Abstract
Aging is associated with deficiencies in the prefrontal cortex, including working memory impairment, and compromised integrity of neuronal dendrites. Although protein kinase C (PKC) is implicated in structural plasticity, and overactivation of PKC results in working memory impairments in young animals, the role of PKC in prefrontal cortical impairments in the aged has not been examined. This study provides the first evidence that PKC activity is associated with prefrontal cortical dysfunction in aging. Pharmacological inhibition of PKC with chelerythrine rescued working memory impairments in aged rats and enhanced working memory in aged rhesus monkeys. Improvement correlated with age, with older monkeys demonstrating a greater degree of improvement following PKC inhibition. Furthermore, PKC activity within the prefrontal cortex was inversely correlated with the length of basal dendrites of prefrontal cortical neurons, as well as with working memory performance in aged rats. Together these findings indicate that PKC is dysregulated in aged animals and that PKC inhibitors may be useful in the treatment of cognitive deficits in the elderly.
Keywords: prefrontal cortex, aging, working memory, protein kinase C, dendritic spines, chelerythrine, dendrites
1. Introduction
Studies of aged animals and humans show prominent deficits in prefrontal cognitive functions including working memory [1,2,6,11,32,33,35,43,46,54]. Impairments in these executive operations compromise the regulation of thought, emotion and behavior, and ultimately jeopardize independence and quality of life. Prefrontal cortical cognitive operations have become especially important in the Information Age, when one must steer through a constant barrage of distractions even to perform simple tasks. Elucidating mechanisms underlying prefrontal cortical decline is critical for the treatment of debilitating cognitive deficits in the elderly.
Goldman-Rakic [22]described working memory microcircuits as local networks, comprised of pyramidal cells engaged in recurrent excitation, creating persistent firing, and GABAergic interneurons, which provide spatial tuning. Prefrontal cognitive impairments may stem from disruptions to the structural components of such microcircuits [30]. Studies of humans and animals have established that advanced aging is associated with reduced neuropil [52] including reduced dendritic arborizations and spine density of prefrontal cortical pyramidal cells [17,19,25,26,34,37,40,47,50,51] as well as 40-50% reduction in synapse density in superficial layers of the prefrontal cortex [39,40]. Thus, the anatomical substrates of prefrontal networks are affected in the aged. These changes are likely reflected in the loss of prefrontal gray matter, which has been reported in aged humans [23,44].
Protein kinase C (PKC) comprises a family of kinases that regulate of a variety of nervous system functions, including cell-cell communication, cytoskeletal reorganization and neuronal housekeeping via phosphorylation of myriad substrates. More than 10 isoforms of PKC have been identified, and the majority of these isoforms are present within the brain (reviewed in [7]). PKCs are classified based on cofactors required for activation. Calcium-dependent (conventional) isoforms require both free calcium and diacylglycerol (DAG) for activation. Calcium-independent (novel) isoforms require DAG only, and atypical isoforms are activated independent of DAG and calcium [7,10]. Both calcium-dependent and calcium-independent PKC isoforms regulate the actin cytoskeleton [29] and overactivation of PKC results in spine loss and altered spine morphology in vitro [14] suggesting a role for PKC overactivation in structural deficits in the aged prefrontal cortex. PKC also plays a role in prefrontal cognitive deficits. Overactivation of PKC via Gq receptor stimulation produces working memory impairments in young animals [13]. This signaling cascade results in increased activation of PKCα as well as increased total PKC activity within the prefrontal cortex [13], suggesting additional isoforms may also be activated. Accordingly, PKC inhibitors targeting a wide array of PKC isoforms improve working memory performance and reverse working memory impairments in rats and monkeys [13,45].
Although no studies have examined PKC activation within the aged prefrontal cortex, studies of the hippocampus [15,16] and the entire cortex [8-10] indicate that the cellular distribution and quantity of activated PKC is altered in the aged brain. Studies relating PKC and cognitive status in the aged have focused largely on the hippocampus. PKC is crucial for hippocampal memory formation [31,38,48,56,57] and alterations in PKCγ contribute to deficits in hippocampal mediated memory in the aged [5,8,9,15,16,21]. It is important to recognize that the hippocampus and the prefrontal cortex are regulated differently and therefore knowledge accumulated from studies of the hippocampus cannot be assumed to generalize to the prefrontal cortex.
The present study sought to clarify the relationship between activation of PKC and structural and functional integrity of the aged prefrontal cortex. We hypothesized that cognitive impairments in the aged involve dysregulation of PKC in the prefrontal cortex. Since structural changes are pronounced in the aged, we also examined dendritic length and spine density within layer III prefrontal pyramidal neurons, the putative site of the network interactions that maintain information over a delay. Our findings indicate that working memory performance and dendritic integrity are inversely related to the activity of PKC in the aged prefrontal cortex, and that inhibition of PKC activity may be useful in the treatment of prefrontal impairments in the elderly.
2. Materials and Methods
2.1 Working memory performance and PKC inhibition in non-human primates
Thirteen female rhesus monkeys (Macaca mulatta) ranging in age from 18 to 35 years were individually housed and maintained on a diet of Purina monkey chow (St. Louis, MO). All animal procedures were approved by the Yale Animal Care and Use Committee and were in accordance with the National Institute of Health’s Guide for Care and Use of Laboratory Animals.
The monkeys were trained and tested on the spatial delayed response task as described previously [4]. Briefly, the animal watched as the experimenter baited one of 2 to 4 food-wells with a food reward. The number of food-wells varied depending on the monkey’s performance level. After baiting, food-wells were covered with identical plaques, and an opaque screen was lowered for a delay. Following the delay, the screen was raised and the animal was allowed to choose one well. Delays were titrated as described previously [4] such that each monkey maintained a baseline performance of 60-73% correct.
Chelerythrine chloride (CHEL, LC Laboratories, Woburn, MA) inhibits PKC activation by blocking the site of diacylglycerol/phorbol-ester binding and by inhibiting PKC translocation to the membrane for activation. Oral administration of 30 μg/kg CHEL has previously been shown to reverse working memory impairments following administration of the α-1 noradrenergic receptor antagonist, cirazoline, which activates PKC via the Gq signaling cascade [13]. Pilot studies indicated that higher doses of CHEL impair performance. Thus, the current study examined the effects of doses at or below the 30 μg/kg dose. Monkeys were orally administered 0.3 μg/kg, 3.0 μg/kg or 30.0 μg/kg of CHEL dissolved in water or vehicle (water) 1 h prior to testing by a single experimenter who was unaware of the drug treatment conditions. All animals received all treatments conditions, with at least 7 days between treatments.
Data were analyzed by comparing performance following treatment using one-way repeated-measures ANOVA. Pair-wise comparisons were evaluated using Fischer’s Lest Significant Difference (LSD) test. Values were represented as the mean ± SEM. Linear relationships between treatment response at the optimal dose of CHEL and age of the monkey (years) were analyzed in 10 monkeys with known birthdates using Pearson’s test. In all cases, an α level of 0.05 was considered statistically significant.
2.2 Working memory evaluation in rats
Sixteen aged (23.5-25 months) and 6 young adult (3-5 months) male Sprague-Dawley rats from Harlan (Indianapolis, IN) were used for two studies. Aged rats arrived in two shipments of 6 and 10 rats. We have previously observed variability in performance and health between shipments of aged rats (unpublished observations). To minimize variability, shipments remained separate for the two studies. Rats were single housed in filter-frame cages and kept on a 12 h light/dark cycle. Rats were habituated to a restricted diet (~13 g/day per rat) of Purina rat chow (St. Louis, MO) offered after testing and water was available ad libitum.
Rats were trained and tested on the spatial delayed alternation task in the T-maze (dimensions, 90 × 65 cm), which is reliant upon the medial prefrontal cortex [27,28,55]. The habituation period was defined as the number of training sessions until the rats were eating 12 food rewards at either end of the T-maze, with replacement to the start-box of the maze between rewards. Following habituation, cognitive performance was evaluated as described previously [12]. On the first trial, rats were rewarded for entering either arm. Thereafter, for a total of 12 trials per session, rats were rewarded only if they entered the maze arm that was not previously chosen. Between trials, the choice point was wiped with alcohol to remove any olfactory clues. Delay periods between trials ranged from ~2 s (the minimum possible for delayed alternation, referred to as “0 s”) to 15 s.
2.3 Acute PKC inhibition in aged, cognitively-impaired rats
Aged rats (N = 6) were habituated and trained on the spatial working memory task described above. Animals were allowed a maximum of 20 min to complete all 12 trials. Criterion for enrollment in this study was a mean 2 day performance of >58% correct at the 0 s delay. One rat failed to meet criterion and was eliminated from the study. Delays were titrated in increments of 5 s to maintain animals at a baseline of 58-75% correct. Rats performing below this baseline subsequent to training were tested at the lowest possible delay (0 s). Rats were approximately 24 months old upon initiation of pharmacological testing. Each rat was administered each of 3 treatment conditions: 0.3 mg/kg CHEL, 1.0 mg/kg CHEL or vehicle (water) via subcutaneous injection 45 min prior to testing. Dosing was determined by pilot studies. Treatment order was quasi-randomized and treatments were separated by a minimum of 6 days.
Treatment was administered following 2 consecutive scores at the same delay within 20 percentage-points (considered stable baseline). Due to variability in performance, outcome following treatment was measured as the difference between percent-correct following treatment and previous 2 day baseline. Comparisons were made using one-way ANOVA and pair-wise comparisons were evaluated using Fischer’s LSD test. Values were represented as the mean ± SEM.
2.4 Cognitive characterization of aged, cognitively-unimpaired rats
Young adult (N = 6) and aged (N = 10) rats were trained and cognitively characterized for 20 days on the spatial working memory task described above. All animals were allowed a maximum of 10 min to complete 12 trials. The time ceiling was more stringent to prevent animals from increasing inter-trial delays and confounding group comparisons. Cognitive characterization (20 test days) commenced when rats achieved a 2 day average of at least 67% correct at 0 s delay. One aged rat failed to meet criterion and was eliminated from the study. The 9 aged rats included in this study performed at a higher baseline than the 5 aged rats included in the acute PKC inhibition study illustrating the great variability in cognitive status among aged rats.
Four delays (0, 5, 10 and 15 s) were quasi-randomized within the 12-trail task to prevent ceiling effects. Performance was measured as number of trials correct out of 12. Performance during the last 3 testing sessions was used for correlations with dendritic morphological and PKC activation (see below). Group means were compared using an independent t test and values were represented as the mean ± SEM.
Following the last testing session, rats were decapitated under light isoflurane anesthesia (20 s in a bell jar). Brains were hemisected. In one hemisphere, the prelimbic and infralimbic cortex (medial prefrontal cortex) was dissected, frozen immediately on dry ice and stored at -80° C until processed. The remaining hemisphere was used for dendritic morphology and spine density analyses. Right and left hemispheres were equally distributed between the morphological and the biochemical studies.
2.5 PKC quantification
Protein samples were prepared as previously described [13]. Briefly, medial prefrontal cortex was homogenized in 0.5 ml of ice-cold sample preparation buffer (20 mM Tris, pH 7.5, 1 mM EGTA, 1 mM EDTA, 2.5 mM Na4P2O7, 1 mM β-glycerophosphate, 1 mM dithiothreitol, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail I and II (Sigma-Aldrich, St. Louis, MO)). The homogenate was centrifuged at 100,000 × g for 30 min at 4°C. The resulting supernatant was used as cytosolic fraction, and the pellet was re-suspended in 0.5 ml of sample preparation buffer containing 0.15% Triton X-100 and 150 mM NaCl and centrifuged again at the same settings. The resulting supernatant was used as the membrane fraction. The protein concentration of each fraction was assessed by a Bradford assay and an appropriate volume of the appropriate sample preparation buffer was added to normalize the protein content between samples (0.5 mg/ml).
PKC is activated in its membrane bound state, thus PKC concentration in the membrane fraction was used as the measure of PKC activity. PKC concentration was measured in duplicate in both membrane and cytosolic fractions with the ELISA-based Protein Kinase Assay kit (Cat# 539484, Calbiochem, San Diego, CA) following manufacture’s instructions. The reaction solution (100 μl) contained: 10 μl (5 μg) sample, 25 mM Tris-HCl (pH 7.0), 0.5 mM EDTA, 5 mM β-mercaptoethanol, 2 mM CaCl2, 3 mM MgCl2, 50 μg/ml phosphatidylserine, and 0.1 mM ATP. For negative controls, 2 mM EGTA was substituted for phosphatidylserine. The reaction solution was transferred to pseudosubstrate-coated 96-well plate and incubated at room temperature (RT) for 15 min, after which time stop solution (100 μl, 20% H3PO4) was added to terminate the reaction. Wells were washed by rinsing 5 times with PBS. Biotinylated antibody (100 μl) was added to each well and incubated for 1 h at RT. Wells were washed, peroxidase-conjugated streptavidin (100 μl) was added and allowed to incubate for 1 h at RT. Wells were washed again and substrate solution (100 μl, O-phenylenediamine and H2O2) was added and allowed to incubate for 5 min, followed by stop solution (100 μl). Optical density was measured at 492 nm in Victor 2 Multilabel Counter (Beckman Coulter, Inc., Fullerton, CA).
The optical density of each sample was obtained by averaging duplicates. This value was normalized to the mean density obtained from the young adult rat samples. Membrane and cytosolic samples were normalized separately. Data were analyzed using an independent t test and values were represented as the mean ± SEM.
2.6 Dendritic morphology and dendritic spine quantification
Following a 12 h post-fixation in 4% paraformaldehyde and 0.125% glutaraldehyde, the rat brain hemispheres were sliced coronally (200 μm), incubated in 4,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO) for 5 min to reveal the cytoarchitecture, mounted on nitrocellulose paper and immersed in PBS. Pyramidal cells in layer III of the medial prefrontal cortex were identified and loaded with 5% Lucifer Yellow (Molecular Probes, Eugene, OR) as described previously [18,19,24,41,42]. Five to 10 neurons were injected per slice avoiding overlapping of dendritic trees. Sections were then mounted and coverslipped in PermaFluor (Immunotech, Marseille, France).
In order for a neuron to be included in the analysis, it had to satisfy previously established criteria [18,19,24,41,42]. Neurons were reconstructed 3-dimensionally at 400x using a Zeiss Axiophot 2 microscope equipped with a motorized stage, video camera system, and Neurolucida software (MicroBrightField, Williston, VT). Sholl analysis was performed and results were expressed in terms of dendritic material per radial distance from the soma, in 30 μm increments [49]. Spine quantifications were performed by reconstructing dendritic segments using a Zeiss LSM 410 confocal microscope (Carl Zeiss Microimaging, Inc., Oberkochen, Germany) with 488 nm excitation. Apical and basal dendritic segments 50 μm and 100 μm from the soma were selected with a systematic random design [18,19,24,41,42]. Serial images were collected at 1000x magnification using a 100x Plan-Apochromat 1.4 N.A. objective (Carl Zeiss) and a zoom of 5, and segments were digitally reconstructed at 0.1 μm increments, through the z-axis. Digitized optical stacks were deconvolved with AutoDeblur (AutoQuant, Troy, NY). Spines were manually counted and each branch length was recorded. Values for spine density were expressed as spine number/μm. Comparisons were performed using independent t tests, and values were represented as the mean ± SEM.
3. Results
3.1 Working memory performance and PKC inhibition in non-human primates
CHEL was administered orally to aged rhesus monkeys 1 h prior to spatial delayed response testing. A one-way repeated-measures ANOVA revealed a significant effect of treatment on task performance (F(3, 36) = 5.654, p = 0.003). Pair-wise comparisons indicated that doses of 0.3 μg/kg CHEL significantly improved performance with respect to vehicle (0.3 μg/kg CHEL: 74.5 ± 2.6% correct, Veh: 68.1 ± 1.1% correct, p = 0.013). Vehicle scores did not significantly differ from higher doses of CHEL (3.0 μg/kg CHEL: 71.5 ± 1.9% correct, p = 0.151; 30.0 μg/kg CHEL: 66.1 ± 1.8%, p = 0.406).
Pearson’s test revealed a significant correlation between the age of the monkeys and cognitive performance following treatment with 0.3 μg/kg CHEL, whereby older age was associated with greater improvement (Pearson’s test: r = 0.777, p = 0.008, Fig. 1A). As delays were adjusted to maintain each monkey at 60-73% correct prior to treatment, there were no ceiling effects in the younger aged monkeys. No correlation of age with cognitive performance was observed following vehicle treatment (Pearson test: r = 0.044, p = 0.904, Fig. 1B).
Figure 1.
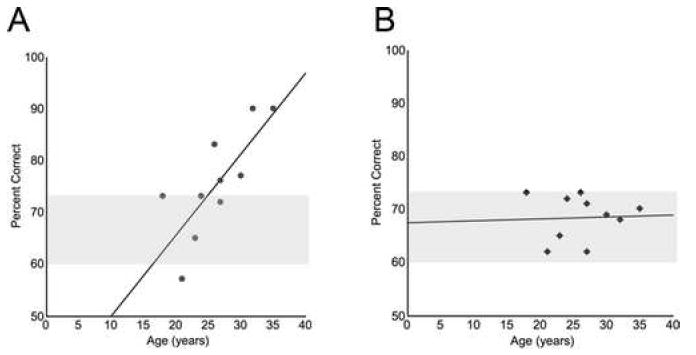
Pearson’s tests revealed a significant positive correlation between age of the monkey (years) and cognitive improvement following 0.3 μg/kg CHEL (r = 0.777, p = 0.008, A). There was not a significant relationship between age of the monkey and performance following vehicle treatment (r = 0.044, p = 0.904, B). All monkeys were maintained at a baseline of 60-73% correct prior to treatment administration (gray bar).
Following 0.3 μg/kg CHEL, performance of the 3 oldest monkeys (mean age = 32.3 years) was 85.6 ± 4.4% correct, which was >20% better than the performance of the 3 youngest aged monkeys following CHEL treatment (mean age = 20.7 years, 65.0 ± 4.8% correct). Performance following vehicle was comparable across these age groups (oldest: 69.0 ± 0.7% correct; youngest: 66.8 ± 3.4% correct).
3.2 Acute PKC inhibition in aged, cognitively-impaired rats
The effect of PKC inhibition with CHEL was evaluated in a group of aged rats with substantial working memory impairment. Mean baseline performance prior to the first treatment was below 60% correct. One-way ANOVA revealed a significant effect of drug treatment (F(2, 12) = 3.98, p = 0.047). Post hoc analysis using Fischer’s LSD test indicated that 1.0 mg/kg CHEL significantly improved performance compared to vehicle (1.0 mg/kg CHEL: 26 ± 6.4% improvement; Veh: 5.4 ± 4.7% improvement, p = 0.047). Performance following 0.3 mg/kg CHEL was not significantly different from vehicle (0.3 mg/kg CHEL: 8.6 ± 5.4% improvement, p = 0.691). PKC inhibition did not appear to influence performance through non-specific motor effects as performance times did not differ as a result of treatment (data not shown).
3.3 PKC activity, dendritic morphology and spine density in aged, cognitively- unimpaired rats
Cognitive characterization
The relationship between PKC inhibition and working memory performance in the aged suggest that impairments in prefrontal cortical function in the aged involve dysregulated PKC activity. To examine this, aged and young adult rats were characterized on the spatial delayed alternation task and PKC activation, dendritic morphology and dendritic spine density within the prefrontal cortex was assessed.
Comparisons in cognitive performance were made using independent t tests. Aged rats took significantly more time to achieve criteria for delayed-alternation testing than young adult rats (young adult: 16.3 ± 1.9 sessions; aged: 24.1 ± 2.5 sessions, p = 0.044). Interestingly, once rats were habituated to the testing procedures, aged and young adult rats were not significantly different in working memory performance (overall working memory: young adult: 65.5 ± 5.0% correct; aged: 69.7 ± 1.5% correct, p = 0.360; last 3-days: young adult: 71.0 ± 5.8 % correct; aged: 77.2 ± 2.4 % correct, p = 0.303).
Prefrontal PKC activation
In an effort to understand whether there are underlying differences in PKC activity in the aged versus young adult prefrontal cortex, an ELISA assay was performed on the prelimbic and infralimbic cortical regions taken from one hemisphere of cognitively characterized aged and young adult rats. Independent t tests did not indicate group differences in PKC activation (young adult: 100 ± 6.8%; aged: 91 ± 6.7%, p = 0.364) or in cytosolic PKC concentration (young adult: 100 ± 28.0%; aged: 78 ± 13%, p = 0.443).
Pearson’s test revealed a significant inverse correlation between working memory performance and PKC activation in aged rats (r = -0.780, p = 0.013, Fig. 2A). Performance was averaged over the last 3 days of the cognitive characterization, just prior to PKC activation measurements. Lower PKC activity levels predicted better cognitive performance in aged rats. No significant relationship between performance and PKC activation was detected among young adult rats (Pearson’s test: r = -0.015, p = 0.977, Fig. 2B).
Figure 2.
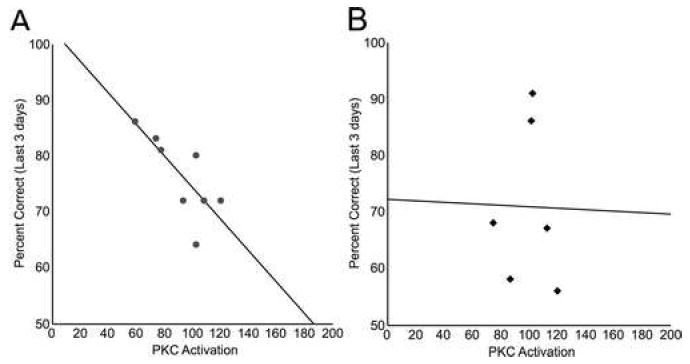
Prefrontal PKC activation predicted working memory impairment in aged rats. PKC activation was determined by evaluating PKC concentration in the membrane fraction of the medial prefrontal cortex of cognitively characterized rats using ELISA. PKC activation inversely correlated with mean working memory performance over the last 3 test sessions in aged rats, with higher membrane-bound PKC concentration predicting poorer performance (r = -0.780, p = 0.013, A). Performance was not predicted by PKC activation in young adult rats (r = -0.015, p = 0.977, B).
Prefrontal pyramidal cell dendritic morphology
Twenty-five neurons from 4 young adult rats and 93 neurons from 9 aged rats were analyzed. To address the possibility of increased variability among neurons within the aged rat brain, a larger sample of neurons from aged animals was included in the analysis.
Several rats failed to have neurons with apical branches longer than 180 μm (radial distance from the soma), therefore 180 μm was the upper limit for all measures of dendritic length. Sholl analysis revealed no significant differences in dendritic material between age groups at any radial distance (Fig. 3A,B). Total dendritic length was obtained by summing all dendritic material included in the Sholl analysis (up to 180 μm in radial distance from the soma). Aged and young adult rats did not significantly differ in total length of apical (young adult: 604.2 ± 95.3 μm; aged: 715.0 ± 35.5 μm; p = 0.198) or basal (young adult: 1258.8 ± 207.8 μm; aged: 1439.0 ± 114.5 μm; p = 0.428) dendrites.
Figure 3.
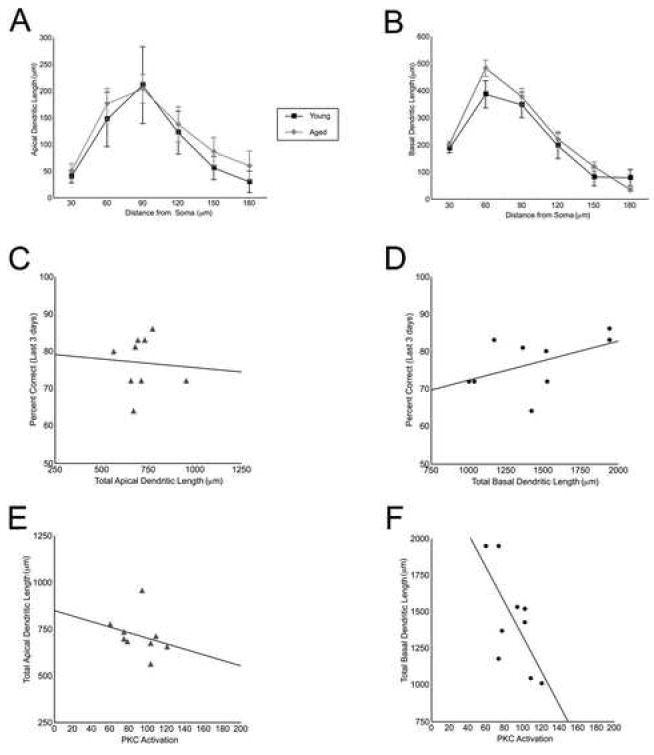
Basal dendritic length correlated with PKC activation and tended to predict working memory performance in aged rats. Apical and basal dendritic length of layer III prefrontal cortical pyramidal neurons was compared in young adult (N = 4 rats, n = 25 neurons) and aged (N = 9 rats, n = 93 neurons) rats. Sholl analysis was used to evaluate quantity of dendritic material in serial bins increasing in increments of 30 μm in radial distance. No significant differences in apical (A) or basal (B) dendritic length between aged (gray lines) and young adult (black lines) rats were observed (values represent means ± SEM.). Measures of total dendritic length were obtained by summating across the Sholl bins and were used for correlations within the aged (C-F). Working memory performance over the last 3 cognitive characterization sessions was not significantly correlated with apical dendritic length (r = -0.068, p = 0.862, C) in aged rats. Basal dendritic length was more strongly related to working memory performance than apical dendritic length. Longer basal dendrites tended to predict better performance, although this correlation did not reach statistical significance (r = 0.495, p = 0.176, D). PKC activation was not significantly correlated with apical dendritic length (r = -0.280, p = 0.466, E). PKC activation inversely correlated with basal dendritic length (r = -0.687, p = 0.041, F). All correlations were performed using Pearson’s test.
The relationship between dendritic length and working memory performance over the last 3 days of testing in aged rats was evaluated with Pearson’s tests. Total apical dendritic length did not correlate with working memory performance in aged rats (r = -0.068, p = 0.862, Fig. 3C). A stronger, albeit non significant, relationship existed between total basal dendritic length and working memory performance in the aged rats, with longer dendrites predicting better working memory performance (r = 0.495, p = 0.176, Fig. 3D). Due to a small sample size, correlations could not be performed in young adult rats.
Pearson’s tests were also used to examine the relationship between PKC activation and dendritic length in aged rats. Apical dendritic length was weakly related to PKC activation in aged rats (r = -0.280, p = 0.466, Fig. 3E). There was a stronger relationship between basal dendrites and PKC activation, with increased PKC activity predicting decreased total length of basal dendrites in aged rats (r = -0.687, p = 0.041, Fig. 3F).
Prefrontal pyramidal cell spine density
Neurons from 5 aged and 3 young adult rats were analyzed for spine density (4 neurons/rat). Spine density was obtained from an average branch length of 30 μm from apical and basal segments 50 μm and 100 μm from the center of the soma.
Aged rats had reduced spine density in proximal regions of apical and basal dendrites (apical, 50 μm: young adult: 1.75 ± 0.12 spines/μm, aged: 1.26 ± 0.07 spines/μm; p = 0.021; basal, 50 μm: young adult: 1.68 ± 0.09 spines/μm, aged: 1.32 ± 0.07 spines/μm; p = 0.023, Fig. 6). Aged rats also had significantly reduced spine density in more distal regions of apical dendrites (apical, 100 μm: young adult: 1.92 ± 0.17 spines/μm, aged: 1.56 ± 0.06 spines/μm; p = 0.023). Differences in spine density did not reach statistical significance in distal regions of basal dendrites (basal, 100 μm: young adult: 1.72 ± 0.17 spines/μm, aged: 1.47 ± 0.09 spines/μm; p = 0.189, Fig. 4). Total apical dendritic spine density (distal and proximal regions) was significantly lower in aged rats relative to young adult rats (young adult: 1.76 ± 0.12 spines/μm; aged: 1.41 ± 0.07 spines/μm, p = 0.036). Differences in total basal dendritic spine density did not reach statistical significance (young adult: 1.69 ± 0.10 spines/μm; aged: 1.32 ± 0.08 spines/μm, p = 0.064).
Figure 4.
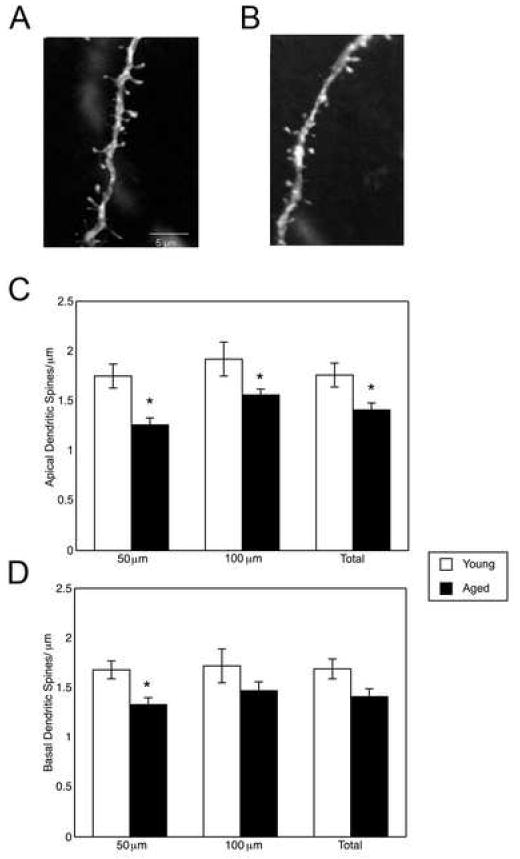
Dendritic spine density of layer III prefrontal cortical pyramidal neurons was reduced in aged relative to young adult rats. Representative reconstructions of dendritic segments are illustrated for young adult (N = 3 rats, n = 4 neurons/rat, A) and aged (N = 5 rats, n = 4 neurons/rat, B) rats. Spine density was evaluated in apical (C) and basal (D) dendrites 50 μm and 100 μm from the center of the soma for each neuron. Spine density was significantly reduced in aged relative to young adult rats in both proximal and distal portions of apical dendrites (proximal: p = 0.021; distal: p = 0.023, C) and in proximal portions of basal dendrites (p = 0.023, D). Spine measures were collapsed across regions of the apical and basal dendrite (“Total”). Total spine density was significantly reduced in apical dendrites of aged relative to young rats (p = 0.036, C). Total spine density differences did not reach statistical significance for basal dendrites (p = 0.064, D). Values represent means ± SEM. *, p < 0.05.
4. Discussion
The findings from this study indicate that activation of PKC is associated with cognitive and morphological deficits of the aged prefrontal cortex. PKC activation within the medial prefrontal cortex predicted poorer working memory performance, and decreased basal dendritic material in aged rats. Inhibition of PKC with systemic administration of CHEL improved working memory performance in aged, cognitively impaired rats and in rhesus monkeys, providing further evidence that PKC dysregulation contributes to prefrontal deficits in the aged.
Low to moderate doses of systemically administered CHEL improved working memory performance in aging rhesus monkeys. Given the multitude of regulatory functions served by PKC throughout the brain [7,31,38,48,56,57], it is not surprising that higher doses of CHEL produced no improvement, as any improving effects of CHEL may have been offset by disruptions in homeostatic mechanisms. The correlation of age of the monkey with performance on the working memory task following an optimal dose of CHEL suggests that cognitive enhancement following CHEL is due to improvements in older monkeys. This effect is not due to ceiling effects as all monkeys were held at a 2-day baseline of 60-73% correct. Instead, these findings indicate that PKC is implicated in prefrontal cognitive operations in the aged, which is consistent with previous findings of PKC dysregulation in the aged brain [8-10,15,16]. In support of this, we found that the oldest 3 monkeys performed more than 20% better than the 3 youngest monkeys following administration of 0.3 μg/kg CHEL.
In order to determine whether PKC inhibition can rescue impairments in working memory in aged rats, we examined the effects of acute CHEL on spatial delayed alternation performance in aged rats with mean pretreatment baselines below 60% correct. CHEL significantly improved performance, suggesting that PKC activation contributes to cognitive impairments in aged subjects. Although CHEL was administered systemically, it is likely that the beneficial actions of CHEL arise in the prefrontal cortex. We have observed that systemic CHEL administration and infusions of CHEL directly to the rat medial prefrontal cortex via an implanted cannula are both capable of reversing stress induced working memory impairments [13] suggesting that the location of action of systemic CHEL is the prefrontal cortex.
We next examined whether basal PKC activity in the prefrontal cortex is associated with working memory performance in aged and young adult rats. Consistent with our findings of CHEL enhancing working memory function in aged rats and monkeys, basal PKC activation within the prefrontal cortex predicted poorer working memory performance in aged but not young adult rats. Working memory is often impaired in aged animals and humans [1,2,6,11,32,33,35,43,46,54], but deficits can be highly variable, with some aged subjects performing as well as young subjects [3,20,36,53]. In our study, variations in PKC activation were robust enough to explain relatively subtle differences in performance amongst cognitively intact aged rats. These findings are consistent with previous reports of PKC-dependent working memory impairments [13,45] and emphasize the role of PKC in working memory function in the aged.
Interestingly, there were no differences in basal levels of cytosolic or membrane-bound PKC between young adult and aged rats. Together with our findings of a significant correlation of PKC activity and working memory performance in the aged, this suggests that the effects of PKC are qualitatively different between the young adult and aged prefrontal cortex. Qualitative differences in PKC regulation have been observed in the young vs. aged hippocampus [15,16]. For example, performance on a hippocampus-mediated task was shown to inversely correlate with cytosolic PKCγ in aged rats. Membrane-bound PKCγ (activation) positively correlated with performance in younger rats, however, elevated membrane-bound PKCγ did not predict enhanced performance among the aged [16]. The PKC assay used in the present study quantified all membrane-bound PKC isoforms. Thus, suppression or elevation of the activity state of specific isoforms could not be resolved. Elucidating how PKC is (dys)regulated in the aged prefrontal cortex will require these rigorous analyses that incorporate both cellular location-specific and isoform-specific PKC activity states in young adult and aged animals.
Previous attempts to quantify PKC (across isoforms) within the entire cortex of aged rats have produced mixed results. Our findings are in agreement with previous reports of similar cytosolic and membrane-bound PKC concentrations in young vs. aged rat cortex [9], but contrast with earlier reports of reduced membrane PKC in aged Sprague-Dawley rat cortex [8]. These discrepancies may reflect variability among aged rats, among isoforms of PKC, or regional differences in PKC regulation. Different cortical areas subserve different functions, thus homogeneity in PKC regulation should not be expected among widespread regions of the cortex. In the present investigation, PKC activation was quantified specifically within the medial prefrontal cortex. Similar membrane and cytosolic concentrations suggest that translocation of PKC to the membrane is not impaired and that the number of functional enzyme molecules is not altered within the aged prefrontal cortex.
A limitation of the present study was the successful performance of the aged rats in the PKC characterization study. There is high variability in the aged population [3,20,36,53]. This sample of aged rats may have come from a relatively unimpaired population. These cohort effects are a weakness of aging studies, which rely on the limited availability of a very small number of animals at any one time. Despite similar cognitive performance and PKC activation measures, the correlative and pharmacological data indicate that PKC is implicated in prefrontal cognitive function in the aged. Inclusion of a more functionally heterogeneous sample of aged animals for correlative studies would provide a better indication as to how PKC activation-levels in the aged compare to the young adult levels and predict working memory across the spectrum of cognitive abilities. Further experiments are also required to determine if PKC regulatory mechanisms, such as inactivation of PKC, or phorbol 12-myristate 13-acetate (PMA) -induced translocation of PKC from the cytosol to the membrane, are altered within the aged prefrontal cortex.
The working memory functions of the prefrontal cortex depend on the interconnectivity of recurrent microcircuits [22]. Pyramidal cells are the principle components of these networks, and dendritic spines are a fundamental anatomical substrate of excitatory network activity. PKC is implicated in neuronal plasticity and is capable of regulating the actin cytoskeleton and therefore the motility of dendritic spines (for review see [29]). Dysregulation of PKC in the aged may provide a mechanistic link to findings of decreased dendritic spine density and dendritic remodeling in the aged prefrontal cortex.
Previous studies have identified prominent reductions in dendritic material and spine density of pyramidal neurons in the aged prefrontal cortex [17,19,25,26,34,37,40,47,50,51]. In our study of cognitively intact aged rats, we found no significant differences in the quantity of apical or basal dendritic material between young and aged rats. We also observed no differences in branch intersections between young adult and aged subjects, suggesting that the complexity of the dendritic arbor was preserved in these aged rats. This conservation of gross dendritic structure may be protective of working memory in these cognitively-intact aged rats. Indeed, combined length of the basal dendrites and working memory performance were positively associated within aged rats, with increased dendritic material predicting better working memory performance, although this relationship fell short of statistical significance. Basal dendritic length was also inversely correlated with PKC activation in aged rats, indicating that PKC activation in aging is associated with structural as well as cognitive deficits. We were unable to evaluate whether these relationships are unique to the aging prefrontal cortex as our sample size of young adult rats was too small to perform the necessary analyses.
Although this group of aged rats was similar to young adult rats in quantitative measures of working memory, PKC, and dendritic length, they showed reduced dendritic spine density along the same neurons that were included in dendritic length analyses. This is consistent with previous reports of spine loss in aging [19,25,37,40]. Spine loss was more pronounced in proximal regions of both basal and apical dendrites. Aged rats exhibited reduced spine density across regions of the apical dendrite, relative to the young adult group. Aged animals also tended to have reduced spine density across regions of the basal dendrite, although differences did not reach statistical significance. Due to small sample sizes, the association of dendritic spine density and PKC or working memory could not be evaluated. Inclusion of larger, more heterogeneous cohorts would enable a multivariate examination of the relationships between PKC activation, dendritic spine density, dendritic branch length, and working memory performance. Such experiments are critical to address the possibility that the basal dendritic arbor is more resilient in aging, and that changes in basal dendrites may compensate for overall loss of dendritic spines.
In summary, inhibition of PKC rescued working memory impairments in both aged rats and monkeys. In aged rats, PKC activity was inversely associated with both cognitive function and basal dendritic length. Taken together, these findings indicate that PKC is associated with prefrontal cortical dysfunction in the aged and suggests that PKC inhibitors may be useful in the treatment of cognitive deficits in the elderly. Future studies examining age-specific alterations in PKC isoforms or directly manipulating PKC activation within the prefrontal cortex are required to elucidate fully the nature of PKC dysregulation as well as the potential mechanistic role of PKC in prefrontal cognitive and structural decline in aging.
Acknowledgments
The authors would like to thank Tracy Sadlon, Lisa Ciavarella, Sam Johnson, and Jessica Thomas for their invaluable technical assistance. The research was supported by NIH grants R37 AG06036 and the Claude D. Pepper Older Americans Independence Center, Yale University School of Medicine (P30 AG21342 NIH/NIA) to A.F.T.A; The National Science Foundation Graduate Research Fellowship and American Foundation of Aging Research Student Research Fellowship to A.R.B.; and NIH grants AG02219 and AG05138 to P.R.H.
Footnotes
Disclosure: Yale University and Amy Arnsten have a license agreement with Marinus Pharmaceuticals for the development of chelerythrine for the treatment of prefrontal cortical disorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1703–9. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando S, Ohashi Y. Longitudinal study on age-related changes of working and reference memory in the rat. Neurosci Lett. 1991;128:17–20. doi: 10.1016/0304-3940(91)90750-n. [DOI] [PubMed] [Google Scholar]
- 3.Arnaiz SL, D’Amico G, Paglia N, Arismendi M, Basso N, del Rosario Lores Arnaiz M. Enriched environment, nitric oxide production and synaptic plasticity prevent the aging-dependent impairment of spatial cognition. Mol Aspects Med. 2004;25:91–101. doi: 10.1016/j.mam.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–98. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes CA, Mizumori SJ, Lovinger DM, Sheu FS, Murakami K, Chan SY, Linden DJ, Nelson RB, Routtenberg A. Selective decline in protein F1 phosphorylation in hippocampus of senescent rats. Neurobiol Aging. 1988;9:393–8. doi: 10.1016/s0197-4580(88)80086-1. [DOI] [PubMed] [Google Scholar]
- 6.Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. J Gerontol. 1978;33:858–71. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- 7.Battaini F. Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol Res. 2001;44:353–61. doi: 10.1006/phrs.2001.0893. [DOI] [PubMed] [Google Scholar]
- 8.Battaini F, Del Vesco R, Govoni S, Trabucchi M. Regulation of phorbol ester binding and protein kinase C activity in aged rat brain. Neurobiol Aging. 1990;11:563–6. doi: 10.1016/0197-4580(90)90118-j. [DOI] [PubMed] [Google Scholar]
- 9.Battaini F, Elkabes S, Bergamaschi S, Ladisa V, Lucchi L, De Graan PN, Schuurman T, Wetsel WC, Trabucchi M, Govoni S. Protein kinase C activity, translocation, and conventional isoforms in aging rat brain. Neurobiol Aging. 1995;16:137–48. doi: 10.1016/0197-4580(94)00154-5. [DOI] [PubMed] [Google Scholar]
- 10.Battaini F, Pascale A. Protein kinase C signal transduction regulation in physiological and pathological aging. Ann N Y Acad Sci. 2005;1057:177–92. doi: 10.1196/annals.1356.011. [DOI] [PubMed] [Google Scholar]
- 11.Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–74. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–4. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Colombo PJ, Gallagher M. Individual differences in spatial memory among aged rats are related to hippocampal PKCgamma immunoreactivity. Hippocampus. 2002;12:285–9. doi: 10.1002/hipo.10016. [DOI] [PubMed] [Google Scholar]
- 16.Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci U S A. 1997;94:14195–9. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69:143–63. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- 18.Duan H, Wearne SL, Morrison JH, Hof PR. Quantitative analysis of the dendritic morphology of corticocortical projection neurons in the macaque monkey association cortex. Neuroscience. 2002;114:349–59. doi: 10.1016/s0306-4522(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 19.Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–61. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- 20.Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–52. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- 21.Gianotti C, Porta A, De Graan PN, Oestreicher AB, Nunzi MG. B-50/GAP-43 phosphorylation in hippocampal slices from aged rats: effects of phosphatidylserine administration. Neurobiol Aging. 1993;14:401–6. doi: 10.1016/0197-4580(93)90098-v. [DOI] [PubMed] [Google Scholar]
- 22.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 23.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–41. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon KM, Wellman CL. Differential effects of cholinergic lesions on dendritic spines in frontal cortex of young adult and aging rats. Brain Res. 2003;992:60–8. doi: 10.1016/j.brainres.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–80. [PubMed] [Google Scholar]
- 27.Kesner RP. Retrospective and prospective coding of information: role of the medial prefrontal cortex. Exp Brain Res. 1989;74:163–7. doi: 10.1007/BF00248289. [DOI] [PubMed] [Google Scholar]
- 28.Kolb B, Gibb R. Anatomical correlates of behavioural change after neonatal prefrontal lesions in rats. Prog Brain Res. 1990;85:241–56. doi: 10.1016/s0079-6123(08)62683-7. [DOI] [PubMed] [Google Scholar]
- 29.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–84. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathis C, Lehmann J, Ungerer A. The selective protein kinase C inhibitor, NPC 15437, induces specific deficits in memory retention in mice. Eur J Pharmacol. 1992;220:107–10. doi: 10.1016/0014-2999(92)90020-5. [DOI] [PubMed] [Google Scholar]
- 32.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–93. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–21. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S, Akiguchi I, Kameyama M, Mizuno N. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol (Berl) 1985;65:281–4. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–9. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- 36.Olton DS, Markowska A, Breckler SJ, Wenk GL, Pang KC, Koliatsos V. Individual differences in aging: behavioral and neural analyses. Biomed Environ Sci. 1991;4:166–72. [PubMed] [Google Scholar]
- 37.Page TL, Einstein M, Duan H, He Y, Flores T, Rolshud D, Erwin JM, Wearne SL, Morrison JH, Hof PR. Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett. 2002;317:37–41. doi: 10.1016/s0304-3940(01)02428-4. [DOI] [PubMed] [Google Scholar]
- 38.Paylor R, Rudy JW, Wehner JM. Acute phorbol ester treatment improves spatial learning performance in rats. Behav Brain Res. 1991;45:189–93. doi: 10.1016/s0166-4328(05)80085-3. [DOI] [PubMed] [Google Scholar]
- 39.Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb Cortex. 2001;11:93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- 40.Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–84. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- 41.Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 43.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–76. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 45.Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–10. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport. 1996;7:1165–9. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- 47.Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 48.Serrano PA, Beniston DS, Oxonian MG, Rodriguez WA, Rosenzweig MR, Bennett EL. Differential effects of protein kinase inhibitors and activators on memory formation in the 2-day-old chick. Behav Neural Biol. 1994;61:60–72. doi: 10.1016/s0163-1047(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 49.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 50.Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp Neurol. 1980;69:164–72. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- 51.Uylings HBM, West MJ, Coleman PD, De Brabander JH, Flood DB. Neuronal and cellular changes in aging brain. In: Clark CM, Trojanowski JQ, editors. Neurodegenerative dementias: Clinical features and pathological mechanisms. Vol. 61. New York: McGraw Hill; 2000. p. 76. [Google Scholar]
- 52.Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer’s disease. Brain and Cog. 2002;49:268–76. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- 53.West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cog. 2002;49:402–19. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- 54.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Wortwein G, Mogensen J, Divac I. Retention and relearning of spatial delayed alternation in rats after ablation of the prefrontal or total non-prefrontal isocortex. Behav Brain Res. 1994;63:127–31. doi: 10.1016/0166-4328(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 56.Yang HC, Lee EH. Protein kinase C activation facilitates memory retention in rats. Chin J Physiol. 1993;36:115–23. [PubMed] [Google Scholar]
- 57.Zhao WQ, Sedman GL, Gibbs ME, Ng KT. Effect of PKC inhibitors and activators on memory. Behav Brain Res. 1994;60:151–60. doi: 10.1016/0166-4328(94)90142-2. [DOI] [PubMed] [Google Scholar]


