Summary
Targeting essential cellular pathways that determine neuronal and vascular survival can foster a successful therapeutic platform for the treatment of a wide variety of degenerative disorders in the central nervous system. In particular, oxidative cellular injury can precipitate several nervous system disorders that may either be acute in nature, such as during cerebral ischemia, or more progressive and chronic, such as during Alzheimer disease. Apoptotic injury in the brain proceeds through two distinct pathways that ultimately result in the early externalization of membrane phosphatidylserine (PS) residues and the late induction of genomic DNA fragmentation. Degradation of DNA may acutely impact cellular survival, while the exposure of membrane PS residues can lead to microglial phagocytosis of viable cells, cellular inflammation, and thrombosis in the vascular system. Through either independent or common pathways, the Wingless/Wnt pathway and the serine-threonine kinase Akt serve central roles in the maintenance of cellular integrity and the prevention of the phagocytic disposal of cells "tagged" by PS exposure. By selectively governing the activity of specific downstream substrates that include GSK-3β, Bad, and β-catenin, Wnt and Akt serve to foster neuronal and vascular survival and block the induction of programmed cell death. Novel to Akt is its capacity to protect cells from phagocytosis through the direct modulation of membrane PS exposure. Intimately linked to the activation of Wnt signaling and Akt is the maintenance of mitochondrial membrane potential and the regulation of Bcl-xL, mitochondrial energy metabolism, and cytochrome c release that can lead to specific cysteine protease activation.
Keywords: Akt, Apoptosis, Oxidative Stress, Phosphatidylserine, Wingless
Oxidative stress as a precipitant of neuronal and vascular injury
Cellular injury in either neurons or cerebral endothelial cells (ECs) can occur through a variety of insults. In particular, oxidative stress through the generation of free radicals has been established as an important pathological component of several central nervous system disorders, such as Alzheimer disease and cerebral ischemia (Anderson et al., 2001; Maiese and Chong, 2003). Overproduction of reactive oxygen species (ROS) in cells leads to oxidative stress that ultimately results in cellular damage.
ROS consist of oxygen free radicals and similar agents that include superoxide free radicals, hydrogen peroxide, singlet oxygen, NO, and peroxynitrite. Oxygen free radicals are agents that contain unpaired electrons and function as electron acceptors to result in the oxidation of other molecules by accepting electrons. Superoxide radical, a product of an oxygen molecule with one additional electron, is an oxygen free radical that can lead to hydroxyl radical formation through hydrogen peroxide. Hydroxyl radicals are generated from hydrogen peroxide through the Haber-Weiss reaction in the presence of ferrous iron. Hydroxyl radicals also may be formed through a chemical reaction between the superoxide radical and nitric oxide (NO).NO interacts with the superoxide radical to yield peroxynitrite that generates a nitrosyl radical. Regeneration of hydroxyl radicals can then ensue, since nitrosyl radicals decompose to form hydroxyl radicals. Both NO and the peroxynitrite species are capable of leading to cell damage through cell membrane lipid destruction and cleavage of DNA (Vincent and Maiese, 1999b; Wang et al., 2003).
The brain is extremely sensitive to oxidative stress due to its enriched amount of unsaturated fatty acid, higher oxygen metabolic rate, and its weaker defense system against ROS. ROS lead to cell injury through a number of mechanisms, such as those involving the peroxidation of cellular membrane lipids (Siu and To, 2002), the cleavage of DNA during the hydroxylation of guanine and methylation of cytosine (Lee et al., 2002), and the oxidation of proteins that yield protein carbonyl derivatives and nitrotyrosine (Adams et al., 2001). Agents such as peroxynitrite also have been found to inhibit complex enzymes in the electron transport chain of the mitochondria resulting in the blockade of mitochondrial respiration (Yamamoto et al., 2002).
Early and late programs for apoptosis
Apoptosis, also known as programmed cell death (PCD), has been suggested to be involved in cellular injury in neurodegenerative diseases (Luth et al., 2002). Both neuronal and vascular PCD proceeds through two distinct pathways that are functionally independent leading to DNA fragmentation and membrane phosphatidylserine (PS) exposure. DNA degradation may immediately alter cellular integrity (Jessel et al., 2002), while the exposure of membrane PS residues can lead to microglial phagocytosis of viable cells (Hoffmann et al., 2001; Chong et al., 2003c; Kang et al., 2003a,b). Exposure of membrane PS residues is believed to occur prior to a later phase of genomic DNA degradation (Denecker et al., 2000) and serves to identify injured cells for microglial phagocytosis of viable cells (Hoffmann et al., 2001; Chong et al., 2002b). In ECs, the exposure of membrane PS residues can play a more formidable role by resulting in cellular inflammation and thrombosis (Dombroski et al., 2000).
ROS can precipitate PCD in neurons and ECs through several cellular pathways. In neurons, ROS can destroy cellular DNA integrity and membrane PS asymmetry. Oxidative stress, such as NO or hydrogen peroxide, results in nuclei condensation and DNA fragmentation (Vincent and Maiese, 1999b; Goldshmit et al., 2001; Chong et al., 2003c; Pugazhenthi et al., 2003). Externalization of membrane PS residues in neurons can occur during toxic insults from anoxia (Chong et al., 2002a), NO exposure (Chong et al., 2003c), or the administration of agents that induce the production of ROS, such as 6-hydroxydopamine (Salinas et al., 2003). ECs that are exposed to oxidative stress also suffer both DNA fragmentation and membrane PS externalization during exposure to specific toxins, such as hypoxia, oxidants, and free radicals (Aoki et al., 2001; Burlacu et al., 2001; Lin and Maiese, 2001; Chong et al., 2002a,b).
For effective therapeutic strategies, protection against PCD should be broad in nature by addressing the separate components of genomic DNA destruction and cellular membrane PS exposure. Current techniques now offer the ability to monitor the induction and change in PS exposure in individual living cells that can determine whether early apoptotic exposure of PS is reversible (Vincent and Maiese, 1999a; Maiese and Vincent, 2000b). Several agents, such as benzothiazole compounds (Maiese and Vincent, 2000a; Maiese and Vincent, 2000b) and Bcl-2 (Fabisiak et al., 2000, can prevent the induction of membrane PS exposure. Yet, other agents such as erythropoietin (Choudhury, 1999; Chong et al., 2002a, 2003b), metabotropic glutamate receptor agonists (Vincent et al., 1999; Lin and Maiese, 2001), and nicotinamide (Lin et al., 2000; Maiese and Chong, 2003) have been shown to also reverse the onset of cellular membrane PS exposure and block further induction of the apoptotic cascade. For example, post-treatment strategies with erythropoietin demonstrate that neuronal and EC membrane PS exposure is reversible in nature, but resides in a fixed time frame. Within a 6 hour period post the onset of a toxic exposure, erythropoietin can modulate critical cellular pathways prior to the induction of cellular mechanisms that can destine a cell to enter a committed apoptotic pathway. This fixed time frame for protection by agents such as erythropoietin most likely coincides with the progressive induction of secondary cellular pathways during a 6 hour time span, such as cytochrome c release and cysteine protease induction (Uehara et al., 1999; Lin and Maiese, 2001; Chong et al., 2003c).
Winning the survival game through the Wingless/Wnt pathway
The wingless/Wnt gene family encodes a group of secreted glycoproteins that play critical roles during embryonic development as well as tumorigenesis (Kawakami et al., 2001; Lustig and Behrens, 2003). Wnt proteins have been categorized into two groups named canonical and noncanonical which function through different signaling pathways. Canonical Wnts include Wnt-1, Wnt-3a, and Wnt-8 and function through β-catenin-dependent pathways. The noncanonical Wnts consist of Wnt-4, Wnt-5a, and Wnt-11 and function through non-β-catenin-dependent pathways, such as the planar cell polarity pathway and the Wnt-calcium dependent pathway (Slusarski et al., 1997; Tada and Smith, 2000). Wnt-1 is the best-characterized member of Wnt family. Wnt-1 was first identified as a proto-oncogene in mammary carcinomas through induction of mouse mammary tumor virus. In addition to its role in mammary neoplasms, Wnt-1 plays a critical role in neuronal development (Tang et al., 2002).
Interestingly, Wnt-1 signaling has been identified as one pathway that can prevent cellular apoptosis as well as lead to cell regeneration (Polesskaya et al., 2003). Wnt binds to the transmembrane receptor Frizzled and the co-receptor lipoprotein related protein 5 and 6 (LRP-5/6) (Wehrli et al., 2000) followed by recruitment of disheveled, the cytoplasmic bridging molecule, to inhibit glycogen synthase kinase (GSK-3b) (Ikeda et al., 1998; Papkoff and Aikawa, 1998). The inhibition of GSK-3β prevents phosphorylation of β-catenin and its degradation. The free β-catenin translocates to the nucleus where it activates lymphocyte enhancer factor (Lef) and T cell factor (Tcf) (Ishitani et al., 2003) leading to stimulation of Wnt – response genes. In some tumor cell lines, Wnt-1 prevents apoptosis through β-catenin/Tcf transcription mediated pathways (Chen et al., 2001; Rhee et al., 2002). Over-expression of exogenous Wnt-1 results in the protection of cells against c-Myc induced apoptosis through induction of β-catenin, cyclooxygenase-2, and Wnt-1 induced secreted protein (WISP-1) (You et al., 2002). In studies with chemotherapeutic agents, Wnt-1 signaling also can inhibit apoptosis through prevention of cytochrome c release from mitochondria and the subsequent inhibition of caspase 9 activation (Chen et al., 2001).
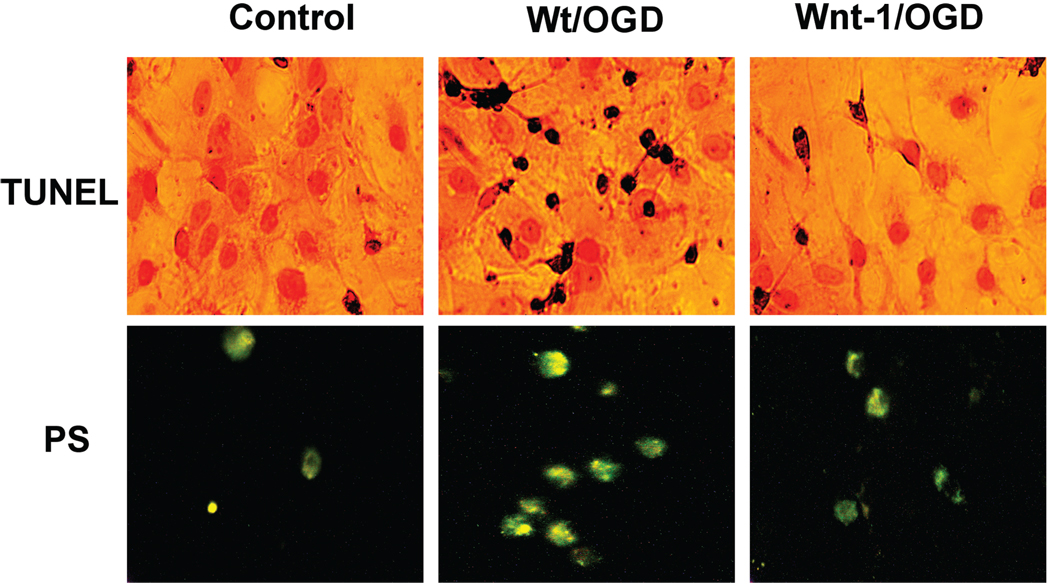
In the central nervous system, Wnt-1 also may function to prevent apoptosis during neuronal or vascular injury. Wnt-1 expression has been demonstrated in ECs (Wright et al., 1999) as well as in the brains of individuals affected by neuropsychiatric disorders (Miyaoka et al., 1999). More recent studies suggest that Wnt signaling may foster specific protection against cellular destruction and inflammatory injury by maintaining genomic DNA integrity and cellular membrane PS asymmetry. Our current studies illustrate that Wnt-1 transfection in primary hippocampal neurons protects cells against oxygen-glucose deprivation (OGD) resulting in an increase in cell survival and a decrease in percent PS exposure and DNA fragmentation following OGD exposure (Fig. 1).
Fig. 1.
Wnt-1 maintains genomic DNA integrity and membrane phosphatidylserine (PS) asymmetry during oxygen-glucose deprivation (OGD). Representative images illustrate DNA fragmentation with terminal deoxynucleotidyl transferase nick end labeling (TUNEL) and phosphatidylserine (PS) exposure with annexin V phycoerythrin labeling in wildtype (Wt/OGD) and Wnt-1 transfected (Wnt-1/OGD) hippocampal neurons 24 hours following exposure to OGD. OGD was performed by replacing media with glucose-free HBSS containing 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 1mM NaH2PO4, 0.9 mM CaCl2, and 10 mg/L phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 °C for 3 hours. OGD induced DNA fragmentation and membrane PS exposure in wildtype cells (Wt/NO) while there was no injury present in Wnt-1 transfected cells.
Consistent with the cytoprotective potential of Wnt signaling, absence or dysfunction in Wnt signaling can precipitate neurodegeneration. The disorder retinitis pigmentosa leads to a progressive apoptotic loss of photoreceptors that has been associated with disruptions in Wnt signaling and excess secretion of Frizzled-related protein-2, suggesting that impairments in the Wnt signaling pathway may be involved in retinal neurodegeneration (Jones et al., 2000). Loss of Wnt signaling also appears to play a role in more widespread neurodegenerative disorders, such as Alzhemer disease. Neurotoxicity of β-amyloid deposition of the 39–42 amino acid peptide (Aβ) in hippocampal neurons during Alzheimer disease has been linked to increased levels of GSK-3β and loss of β-catenin. Decreased production of Aβ can occur during the enhancement of protein kinase C (PKC) activity (Savage et al., 1998) which may be controlled by the Wnt pathway (Garrido et al., 2002). Furthermore, the proteolytic processing of the beta-amyloid precursor protein (APP) during Alzheimer disease has been closely linked to the Wnt pathway through at least two distinct mechanisms. Presenilin 1 (PS1) is required for the proteolytic processing of APP that can play a pivotal role in the development of Alzheimer disease. PS1 has been shown to down-regulate Wnt signaling and interact with beta-catenin to promote its turnover (Soriano et al., 2001). In addition, dishevelled, a downstream transducer of Wnt signaling, can promote non-amyloidogenic alpha-secretase cleavage of APP to yield secreted APP (sAPP) and inhibit GSK-3β to reduce the phosphorylation of tau. Thus, disheveled may increase neuronal protection during neurodegenerative disorders through sAPP production and reduction in tau phosphorylation (Mudher et al., 2001).
Akt can function solo or partner with Wnt to prevent cellular injury
One potential pathway that may be central for fostering cellular integrity and survival in the central nervous system involves protein kinase B, also referred to as PKBα or Akt after the oncogene v-Akt. Akt is phosphorylated and activated through the phosphoinositide 3 kinase (PI 3-K) pathway. Once recruited to the plasma membrane, PI 3-K phosphorylates glycerophospholipid phosphatidyl-inositol 4,5-bisphosphate and yields phosphatidylinositol 3,4 bisphosphate (PIP2) and phosphatidylinositol 3,4,5 trisphosphate (PIP3). In the cytosol, Akt translocates to the cell membrane as a result of its binding to PIP2 and PIP3 and subsequently becomes activated through phosphorylation by phosphoinositide-dependent kinase 1 (Wick et al., 2000).
Increased activity of Akt can provide protection against neuronal and vascular injury. Maximal activity of Akt is achieved through phosphorylation by phosphoinositide-dependent kinase 1 at Ser473 to confer protection against genomic DNA degradation (Yamaguchi et al., 2001; Chong et al., 2002a; Wick et al., 2002) and membrane PS exposure (Chong et al., 2002a, 2003c; Kang et al., 2003). During oxidative stress, such as injuries involving excitotoxicity (Kim et al., 2002), free radical exposure (Matsuzaki et al., 1999; Chong et al., 2003c), hypoxia (Chong et al., 2002a), or trauma (Murashov et al., 2001), phosphorylation of Akt is enhanced.
This protection against apoptotic injury by Akt may be dependent upon the activity of several substrates, such as Bad, caspase 9, IkB kinase γ, the forkhead transcription factor (FOXOX3a, FHKRL1), and GSK-3β. For example, phosphorylation of Bad leads to the binding of Bad with the cytosolic protein 14-3-3 to release Bcl-xL and allow it to block apoptosis (Masters et al., 2001). As a substrate of Akt, the Forkhead transcription factor also modulates survival in a variety of cell systems (Brunet et al., 1999; Shin et al., 2001; Dijkers et al., 2002). Inhibitory phosphorylation of FOXO3a may prevent apoptosis through several mechanisms, such as blocking FOXO3a transcription during its association with 14-3-3 protein (Brunet et al., 1999), regulating the induction of the cell cycle (Kops et al., 2002), or through the modulation of mitochondrial membrane depolarization and cytochrome c release (Dijkers et al., 2002).
Beyond its independent role to prevent cellular apoptosis, Akt may be required for the Wnt-1 pathway to promote cellular differentiation and survival. As previously described, Wnt-1 can inactivate GSK-3β and block the phosphorylation of β-catenin (Ikeda et al., 1998; Papkoff and Aikawa, 1998). This leads to the activation of β-catenin followed by transcription of its target genes for cellular protection. Akt may be necessary in pathways that involve Wnt-1, since Akt inhibits the activity of GSK-3β through phosphorylation of this protein to promote cell survival (Crowder and Freeman, 2000). Furthermore, neuronal cell differentiation that is dependent upon Wnt signaling appears to become stalled without Akt phosphorylation and the subsequent inactivation of GSK-3β (Fukumoto et al., 2001). In addition, Wnt has been demonstrated through WISP-1 to activate the anti-apoptotic signaling pathway of Akt following genomic DNA damage (Su et al., 2002) and to block cell injury during serum withdrawal through increased Akt phosphorylation and activity (Longo et al., 2002).
Akt offers novel protection against microglial activation and proliferation
Akt also may offer a unique level of cellular protection by modulating membrane PS exposure and microglial activation. Although usually maintained in a quiescent state, microglia can become activated during a variety of pathological insults. Activated microglia may lead to cellular damage through the generation of NO and associated ROS products (Sankarapandi et al., 1998). The secretion of cytokines by microglia also may represent another source of cytotoxicity for this cell population. Microglia produce a variety of cytokines in response to toxic stimulation, such as interleukins and tumor necrosis factor-α (TNF-α). TNF-α production by microglia may be linked to neurodegeneration by increasing the sensitivity of neurons to free radical exposure. For example, Aβ induced microglial secretion of TNF-α during Aβ deposition lead to the neuronal expression of inducible NOS, peroxinitrite production, and neuronal apoptosis (Combs et al., 2001).
Once activated, microglia function to remove cellular debri and apoptotic cells through phagocytosis. A recent body of investigations have elucidated several potential mechanisms that may regulate the phagocytosis of cells that have entered the apoptotic pathway. Some studies point to the generation of annexin I and membrane PS exposure that appears to be necessary to tether an apoptotic cell with a phagocyte (Arur et al., 2003). Secreted factors by either apoptotic or phagocytic cells, such as milk fat globule EGF factor 8 (Hanayama et al., 2002), fractalkine (Hatori et al., 2002), and lipid lysophosphosphatidylcholine (Lauber et al., 2003) also have been shown to assist with the phagocytic removal of injured cells.
Yet, a common denominator that appears to be critical for the removal of apoptotic cells by phagocytic sentries is the translocation of membrane PS residues from the inner cellular membrane to the outer surface (Maiese and Vincent, 2000b; Fadok et al., 2001; Kang et al., 2003). In cells that are without injury, the phospholipids of the plasma membrane are distributed asymmetrically with the outer leaflet of the plasma membrane consisting primarily of choline-containing lipids, such as phosphatidylcholine and sphingomyelin, and the inner leaflets consisting of aminophospholipids that include phosphatidylethanolamine and PS. The disruption of membrane phospholipid asymmetry leads to the externalization of membrane PS residues and serves to identify cells for phagocytosis (Hoffmann et al., 2001; Chong et al., 2003d; Kang et al., 2003; Maiese and Chong, 2003). Under some circumstances, translocation of PS residues may be associated with energy depletion during cellular injury. Membrane PS residues can appear on the external leaflet as a result of reduced aminophospholipid translocase activity (Gleiss et al., 2002) and activation of a phospholipid scramblase that may be calcium independent (Williamson et al., 2001). Maintenance of PS on the inner leaflet of the cell membrane is through the activity of a 120-Da magnesium-dependent ATPase. This ATPase-dependent activity is lost during apoptosis. As a result, the inhibition of the ATP-dependent aminophospholipid translocase during cellular injury can play a significant role in PS externalization (Goldshmit et al., 2001).
Interestingly, Akt can modulate the spatial regulation of actin assembly, suggesting a relationship between Akt and the coordination of cytoskeletal organization (Lemmon et al., 2002). Furthermore, through a series of investigations, Akt has recently been shown to be a necessary component for the modulation of membrane PS externalization and prevent microglial activation (Kang et al., 2003a,b). Initially, microglial activation and proliferation have been shown to occur during oxidative stress that includes free radical exposure (Chong et al., 2003c). In addition, through the use of an antibody to the PS receptor, it has been demonstrated that membrane PS residue exposure is both necessary and sufficient to induce microglial activation and proliferation (Chong et al., 2003c; Kang, 2003; Kang et al., 2003). Furthermore, media taken from cells that overexpress active, phosphorylated Akt during cellular injury leads to a significant reduction in microglial activation and proliferation (Kang et al., 2003a,b). Taken together, this series of studies illustrate that Akt can directly modulate microglial activation and proliferation through the modulation of membrane PS exposure on cells and conceivably prevent the shedding of membrane PS residues that is known to occur during apoptosis (Simak et al., 2002).
Mitochondrial membrane potential (ΔΨm) becomes critical for cell survival
In both neuronal and vascular populations, maintenance of cellular integrity during toxic insults, such as oxidative stress, is unlikely to be determined by only one or two principal mechanisms. More often, preservation of cellular survival requires the intricate association of a series of cellular pathways. One pathway in particular that is closely linked to the activation of Wnt signaling and Akt is the maintenance of mitochondrial membrane potential (ΔΨm). For example, Wnt can prevent the induction of PCD during c-Myc activation (You et al., 2002) and p53-dependent cell death (Su et al., 2002) by inhibiting mitochondrial release of cytochrome c. Similar to Wnt, Akt activation has been shown to be necessary and sufficient to inhibit the release of cytochrome c from mitochondria (Kennedy et al., 1999; Kang et al., 2003b).
Mitochondria are a significant source of superoxide radicals and other ROS that are associated with oxidative stress. Although amino acid biosynthesis, fatty acid oxidation, and steroid metabolism are vital functions of mitochondria, production of ATP through the electron transport chain is considered to be the most critical of mitochondrial functions. Blockade of the electron transfer chain at the flavin mononucleotide group of complex I (NADPH ubiquinone oxidoreductase) or at ubiquinone site of complex III (ubiquinone-cytochrome c reductase) results in the active generation of ROS (Turrens et al., 1985; Liu et al., 2002). Once generated, ROS further impair mitochondrial electron transport and enhance ROS production.
Loss of ΔΨm through the opening of the mitochondrial permeability transition pore represents a significant determinant for cell injury and the subsequent induction of the apoptotic cascade (Bal-Price and Brown, 2000; Lin et al., 2000; Chong et al., 2003b). Oxidative stress through ROS generation leads to the opening of the mitochondrial permeability transition pore and the release of cytochrome c into the cytosol (Maciel et al., 2001). The pro-apoptotic member Bax has been demonstrated to increase the production of ROS from mitochondria and precipitate the release of cytochrome c (Kirkland et al., 2002). Once Bax is translocated to mitochondrial membrane from cytosol, it undergoes conformational alteration resulting in its insertion into the mitochondrial membrane to facilitate cytochrome c release. Bax forms clusters with the formation of Bax multimers that appear to be a prerequisite for cytochrome c release (De Giorgi et al., 2002). Subsequent release of cytochrome c results in the oligomerization of apoptotic protease activating factor-1 (Apaf-1) and promotes the allosteric activation of caspase 9 by forming the Apaf-1 apoptosome (Li et al., 1997). Caspase 9 can subsequently activate caspase 3 (Li et al., 1997) as well as caspase 1 through the intermediary caspase 8 (Takahashi et al., 1999). Together, caspase 1 and caspase 3 lead to both DNA fragmentation and membrane PS exposure (Li et al., 1997; Maiese and Vincent, 2000b; Chong et al., 2002a).
Controlling cytochrome c release directly through mitochondrial membrane pore formation
A number of pathways may assist in preserving cell survival and integrity through the modulation of ΔΨm and the release of cytochrome c during oxidative stress. The Bcl-2 family is one group of proteins that can regulate apoptosis through the modulation of mitochondrial homeostasis. Several Bcl-2 family members have been identified and are functionally categorized into two groups that contain anti-apoptotic protein members (Bcl-2, Bcl-xL) and pro-apoptotic protein members (Bax, Bad, Bak, Mcl-2). The balance between anti-apoptotic and pro-apoptotic Bcl-2 family members can be critical in determining fate of a cell. Bcl-2 and Bcl-xL are localized on the outer mitochondrial membrane, the nuclear envelope, and the endoplasmic reticulum (Krajewski et al., 1993; Hsu et al., 1997). These proteins block apoptosis by preventing mitochondrial cytochrome c release. This process occurs through the modulation of intracellular calcium and the regulation of intracellular pH (Ishaque and Al-Rubeai, 1998). In contrast, the pro-apoptotic member Bax oligomerizes after release from binding to Bcl-2 and then inserts itself into the mitochondrial membrane to trigger cytochrome c release (Eskes et al., 2000).
Trophic factors that have recently been shown to have application in the central nervous system, such as erythropoietin, may modulate the release of cytochrome c directly or through the regulation of the Bcl-2 family member Bcl-xL. At least in erythroid cells, the Bcl-2 member Bcl-xL has been shown to be strongly expressed and necessary for erythropoietin to prevent apoptosis in the later stages of erythroid progenitor cell life (Gregoli and Bondurant, 1997). In neurons, Bcl-xL has been shown to be strongly expressed during erythropoietin administration (Wen et al., 2002). In addition, erythropoietin may require Bcl-xL expression for cytoprotection, since without erythropoietin, Bcl-xL is not expressed and apoptotic cell death results in hematopoietic cells (Silva et al., 1996). More recent work in cerebral vascular cell populations illustrate that up-regulation of Bcl-xL by erythropoietin may be necessary for the prevention of PCD (Chong et al., 2003b).
It is also conceivable that the stabilization of cellular energy metabolism may be an important factor to modulate mitochondrial membrane pore formation, since the maintenance of mitochondrial membrane potential is an ATP facilitated process (Lemeshko and Lemeshko, 2000). Studies with nicotinamide, a precursor for the coenzyme β-nicotinamide adenine dinucleotide (NAD+) and an agent that prevents NAD+ depletion (Klaidman et al., 2001), appear to support such a premise. Nicotinamide can act directly at the level of mitochondrial membrane pore formation to prevent cytochrome c release (Maiese et al., 2001; Chong et al., 2002b; Maiese and Chong, 2003). Nicotinamide is able to reverse mitochondrial membrane depolarization during the induction of mitochondrial permeability transition pore formation by the agents tert-butylhydorperoxide, an oxidative inducer of mitochondrial membrane permeability that impairs mitochondrial ATP synthesis (Imberti et al., 1993), and during the administration of atractyloside, an agent that binds to the mitochondrial adenosine nucleotide translocator to elicit pore formation (Brown et al., 1997).
Both Wnt signaling and Akt also play vital roles in maintaining mitochondrial membrane integrity to prevent the induction of apoptotic pathways. In addition to work illustrating that Wnt can prevent mitochondrial release of cytochrome c (Su et al., 2002; You et al., 2002), several investigations demonstrate that Akt blocks cysteine protease activity through the modulation of mitochondrial membrane potential and cytochrome c release. Akt may control mitochondrial release through the modulation of Bad. Bad is thought to induce apoptosis via the formation of heterodimers with Bcl-xL resulting in the displacement and release of Bax from the binding with Bcl-xL. Bax can then translocate to the mitochondria where it promotes cytochrome c release. Akt phosphorylates Bad, inhibits Bax conformational change, and blocks the translocation of Bax to the mitochondria preventing cytochrome c release and apoptosis (Yamaguchi et al., 2001). Alternatively, Akt may act directly at the level of the mitochondrial membrane and alter mitochondrial pore formation through pathways that are independent from Bcl-xL. In some cell systems, Akt appears to rely on the maintenance of ΔΨm to a greater degree than Bcl-xL to maintain cell survival and prevent cell injury (Plas et al., 2001). Additional work suggests that Akt prevents apoptotic injury through the direct modulation of the inner mitochondrial membrane potential (Dijkers et al., 2002), since Akt is ineffective in fostering cell survival during the direct application of cytochrome c (Kennedy et al., 1999). Investigations that have employed neuronal or EC clones to either overexpress the myristoylated (active) form of Akt or a dominant-negative Akt mutant that lacked kinase activity further support the premise that Akt directly maintains mitochondrial membrane potential and prevents the release of cytochrome c during oxidative stress injury (Chong et al., 2003a; Kang et al., 2003a,b).
Conclusion
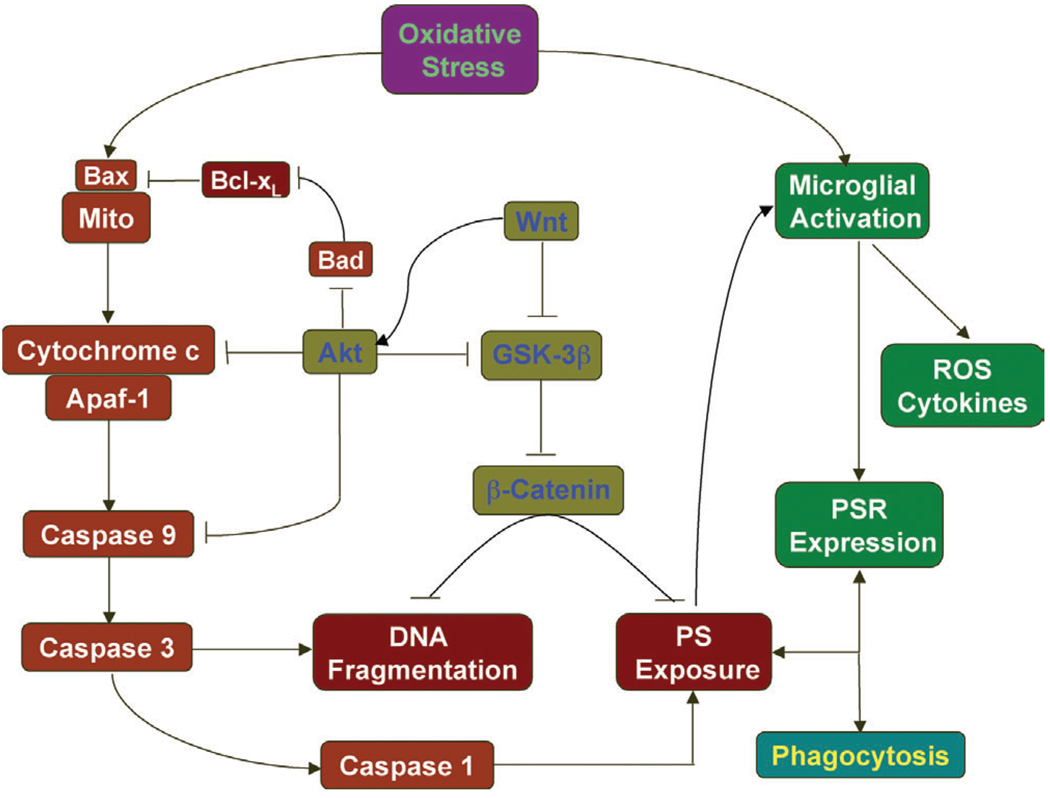
Interest in cellular mechanisms that modulate neuronal and vascular survival in the central nervous system continues to gain significant attention. Knowledge of the cellular elements that not only precipitate cellular injury, but also foster cellular integrity becomes essential for future development of therapeutic strategies against neurodegenerative disorders (Fig. 2). Neuronal and vascular apoptotic injury proceeds through two independent pathways that consist of the early externalization of membrane PS residues and the later destruction of genomic DNA. In neurons, exposure of membrane PS residues primarily serves to identify injured cells for microglial phagocytosis. In ECs, externalization of membrane PS residues promotes cell demise through additional pathways that involve cellular inflammation and thrombosis. Pivotal to the modulation of cellular integrity and phagocytic disposal of injured cells are the signaling pathways of the proto-oncogene Wnt and the serine-threonine kinase Akt. Through either distinct or common pathways, Wnt and Akt act upon downstream substrates, such as GSK-3β, Bad, and WISP-1 to block the induction of apoptotic cellular injury. Novel to the Akt pathway is the ability of Akt to protect cells from inflammatory injury and phagocytic removal through the direct modulation of cellular membrane PS externalization. One particular pathway that is closely tied to the protective capacities of both Wnt and Akt is the maintenance of ΔΨm and the central modulation of Bcl-xL, mitochondrial energy reserves, and cytochrome c release. By elucidating and targeting the critical elements that govern neuronal and vascular survival, we can eventually foster successful clinical applications for the treatment of degenerative disorders in the central nervous system.
Fig. 2.
Cellular modulators of degenerative disease in the central nervous system. Apoptotic injury in either neuronal or vascular cells proceeds through two independent pathways that ultimately result in the early externalization of membrane phosphatidylserine (PS) residues and the late induction of genomic DNA fragmentation. Externalization of membrane PS residues can precipitate microglial activation, the phagocytosis of injured cells, and thrombosis in the vascular system. Essential to the maintenance of genomic DNA integrity are the signaling pathways of the proto-oncogene Wnt and the serine-threonine kinase Akt. Through either distinct or common pathways, Wnt and Akt act upon downstream substrates, such as GSK-3β, Bad, and b-catenin to block the induction of programmed cell death. Unique to Akt is its ability to prevent phagocytosis of cells through the direct modulation of cellular membrane PS externalization. Closely tied to the protective capacities of both Wnt and Akt is the maintenance of ΔΨm and the central modulation of Bcl-xL, mitochondrial energy reserves, and cytochrome c release that can lead to specific cysteine protease activation of caspase 1, 3, and 9.
Acknowledgements
This research was supported by the following grants (KM): American Heart Association (National), Janssen Neuroscience Award, Johnson and Johnson Focused Investigator Award, LEARN Foundation Award, MI Life Sciences Challenge Award, and NIH NIEHS (P30 ES06639).
References
- Adams S, Green P, Claxton R, Simcox S, Williams MV, Walsh K, Leeuwenburgh C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front Biosci. 2001;6:A17–A24. doi: 10.2741/adams. [DOI] [PubMed] [Google Scholar]
- Anderson I, Adinolfi C, Doctrow S, Huffman K, Joy KA, Malfroy B, Soden P, Rupniak HT, Barnes JC. Oxidative signaling and inflammatory pathways in Alzheimer's disease. Biochem. Soc. Symp. 2001;67:141–149. doi: 10.1042/bss0670141. [DOI] [PubMed] [Google Scholar]
- Aoki M, Nata T, Morishita R, Matsushita H, Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T, Kaneda Y. Endothelial apoptosis induced by oxidative stress through activation of nf-kappab: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38:48–55. doi: 10.1161/01.hyp.38.1.48. [DOI] [PubMed] [Google Scholar]
- Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J. Neurochem. 2000;75:1455–1464. doi: 10.1046/j.1471-4159.2000.0751455.x. [DOI] [PubMed] [Google Scholar]
- Brown J, Higo H, McKalip A, Herman B. Human papillomavirus (HPV) 16 E6 sensitizes cells to atractyloside-induced apoptosis: role of p53, ICE-like proteases and the mitochondrial permeability transition. J. Cell. Biochem. 1997;66:245–255. doi: 10.1002/(sici)1097-4644(19970801)66:2<245::aid-jcb11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Burlacu A, Jinga V, Gafencu AV, Simionescu M. Severity of oxidative stress generates different mechanisms of endothelial cell death. Cell. Tissue Res. 2001;306:409–416. doi: 10.1007/s004410100424. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002a;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J. Vasc. Res. 2002b;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Maiese K. Erythropoietin: Cytoprotection in vascular and neuronal cells. Curr. Drug. Targets-Cardiovas. Hemat. Dis. 2003a;3:141–154. doi: 10.2174/1568006033481483. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J. Cereb. Blood Flow. Metab. 2003b;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharmacol. 2003c;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol. Histopathol. 2003d;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Glycogen synthase kinase-3 beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J. Biol. Chem. 2000;275:34266–34271. doi: 10.1074/jbc.M006160200. [DOI] [PubMed] [Google Scholar]
- De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- Denecker G, Dooms H, Van Loo G, Vercammen D, Grooten J, Fiers W, Declercq W, Vandenabeele P. Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 2000;465:47–52. doi: 10.1016/s0014-5793(99)01702-0. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein Iimmune mice. J. Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabisiak JP, Tyurin VA, Tyurina YY, Sedlov A, Lazo JS, Kagan VE. Nitric oxide dissociates lipid oxidation from apoptosis and phosphatidylserine externalization during oxidative stress. Biochemistry. 2000;39:127–138. doi: 10.1021/bi9912544. [DOI] [PubMed] [Google Scholar]
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16:1982–1984. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- Gleiss B, Gogvadze V, Orrenius S, Fadeel B. Fas-triggered phosphatidylserine exposure is modulated by intracellular ATP. FEBS Lett. 2002;519:153–158. doi: 10.1016/s0014-5793(02)02743-6. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J. Biol. Chem. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- Gregoli PA, Bondurant MC. The roles of Bcl-X(L) and apopain in the control of erythropoiesis by erythropoietin. Blood. 1997;90:630–640. [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hatori K, Nagai A, Heisel R, Ryu JK, Kim SU. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002;69:418–426. doi: 10.1002/jnr.10304. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotc cells. J. Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberti R, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial and glycolytic dysfunction in lethal injury to hepatocytes by t-butylhydroperoxide: protection by fructose, cyclosporin A and trifluoperazine. J. Pharmacol. Exp. Ther. 1993;265:392–400. [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Use of intracellular pH and annexin-V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J. Immunol. Methods. 1998;221:43–57. doi: 10.1016/s0022-1759(98)00166-5. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol. Cell. Biol. 2003;23:1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J. Cell. Mol. Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000;11:3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- Kang J-Q, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through the maintenance of membrane asymmetry and modulation of cysteine protease activity. J. Neurosci. Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol. Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Proteinckinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL. A Bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J. Neurosci. 2002;22:6480–6490. doi: 10.1523/JNEUROSCI.22-15-06480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaidman LK, Mukherjee SK, Adams JD., Jr. Oxidative changes in brain pyridine nucleotides and neuroprotection using nicotinamide. Biochim. Biophys. Acta. 2001;1525:136–148. doi: 10.1016/s0304-4165(00)00181-1. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell Biol. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Lee DH, O'Connor TR, Pfeifer GP. Oxidative DNA damage induced by copper and hydrogen peroxide promotes CG→TT tandem mutations at methylated CpG dinucleotides in nucleotide excision repair-deficient cells. Nucleic Acids Res. 2002;30:3566–3573. doi: 10.1093/nar/gkf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeshko SV, Lemeshko VV. Metabolically derived potential on the outer membrane of mitochondria: a computational model. Biophys. J. 2000;79:2785–2800. doi: 10.1016/S0006-3495(00)76518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J. Cereb. Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J. Cereb. Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J. Cancer Res. Clin. Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953:135–143. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca(2+)-induced membrane permeability transition in brain mitochondria. J. Neurochem. 2001;79:1237–1245. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends. Pharmacol. Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Lin S, Chong ZZ. Elucidating neuronal and vascular injury through the cytoprotective agent nicotinamide. Curr. Med. Chem-Imm. Endoc. Metab. Agents. 200;11:257–267. [Google Scholar]
- Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol. Neurobiol. 2000a;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J. Neurosci Res. 2000b;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Masters SC, Yang H, Datta SR, Greenberg ME, Fu H. 14-3-3 inhibits Bad-induced cell death through interaction with serine-136. Mol. Pharmacol. 2001;60:1325–1331. doi: 10.1124/mol.60.6.1325. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 1999;73:2037–2046. [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr. Res. 1999;38:1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, Killick R, Iqbal T, Raymond L, Varndell I, Sheppard P, Makoff A, Gower E, Soden PE, Lewis P, Murphy M, Golde TE, Rupniak HT, Anderton BH, Lovestone S. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J. Neurosci. 2001;21:4987–4995. doi: 10.1523/JNEUROSCI.21-14-04987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Ul Haq I, Hill C, Park E, Smith M, Wang X, Goldberg DJ, Wolgemuth DJ. Crosstalk between p38, Hsp25 and Akt in spinal motor neurons after sciatic nerve injury. Brain Res. Mol. Brain Res. 2001;93:199–208. doi: 10.1016/s0169-328x(01)00212-1. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta-activity and beta-catenin signaling in mammary epithelial cells. Biochem. Biophys. Res. Commun. 1998;247:851–858. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J, Neurochem. 2003;84:982–996. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J. Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I, Bakin AV, Rodeck U, Brunet A, Arteaga CL. Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol. Biol. Cell. 2001;12:3328–3339. doi: 10.1091/mbc.12.11.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Grillot D, Benito A, Richard C, Nunez G, Fernandez-Luna JL. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood. 1996;88:1576–1582. [PubMed] [Google Scholar]
- Simak J, Holada K, Vostal JG. Release of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecin. BMC Cell Biol. 2002;3:11. doi: 10.1186/1471-2121-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AW, To CH. Nitric oxide and hydroxyl radical-induced retinal lipid peroxidation in vitro. Clin. Exp. Optom. 2002;85:378–382. [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J. Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp. Cell Res. 1999;249:291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Tang K, Yang J, Gao X, Wang C, Liu L, Kitani H, Atsumi T, Jing N. Wnt-1 promotes neuronal differentiation and inhibits gliogenesis in P19 cells. Biochem. Biophys Res. Commun. 2002;293:167–173. doi: 10.1016/S0006-291X(02)00215-2. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Uehara T, Kikuchi Y, Nomura Y. Caspase activatation accompanying cytochrome c release from mitochondria is possibly involved in nitric oxide-induced neuronal apoptosis in SH- SY5Y cells. J. Neurochem. 1999;72:196–205. doi: 10.1046/j.1471-4159.1999.0720196.x. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J. Histochem. Cytochem. 1999a;47:661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp. Cell Res. 1999b;246:290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp. Neurol. 1999;155:79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Ho YJ. Oxidative neurotoxicity in rat cerebral cortex neurons: synergistic effects of H2O2 and NO on apoptosis involving activation of p38 mitogen-activated protein kinase and caspase-3. J. Neurosci. Res. 2003;72:508–519. doi: 10.1002/jnr.10597. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J. Neurosci. Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J. Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- Williamson P, Christie A, Kohlin T, Schlegel RA, Comfurius P, Harmsma M, Zwaal RF, Bevers EM. Phospholipid scramblase activation pathways in lymphocytes. Biochemistry. 2001;40:8065–8072. doi: 10.1021/bi001929z. [DOI] [PubMed] [Google Scholar]
- Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 2001;276:5256–5264. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Maruyama W, Kato Y, Yi H, Shamoto-Nagai M, Tanaka M, Sato Y, Naoi M. Selective nitration of mitochondrial complex I by peroxynitrite: involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J. Neural Transm. 2002;109:1–13. doi: 10.1007/s702-002-8232-1. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]