virus strain SC35M lacking interferon antagonist NS1 in mice and chickens. J Gen Virol. 2007;88:1403–1409. doi: 10.1099/vir.0.82764-0. [DOI] [PubMed] [Google Scholar]
- 106.Wu L, Estrada O, Zaborina O, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309:774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 107.Meduri GU, Kanangat S, Stefan J, Tolley E, Schaberg D. Cytokines IL-1β, IL-6, and TNF-α enhance in vitro growth of bacteria. Am J Respir Crit Care Med. 1999;160:961–967. doi: 10.1164/ajrccm.160.3.9807080. [DOI] [PubMed] [Google Scholar]
- 108.Lee JH, Del Sorbo L, Khine AA, et al. Modulation of bacterial growth by tumor necrosis factor-alpha in vitro and in vivo. Am J Respir Crit Care Med. 2003;168:1462–1470. doi: 10.1164/rccm.200302-303OC. [DOI] [PubMed] [Google Scholar]
- 109.Tal G, Mandelberg A, Dalal I, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 110.Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc Natl Acad Sci U S A. 2005;102:2487–2489. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires’ disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inoue Y, Shimojo N, Suzuki Y, et al. CD14 -550 C/T, which is related to the serum level of soluble CD14, is associated with the development of respiratory syncytial virus bronchiolitis in the Japanese population. J Infect Dis. 2007;195:1618–1624. doi: 10.1086/516790. [DOI] [PubMed] [Google Scholar]
- 113.Ku CL, von Bernuth H, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ku CL, Picard C, Erdös M, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44:16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khor CC, Chapman SJ, Vannberg FO, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roy S, Knox K, Segal S, et al. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002;359:1569–1573. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 117.Gomi K, Tokue Y, Kobayashi T, et al. Mannose-binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest. 2004;126:95–99. doi: 10.1378/chest.126.1.95. [DOI] [PubMed] [Google Scholar]
- 118.Gong MN, Zhou W, Williams PL, Thompson BT, Pothier L, Christiani DC. Polymorphisms in the mannose binding lectin-2 gene and acute respiratory distress syndrome. Crit Care Med. 2007;35:48–56. doi: 10.1097/01.CCM.0000251132.10689.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jönsson G, Truedsson L, Sturfelt G, Oxelius VA, Braconier JH, Sjöholm AG. Hereditary C2 deficiency in Sweden: frequent occurrence of invasive infection, atherosclerosis, and rheumatic disease. Medicine (Baltimore) 2005;84:23–34. doi: 10.1097/01.md.0000152371.22747.1e. [DOI] [PubMed] [Google Scholar]
- 120.Lahti M, Lofgren J, Marttila R, et al. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Löfgren J, Rämet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185:283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- 122.Adamzik M, Frey UH, Rieman K, et al. Insertion/deletion polymorphism in the promoter of NFKB1 influences severity but not mortality of acute respiratory distress syndrome. Intensive Care Med. 2007;33:1199–1203. doi: 10.1007/s00134-007-0649-4. [DOI] [PubMed] [Google Scholar]
- 123.Chapman SJ, Khor CC, Vannberg FO, et al. I{kappa}B genetic polymorphisms and invasive pneumococcal disease. Am J Respir Crit Care Med. 2007;176:181–187. doi: 10.1164/rccm.200702-169OC. [DOI] [PubMed] [Google Scholar]
- 124.Zhai R, Zhou W, Gong MN, et al. Inhibitor kappaB-alpha haplotype GTC is associated with susceptibility to acute respiratory distress syndrome in Caucasians. Crit Care Med. 2007;35:893–898. doi: 10.1097/01.CCM.0000256845.92640.38. [DOI] [PubMed] [Google Scholar]
- 125.Schaaf B, Rupp J, Müller-Steinhardt M, et al. The interleukin-6 -174 promoter polymorphism is associated with extrapulmonary bacterial dissemination in Streptococcus pneumoniae infection. Cytokine. 2005;31:324–328. doi: 10.1016/j.cyto.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 126.Wattanathum A, Manocha S, Groshaus H, Russell JA, Walley KR. Interleukin-10 haplotype associated with increased mortality in critically ill patients with sepsis from pneumonia but not in patients with extrapulmonary sepsis. Chest. 2005;128:1690–1698. doi: 10.1378/chest.128.3.1690. [DOI] [PubMed] [Google Scholar]
- 127.Gong MN, Thompson BT, Williams PL. Interleukin-10 polymorphism in position -1082 and acute respiratory distress syndrome. Eur Respir J. 2006;27:674–681. doi: 10.1183/09031936.06.00046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yasuda H, Okinaga S, Yamaya M, et al. Association of susceptibility to the development of pneumonia in the older Japanese population with haem oxygenase-1 gene promoter polymorphism. J Med Genet. 2006;43:e17. doi: 10.1136/jmg.2005.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 130.Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113:620–626. doi: 10.1016/j.jaci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–619. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]