Abstract
Current fluctuations in pure lipid membranes have been shown to occur under the influence of transmembrane electric fields (electroporation) as well as a result from structural rearrangements of the lipid bilayer during phase transition (soft perforation). We demonstrate that the ion permeability during lipid phase transition exhibits the same qualitative temperature dependence as the macroscopic heat capacity of a D15PC/DOPC vesicle suspension. Microscopic current fluctuations show distinct characteristics for each individual phase state. Although current fluctuations in the fluid phase show spikelike behavior of short timescales (∼2 ms) with a narrow amplitude distribution, the current fluctuations during lipid phase transition appear in distinct steps with timescales of ∼20 ms. We propose a theoretical explanation for the origin of timescales and permeability based on a linear relationship between lipid membrane susceptibilities and relaxation times near the phase transition.
Introduction
The permeability of cell membranes to ions, proteins, and other transmitters, and the regulation of these processes, is of crucial importance in maintaining basic cell functions. In this context, ion channel proteins have been found to play a major role in the regulation of ionic transport across the membrane (1). In addition to the characteristics of the channel, proteins themselves as an influence of the membrane phase state on channel function have been identified (2,3), indicating the importance of the lipid bilayer for transmembrane currents. Boheim et al., for example, reported on the slowing down of protein induced ion channels during the lipid phase transition (4,5). The first indication of thermodynamically induced changes in permeability was already given by Papahadjopoulos et al. in 1973 (6), who found a maximum leakage of radioactive labeled sodium ions during lipid phase transition.
The application of high electric fields (typically ∼107–108 V/m) presents another mechanism for overcoming the lipid membrane barrier, inducing current fluctuations in the absence of channel forming peptides (electroporation) (7–11).
A third mechanism (in addition to ion channels, pores, and electroporation) to cross the lipid membrane barrier has been elucidated by Antonov et al. (12). They reported that quantized ion-conducting channels appear in unmodified 1,2-distearoyl-sn-glycero-3-phosphocholine membranes close to the phase transition and demonstrated more recently, that the conductance of different ions is in accordance with the well-known Hofmeister series (13). Similarly, Kaufmann and Silmann (14) as well as the literature (2,15–18) were able to demonstrate a close relationship between the physical state of the bilayer and ion current fluctuations. This relationship is also the subject of an accompanying article by Blicher et al.(19).
In this article, we study the origin of ion current fluctuations in protein free lipid bilayers by a thorough comparison of timescales and amplitudes of ion current fluctuations with the heat capacity profile of lipid membranes. Although current fluctuations in the fluid phase appear as short (∼2 ms) spikes, they last for ∼20 ms as discrete steplike currents in the phase transition regime including long-lasting events of ∼100 ms. Based on a model recently proposed by Grabitz et al. (20), we suggest that the increased timescales at Tm have their origin in the extended relaxation times generally found during lipid membrane phase transitions and originating from the flat thermodynamic potential and consequent weak restoring forces driving the system back to its equilibrium near Tm.
Materials and Methods
Lipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Tm = −4°C) and 1,2 dipentadecanoyl-sn-glycero-3-phosphocholine (D15PC, Tm = 33°C) dissolved in chloroform were purchased from Avanti Polar Lipids (Birmingham, AL) and used without further purification. Hexadecane was dissolved in pentane to achieve a final concentration of 2.5% for the prepainting solution.
We performed current measurements using a patch-clamp amplifier (Cornerstone Series) from Dagan (Minneapolis, MN). The temperature was controlled during the experiments with the aid of a standard heat bath (Julabo, Seelbach, Germany). Heat capacity profiles of small unilamellar vesicles were recorded using a VP-DSC calorimeter from Microcal (Avestin, Ottawa, Canada).
To obtain lipid mixtures, lipids dissolved in chloroform were mixed in the desired proportion. Aqueous solution of lipids for calorimetric measurements were obtained as described previously (21). The experiments on the artificial membranes (in this case, black lipid membranes, or BLMs) were performed by using a setup consisting of two Teflon chambers separated by a Teflon foil of 25-μm thickness with a 150-μm diameter hole. The hole was produced by drilling it into the foil. Lipid membranes were obtained by prepainting the Teflon foil with the hexadecane solution and 15 min of waiting until the pentane was evaporated. Subsequently, both chambers were filled up to a level sufficiently far below the hole with a 200-mM NaCl solution. Ten microliters of lipid solution was spread on the water surface and after a waiting time of 15 min, the electrolyte level was raised above the hole. Before experiments, the bilayer was characterized by measuring the resistance and the capacity of the membrane.
Results and Discussion
Theory
In thermodynamics, the different functions of state (enthalpy, energy, etc.), the susceptibilities (heat capacity, compressibilities, etc.) and the response times are coupled via the fluctuation-dissipation theorem (22). For lipid membranes, it has been shown by Grabitz et al. (20) and Seeger et al. (23) that the relaxation timescale τ after a pressure perturbation is proportional to the excess heat capacity Δcp such that
| (1) |
where T is the temperature in Kelvin and L is a phenomenological coefficient independent of temperature (see Grabitz et al. (20), and Seeger et al. (23), for details). For 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) multilamellar vesicles (MLV), for instance, L = 6.6 x 108 J K/mol and for DMPC large unilamellar vesicle (LUV) it is L = 15.7 x 108 J K/mol. This equation implies that relaxation is slow if the system is at the heat capacity maximum, which should be very pronounced in lipid membranes exhibiting a phase transition. For multilamellar dipalmitoylphosphatidlycholine (DPPC) vesicles, this time can be as long as 30 s. According to Onsager (24,25), each fluctuation can be considered as a perturbation of the system away from the entropy maximum. Therefore, fluctuation lifetimes and relaxation times are the same.
According to Einstein (26), fluctuations in the thermodynamic variable xi are related to the curvature of the entropy potential S by
or for xi = xj, in the often experimentally more convenient free energy (G) expression
where kb is the Boltzmann constant and T the temperature.
This can be used to show that heat capacity and fluctuations in enthalpy H are proportional,
around the entropy maximum (R is the gas constant). Along the same lines, one can show that the lateral compressibility κT is given by
| (2) |
i.e., it is proportional to the fluctuations in area A. Heimburg (27) and Ebel et al. (28) have shown that changes in heat, in volume, and in area in lipid transitions are proportional functions. This leads to the relation
| (3) |
meaning that the lateral compressibility changes are proportional to the excess heat capacity (γ is a material constant of ∼1 m2/J (27)). This further implies that it does not matter whether one considers a perturbation in enthalpy (after a temperature jump) or a perturbation in volume or area. The fluctuations in volume and area have the same lifetimes as those of the heat.
Pore formation in absence of channel-forming proteins occurs because of thermal area fluctuations. According to Nagle and Scott (29), the work to create a pore in the membrane is proportional to its lateral compressibility, which is proportional to the excess heat capacity (Eq. 3). As a consequence, pore formation is facilitated in the melting regime. Membranes are expected to be more permeable at the heat capacity maximum (for details, see(19)). Equating the fluctuation lifetime with the lifetime of pores, this implies that the pore opening times are proportional to the heat capacity. This relation has already been discussed in Seeger et al. (23), and in this article we will demonstrate that this relation leads to reasonable predictions. Both permeability and mean pore open time are at maximum in the chain melting transition. Experimental values for pore lifetimes are close to those estimated from Eq. 1.
Permeability changes during lipid membrane phase transition
To study the temperature dependence of lipid bilayers, a BLM was prepared at 40°C well above the phase transition of the lipid mixture used (5:95 DOPC/D15PC). Before the BLM experiments, the heat capacity of the lipid mixture has been measured (inset of Fig. 1), revealing a melting transition at Tm ≈ 30°C. To assure bilayer formation, the electric capacitance of the membrane film has been monitored. The bilayer was then left to equilibrate for 15 min. After film formation, the stability of the bilayer was tested by a stepwise increase of the clamp voltage until significant current fluctuation occurred (i.e., threshold voltage). Subsequently, the applied voltage VM was decreased until the current fluctuations disappeared, leaving the system close to the conducting state. After 10 min waiting for equilibration, the temperature was lowered at a rate of 0.2°C/min at constant clamp voltage. Fig. 1 shows the overall transmembrane conductivity (averaged over 60 s) at VM = 400 mV as a function of temperature. At T = 36°C the transmembrane conductivity begins to rapidly rise to reach a maximum at T ≈ 31.5°C. Clearly, ion permeability and heat capacity follow the same qualitative temperature dependence with a full-width at half height of 3–6 K. The slight shift of Itot (T) toward higher temperatures is probably due to the different sample preparations. After sonification, small unilamellar vesicles are believed to be under some tension, whereas BLMs are rather relaxed. Tension, however, tends to increase the membrane area, thereby supporting a lowering in Tm. Finally, uncontrollable lipid accumulation around the septum might be an additional source of changes in Tm. We were able to observe the same correlation between heat capacity and ion permeability for D15PC/DMPC mixtures (see Supporting Material). However, the introduction of DOPC in our lipid mixtures significantly increased the stability of the planar lipid membrane. This can also be seen in Fig. 2 b, where the bilayer resists membrane potentials as high as 1000 mV.
Figure 1.
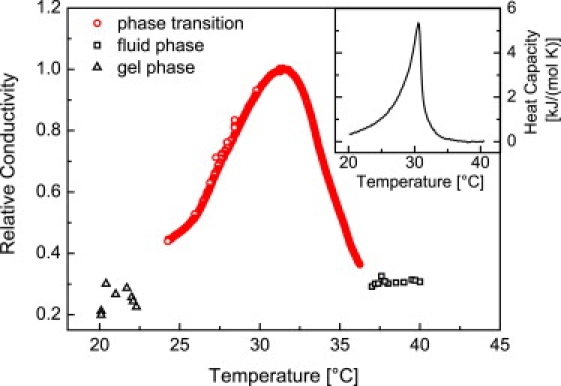
Relative conductance of a D15PC/DOPC (95:5) mixture as a function of temperature at VM = 400 mV. A maximum is observed between 35°C and 25°C. The current trace clearly correlates with the heat capacity profile (see inset). The maximum corresponds to a conductivity of 2 x 10−7 S/cm2. Conductivity measurements outside the transition region in the fluid (squares) and gel phase (triangles) have been performed on individual BLMs and are further described in the Supporting Material.
Figure 2.
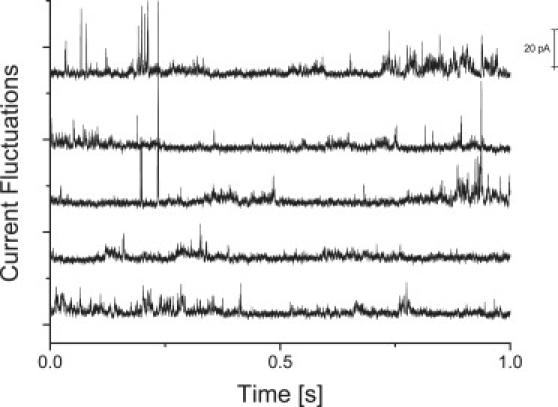
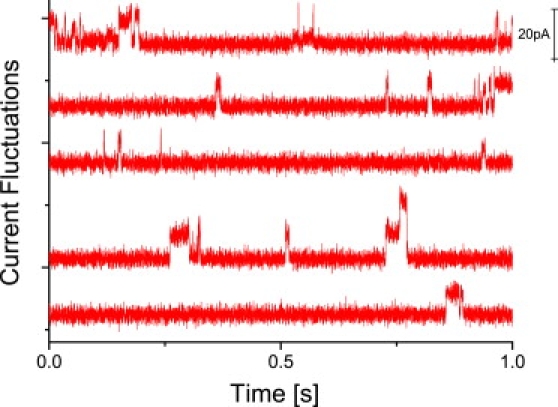
Typical current traces of a D15PC/DOPC (95:5) (top panel) in the fluid phase (33°C, VM = 100 ∼ mV) and (bottom panel) in the phase transition regime (31.5°C, VM = 1000 mV). In the fluid phase, spikelike current fluctuations on short timescales are observed. In the phase transition regime, however, quantized fluctuations appear at longer timescales. Note that the typical timescales are strongly increased as compared with the top panel.
One possible origin of the increase in overall permeability is the increasing number of lattice defects at the gel/fluid phase boundary. This was suggested by several theoretical findings (30) and is supported by the work of Papahadjopoulos et al. (6), who found a maximum leakage of radioactive labeled sodium ions during lipid phase transition. An alternative approach to explain the increased permeability in the phase transition region was given by Kaufmann et al. (31) as well as by Nagel and Scott (29) and Blicher et al. (19), who address the changes in lateral compressibility κT and their relation to the fluctuations in area 〈∂A2〉 (Eq. 2). Considering such area fluctuations as a source of defects excellently agrees with the correlation between heat capacity and conductivity.
Current characteristics strongly depend on lipid phase state
To unravel the origin of the transient conductivity behavior, we prepared BLMs from a DOPC/D15PC (5:95) mixture both in the fluid phase (40°C) and in the phase transition region (27°C). After a stepwise increase in VM until values were reached at which current fluctuations were observed, the voltage VM was again slightly reduced, resulting in stable current fluctuations over several minutes.
In the fluid phase (Fig. 2 a, T = 33°C, VM = 100 mV), spikelike events were observed and did not change their characteristics at even higher temperatures. The current fluctuations in the phase transition regime (Fig. 2 b, T = 31,5°C, VM = 1000 mV), however, appear rather steplike and quantized, with longer opening times. Again, the bilayer exhibited very high stability (the relation between applied voltage and initial resistance will be addressed in a separate article). For the DMPC/D15PC mixture, for example, strong current fluctuations were found at ∼500 mV and below (see Supporting Material). An analysis of the current amplitudes results in a rather broad distribution with two small maxima at ∼12 pS and 24 pS in the fluid state (Fig. 3 a). In the phase transition regime, these maxima become very pronounced (Fig. 3 b). In addition, the gel state exhibited some current fluctuations, which, however either slowly disappeared when waiting for extended times or led to membrane rupture (see Supporting Material). Distinct quantized current fluctuations in lipid membranes have been reported before (2,12–18,29), ranging between ∼1 pA and ∼1 nA close to the phase transition temperature. However, so far, no clear relation among current amplitudes, distribution, and fluctuations for the different thermodynamic states of the lipid membrane has been given.
Figure 3.
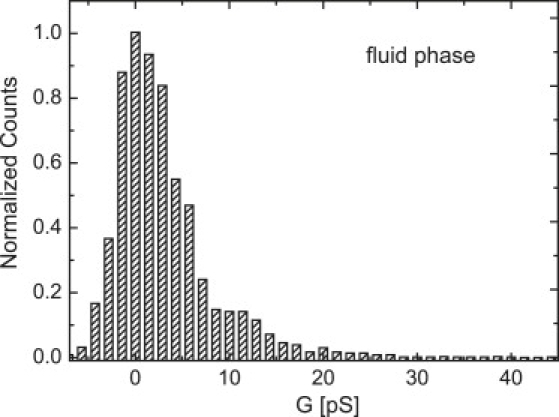
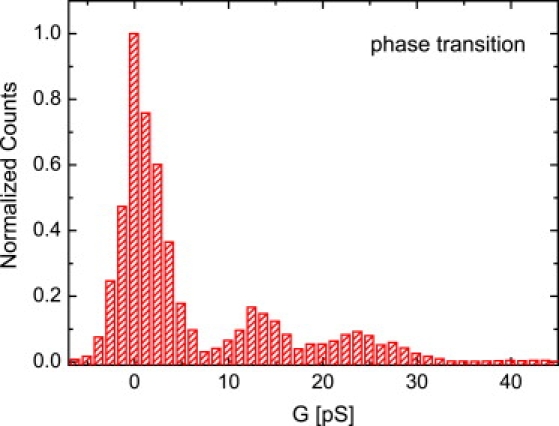
Conductivity histogram of current fluctuations in (top panel) the fluid phase (T = 33°C, VM = 100 mV) and (bottom panel) the phase transition regime (T = 31.5°C, VM = 1000 mV). The peak at G = 0 pS corresponds to the baseline. In the fluid phase, no significant peaks appear. In the phase transition regime, distinct maxima around G = 12 pS and G = 24 pS appear, with some additional substructure around G = 20 and G = 27 pS. Data were collected from three individual bilayers by analyzing a 200 s long trace. The observed maxima agree in all measurements within a range of 20%.
The existence of current fluctuations in both states suggests that the nature of the underlying mechanism for these current fluctuations is the same, but the specific characteristics seem to be determined by the physical properties of the membrane. In agreement with our results, we believe, that fluctuations in area 〈∂A2〉 are the origin of lattice defect formation and hence permeating ions. The origin of the steplike shape of the current fluctuations remains unclear, yet is probably related to the physical properties of the liquid crystal. In principle, and based on our experimental results, one could model the pore size necessary for an ion to pass the membrane. For a one-step fluctuation (Fig. 2 b), this suggests a single pore diameter of ∼1 nm, using a cylindrical pore approximation assuming free instead of surface-bound water within the pore.
Timescales of current fluctuation increase during phase transition
To unravel the relationship between thermodynamic behavior and the observed fluctuations, we analyzed the typical timescales of the opening times. Peaks were identified and characterized with a threshold criterion on the same set of data used to describe the conductance characteristics above (Fig. 3). In Fig. 4, we show the resulting data for the fluid phase (circles) and the phase transition (triangles). The logarithmic plot already reveals that the opening times are significantly increased within the phase transition regime. In the fluid phase, we found a mean average opening time of ∼3 ms (exponential fit). The behavior alters significantly when the phase transition region is entered. The mean average timescales shift by almost an order of magnitude to ∼20 ms, including long-lasting states of up to ∼100 ms. We also observed a broad variety of events including short events in the transition region and long fluctuations in the fluid state during measurements over extended periods of times. This is also reproduced by the broad distributions shown in Fig. 3, and reflects uncontrollable variations in the lipid membrane structure and dynamics like, for example, the pool of lipids accumulating around the septum or the degree of asymmetry. However, the tendency toward increased timescales during lipid phase transitions is obvious and reproducible in every experiment. Importantly, these enhanced timescales are in line with the increasing relaxation times of the membrane in this regime (20) and originate most likely from the small restoring forces of the flat thermodynamic potential around Tm. Experimentally, increased relaxation times during the main phase transition of artificial membranes have been reported before by Tsong (32) as well as by Blume and Hillmann (33). Since the relaxation times reflect the reorganization of lipids in the membrane, one would expect that pore nucleation, pore opening, and closing, respectively, follow the same time evolution as lipid relaxation.
Figure 4.
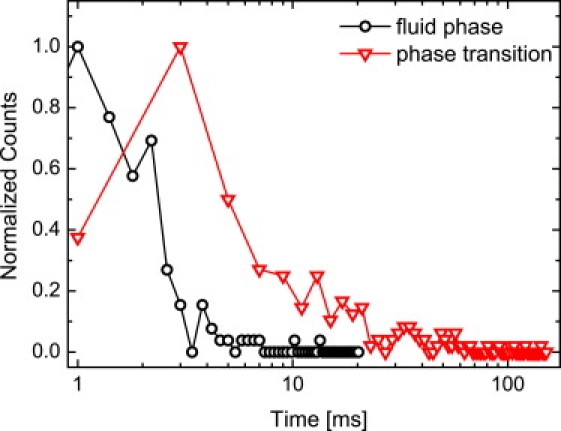
Logarithmic plot of the timescales of the current fluctuation in the fluid phase (triangles) and the phase transition regime (circles). The transmembrane currents have been measured under constant membrane potential Vm. The timescales of the fluid phase follow an exponential decay, whereas those in the transition can only be approximated by a double-exponential decay. The average lifetimes τ center around τ = 3 ms in the fluid and τ = 20 ms in the phase transition. This is in good agreement with our theoretical prediction.
Taking the phenomenological coefficient L for DMPC MLV (as given above) and numbers for cp from Fig. 1 (at ∼31°C), Eq. 1 yields lifetimes of 165 ms, whereas using the L from DMPC LUV yields lifetimes of 73 ms. Considering the transition half-width of only 2°C, this is very close to the experimentally found average lifetime of 20 ms (Fig. 4).
Conclusion
The behavior of current fluctuations in an unmodified phospholipid membrane under a constant voltage was found to be strongly sensitive to the phase state. Whereas fluctuations in the fluid phase reveal an unordered pattern on short timescales, in the phase-transition region, quantized fluctuations on much longer timescales occur. Using the fluctuation dissipation theorem and the linear relationship between heat capacity and area compressibility, we were able to predict the correct timescales and identify weak restoring forces of the flat thermodynamic potential as the origin of the extended timescales during lipid phase transition. Therefore, this article demonstrates the thermodynamic potential of lipid membranes to be the physical origin of lipid membrane current fluctuations.
Acknowledgments
We thank Dr. K. Kaufmann (Göttingen) for very helpful discussions and draw the reader's attention to his earlier work (31). M.F.S. personally thanks K. Kaufmann, who inspired him to work in this field, described the fundamental difficulty of transport across lipid membranes, and outlined the solution and its thermodynamic origin in his book in 1989 (31).
Financial support by the Deutsche Forschungsgemeinschaft (No. SPP 1313) and the German Excellence Initiative via the “Nanosystems Initiative Munich (NIM)” is gratefully acknowledged. C.L. thanks the Bavarian Science Foundation for financial support.
Supporting Material
References
- 1.Neher E., Sackmann E. Single-channel currents recorded from membrane of denervated frog muscle fibers. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- 2.Boheim G., Hanke W., Eibl H. Lipid phase transition in planar bilayer membrane and its effect on carrier- and pore-mediated ion transport. Proc. Natl. Acad. Sci. USA. 1980;77:3403–3407. doi: 10.1073/pnas.77.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B., Hermansson M., Gyorke S., Somerharju P., Virtanen J.A. Regulation of calcium channel activity by lipid domain formation in planar lipid bilayers. Biophys. J. 2003;85:933–942. doi: 10.1016/S0006-3495(03)74532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boheim G., Hanke W., Überschär S., Eibl H. Alamethicin pore formation in planar lipid bilayers above and below lipid phase transition temperature. In: Antolini R., Gliozzi A., Gorio A., editors. Model Systems and Reconstitution. Raven Press; New York: 1982. Transport in Biomembranes. [Google Scholar]
- 5.Boheim G., Hanke W., Überschär S., Eibl H. Pore forming ionophores induce lateral phase separation in planar lipid bilayers below lipid phase transition temperature. In: Spach G., editor. Physical Chemistry of Transmembrane Ion Motions. Elsevier; Amsterdam, The Netherlands: 1983. [Google Scholar]
- 6.Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–340. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 7.Abidor I.G., Arakelyan V.B., Chernomordik L.V., Chizmadzhev Y.A., Pastushenko V.F. Electric breakdown of bilayer lipid membranes. I. The main experimental facts and their qualitative discussion. Bioelectrochem. Bioenerg. 1979;6:37–52. [Google Scholar]
- 8.Weaver J.C., Chizmadzhev Y.A. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 1996;41:135–160. [Google Scholar]
- 9.Neumann E., Boldt E. Membrane electroporation: biophysical and biotechnical aspects. In: Cleary S.F., Hawdridge F.M., editors. Charge and Field Effects in Biosystems II.M.J. Allen. Plenum Press; New York: 1989. [Google Scholar]
- 10.Winterhalter M., Helfrich W. Effect of voltage on pores in membranes. Phys. Rev. A. 1987;36:5874–5876. doi: 10.1103/physreva.36.5874. [DOI] [PubMed] [Google Scholar]
- 11.Chermomordik L. Electropores in lipid bilayers and cell membranes. In: Chang D.C., Chassy B.M., Saunders J.A., Sowers A.E., editors. Guide to Electroporation and Electrofusion. Academic Press; New York: 1992. [Google Scholar]
- 12.Antonov V.F., Petrov V.V., Molnar A.A., Predvoditelev D.A., Ivanov A.S. The appearance of single ion channels in unmodified lipid bilayer membrane at the phase transition temperature. Nature. 1980;283:585–588. doi: 10.1038/283585a0. [DOI] [PubMed] [Google Scholar]
- 13.Antonov V.F., Anosov A.A., Norik V.P., Smirnova E.Y. Soft perforation of planar bilayer lipid membranes of dipalmitoylphosphatidylcholine at the temperature of the phase transition from the liquid crystalline to the gel state. Eur. Biophys. J. 2005;34:155–162. doi: 10.1007/s00249-004-0438-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann K., Silman I. The induction by protons of ion channels through lipid bilayer membranes. Biophys. Chem. 1983;18:89–99. doi: 10.1016/0301-4622(83)85002-9. [DOI] [PubMed] [Google Scholar]
- 15.Antonov V.F., Shevchenko E.V., Kozhomkulov E.T., Molnar A.A., Smirnova E.Y. Capacitive and ionic currents in BLM from phosphatidic acid in Ca2+-induced phase transition. Biochem. Biophys. Res. Commun. 1985;133:1098–1103. doi: 10.1016/0006-291x(85)91249-5. [DOI] [PubMed] [Google Scholar]
- 16.Gögelein H., Koepsell H. Channels in planar bilayers made from commercially available lipids. Pflugers Arch. 1984;401:433–434. doi: 10.1007/BF00584351. [DOI] [PubMed] [Google Scholar]
- 17.Woodbury D.J. Pure lipid vesicles can induce channel-like conductances in planar bilayers. J. Membr. Biol. 1989;109:145–150. doi: 10.1007/BF01870853. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa K., Fujimoto T., Shimooka T., Terada H., Kumazawa N. Electrical oscillation and fluctuations in phospholipid membranes. Phospholipids can form a channel without protein. Biophys. Chem. 1988;29:293–299. doi: 10.1016/0301-4622(88)85051-8. [DOI] [PubMed] [Google Scholar]
- 19.Blicher A., Wodzinska K., Fidorra, M.Winterhalter M., Heimburg T. The temperature dependence of lipid membrane permeability, its quantized nature, and the influence of anesthetics. Biophys. J. 2009;96:4581–4591. doi: 10.1016/j.bpj.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabitz P., Ivanova V.P., Heimburg T. Relaxation kinetics of lipid membranes and its relation to the heat capacity. Biophys. J. 2002;82:299–309. doi: 10.1016/S0006-3495(02)75395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider M.F., Marsh D., Jahn W., Kloesgen B., Heimburg T. Network formation of lipid membranes: triggering structural transitions by chain melting. Proc. Natl. Acad. Sci. USA. 1999;96:14312–14317. doi: 10.1073/pnas.96.25.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo R. The fluctuation-dissipation theorem. Rep. Prog. Phys. 1966;29:255–284. [Google Scholar]
- 23.Seeger H.M., Gudmundsson M.L., Heimburg T. On the influence of anesthetics, neurotransmitters and antibiotics on the relaxation processes in lipid membranes. J. Phys. Chem. B. 2007;111:13858–13866. doi: 10.1021/jp075346b. [DOI] [PubMed] [Google Scholar]
- 24.Onsager L. Reciprocal relations in irreversible processes I. Phys. Rev. 1931;37:405–426. [Google Scholar]
- 25.Onsager L. Reciprocal relations in irreversible processes II. Phys. Rev. 1931;38:2265–2279. [Google Scholar]
- 26.Einstein A. The theory of critical opalescence of homogenous liquids and liquid mixtures near the critical state [Theorie der Opaleszenz von homogenen Flüssigkeiten und Flüssigkeitsgemischen in der Nähe des kritischen Zustandes] Ann. Phys. 1910;25:205–226. [Google Scholar]
- 27.Heimburg T. Mechanical aspects of membrane thermodynamics. Estimation of the mechanical properties of lipid membranes close to the chain melting transition from calorimetry. Biochim. Biophys. Acta. 1998;1415:147–162. doi: 10.1016/s0005-2736(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 28.Ebel H., Grabitz P., Heimburg T. Enthalpy and volume changes in lipid membranes. I. The proportionality of heat and volume changes in the lipid melting transition and its implication for the elastic constants. J. Phys. Chem. B. 2001;105:7353–7360. [Google Scholar]
- 29.Nagle J.F., Scott H.L. Lateral compressibility of lipid mono and bilayers: theory of membrane permeability. Biochim. Biophys. Acta. 1978;513:236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- 30.Cruzeiro-Hansson L., Ipsen J.H., Mouritsen O.G. Intrinsic molecules in lipid membranes change the lipid-domain interfacial area: cholesterol at domain interfaces. Biochim. Biophys. Acta. 1989;979:166–176. doi: 10.1016/0005-2736(89)90432-x. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann, K., W. Hanke, and A. Corcia. 1989. BOOK 3: Ion Channel Fluctuations in Pure Lipid Bilayer Membranes: Control by Voltage. http://membranes.nbi.dk/Kaufmann/. Caruaru, Brazil.
- 32.Tsong T.Y. Kinetics of the crystalline-liquid crystalline phase transition of dimyristol l-a-lecithin bilayers. Proc. Natl. Acad. Sci. USA. 1974;71:2684–2688. doi: 10.1073/pnas.71.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blume A., Hillmann M. Dimyristoylphosphatidic acid/cholesterol bilayers. Thermodynamic properties and kinetics of the phase transition as studied by the pressure jump relaxation technique. Eur. Biophys. J. 1986;13:343–353. doi: 10.1007/BF00265670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


