Abstract
A family of gas-transporting proteins, the Mep/Amt/Rh glycoprotein family, has been identified recently. These are integral membrane proteins, are widely expressed in sites of gas transport, and are known to transport the gaseous molecule, NH3, and recent evidence indicates they can transport CO2. Because the mammalian lung is a critical site for gas transport, the current studies examine the expression of the nonerythroid members of this extended family, Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg), in the normal mouse lung. Real-time RT-PCR and immunoblot analysis demonstrated both Rhbg and Rhcg mRNA and protein expression, respectively. Immunohistochemistry demonstrated both Rhbg and Rhcg were expressed in bronchial and bronchiolar epithelial cells. Rhbg was expressed by Clara cells, specifically, whereas all bronchial/bronchiolar epithelial cells, with the exception of goblet cells, expressed Rhcg. Rhbg expression was basolateral, whereas Rhcg exhibited apical and intracellular immunolabel, polarized expression similar to that observed in Rhbg- and Rhcg-expressing epithelial cells in other organs. There was no detectable expression of either Rhbg or Rhcg in alveolar endothelial or epithelial cells, in pneumocytes or in vascular tissue. In vitro studies using cultured bronchial epithelial cells confirm Rhbg and Rhcg expression, demonstrate that saturable, not diffusive, transport is the primary mechanism of ammonia/methylammonia transport, and show that the saturable transport mechanism has kinetics similar to those demonstrated previously for Rhbg and Rhcg. These findings suggest Rhbg and Rhcg may contribute to bronchial epithelial cell ammonia metabolism and suggest that they do not contribute to pulmonary CO2 transport.
Keywords: ammonia, CO2, transport
increasing evidence suggests both that plasma membranes have limited gas permeability and that specific proteins facilitate gaseous molecule movement across plasma membranes. Examples of plasma membranes with low gas permeability include the apical plasma membrane of gastric parietal and chief cells, which have only minimal permeability to CO2 and NH3 (6, 65), colonic epithelia, which have very low permeability to the gaseous molecule, NH3 (60), and renal tubular epithelial cells, where direct measurements have shown limited NH3 permeability (17, 23, 72) that leads to the development of significant NH3 gradients (22).
The identification of limited diffusive gas permeability suggests that carrier-mediated gas movement could be an important mechanism for gas transport in many sites. Indeed, several studies confirm exactly this thesis. For example, aquaporin-1 (AQP1)-mediated CO2 transport appears necessary for renal proximal tubule solute transport (7) and may mediate as much as 50–60% of erythrocyte transmembrane CO2 transport (5, 10, 16, 18). AQP1 may also mediate O2 transport (13). In the kidney, carrier-mediated mechanisms are the primary mechanism of ammonia1 movement across the plasma membranes of renal proximal tubule, thick ascending limb of the loop of Henle and collecting duct epithelial cells (4, 26, 27, 38, 68). Thus understanding the cellular mechanisms of gas movement involves consideration of the proteins present and their capacity to transport specific gaseous molecules.
The Mep/Amt/Rh glycoprotein protein family is a recently recognized protein family that transports gas molecules. It was originally identified in yeast and plant cells (48, 52), and subsequent studies have shown its expression in essentially all living organisms (46, 67). Rh glycoproteins are recently identified mammalian members of this extended family (41, 43, 47). Rh A glycoprotein (RhAG/Rhag2 ) is expressed exclusively in erythrocytes (42, 66), whereas the nonerythroid Rh glycoproteins, Rh B glycoprotein (RhBG/Rhbg) and Rh C glycoprotein (RhCG/Rhcg), are widely expressed (67).
The gas transport characteristics of the nonerythroid Rh glycoproteins have been extensively studied. All three known Rh glycoproteins, Rhag, Rhbg, and Rhcg, can transport ammonia, with the majority of studies identifying the gaseous molecule, NH3, as the transported species (2, 3, 44, 45, 49, 51, 55, 70, 71). Rh glycoproteins also transport methylammonia (MA), but not Na+, K+, or other known cations, and there are currently no known specific Rh glycoprotein transport inhibitors. Although some studies suggest that Rh glycoproteins can also transport NH4+ (2, 3, 51), whether this involves parallel NH3 and H+ transport or specific movement of the molecular species NH4+ has not been determined. Members of this family also transport CO2. Both the bacterial protein, AmtB, and the erythroid-specific Rh glycoprotein, RhAG, transport CO2 when expressed in the Xenopus oocyte (50, 51). Erythrocytes from humans with Rhnull disease, which results from genetic abnormalities in RhAG expression, exhibit decreased CO2 transport (15). In the green algae, Chlamydomonas reinhardtii, a unicellular model organism, the Rh glycoprotein, Rh1, does not contribute to extracellular ammonia transport (62). Instead, Rh1 appears to be involved in CO2 metabolism as increasing extracellular CO2 increases Rh1 expression, and inhibiting Rh1 expression inhibits the normal increase in growth rates in response to elevated extracellular CO2 (62). Finally, X-ray crystallographic analysis of Nitrosomonas europaea Rh protein identifies a putative CO2 binding site adjacent to the intracellular exit site of the channel (39).
In mammals, the lung is a major organ for gas exchange with the environment. Characterizing Rh glycoprotein expression in the lung might provide important information regarding the molecular mechanisms of pulmonary gas transport. Therefore, the current studies examine the expression of the nonerythroid Rh glycoproteins, Rhbg and Rhcg, in the murine lung. Because bronchial epithelial cells express Rhbg and Rhcg, we then examined whether cultured bronchial epithelial cells express Rhbg and Rhcg and whether ammonia transport occurred through diffusive or transporter-mediated mechanisms.
METHODS
Mice.
Normal BALB/c mice were obtained from Harlan Sprague Dawley (Indianapolis, IN) and were maintained on a normal mouse diet and ad libitum water intake until the day of study. All animal use was in compliance with the American Physiological Society's Guiding Principles in the Care and Use of Animals, and animal use protocols were approved by the North Florida/South Georgia Veterans Administration Institutional Animal Care and Use Committee.
Cell culture.
Immortalized BEAS-2B bronchial epithelial cells were maintained in culture using standard techniques.
Antibodies.
Affinity-purified Rhbg and Rhcg antibodies were generated in this laboratory and have been characterized previously (24, 45, 58, 59, 64). Antibodies to Clara cell secretory protein were obtained from Chemicon (Temecula, CA).
Membrane protein preparation.
Animals were euthanized with intraperitoneal pentobarbital sodium, and lung tissue was rapidly removed and stored frozen at −70°C until used. Tissues were homogenized in buffer A (in mM: 50 sucrose, 10 Tris buffer, 1 EDTA, pH 7.4) with a glass Dounce and then diluted in buffer B (in mM: 250 sucrose, 10 Tris buffer, and 1 EDTA, pH 7.4) containing Halt Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL). The sample was then centrifuged at 1,000 g for 5 min at 4°C. The pellet was resuspended in buffer B and again centrifuged at 1,000 g. The combined supernatants were then centrifuged at 21,000 g for 30 min at 4°C. The 21,000 g pellet was finally resuspended in buffer B. An aliquot was obtained for protein determination using a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL), and the remainder was stored at −70°C until used. For BEAS-2B cells, we extracted proteins using M-PER Mammalian Protein Extraction Reagent (Pierce Biotechnology) using the manufacturer's recommended procedure.
mRNA extraction.
Female mice weighing 17–20 g were anesthetized with sodium pentobarbital (50 mg/kg ip). After euthanasia, the lungs were infused in situ with PBS via the trachea, removed rapidly, placed into RNAlater (Qiagen, Valencia, CA), and stored in a −70°C freezer until used. Total RNA was extracted from lung tissue and cultured cells using RNeasy Mini Kit (Qiagen) and stored in a −70°C freezer until used.
Tissue preparation for immunohistochemical localization.
Mice were anesthetized with inhalant isoflurane by face mask. The lungs were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and immersed for 24–48 h at 4°C in the same fixative. Lung samples were embedded in polyester wax (polyethylene glycol 400 distearate; Polysciences, Warrington, PA), and 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Real-time RT-PCR.
We performed real-time RT-PCR as described in detail previously (28, 58, 69). Briefly, the forward primer for mouse Rhbg was 5′-GCCTGCAGAGTGTGTTTCCA-3′, the reverse primer was 5′-GAGCTGATACACGGCCTGAGA-3′, and the fluorescent probe was 6FAM-TGGCACTCCGCTGACCCTTGG-TAMRA. For mouse Rhcg, the forward primer was 5′-GGATACCCCTTCTTGGACTCTTC-3′, the reverse primer was 5′-TGCCTTGGAACATGGGAAAT-3′, and the fluorescent probe was 6FAM-AGCCTCCGCCTGCTCCCCAAC-TAMRA. We used primers and TaqMan probes for RhBG (Hs00220735_m1) and for RhCG (Hs00170975_m1) obtained from Applied Biosystems (Foster City, CA) for studies examining BEAS-2B mRNA. RNA was reverse transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) and random primers. Real-time RT-PCR was then performed on an ABI Prism GeneAmp 7500 Sequence Detection System. Amplification was performed using a 25-μl total fluid volume and TaqMan RT-PCR Master Mix Reagents (Applied Biosystems). Results were analyzed using GeneAmp 7500 SDS software version 1.3 (Perkin-Elmer Applied Biosystems).
RT-PCR procedure.
RNA was reverse transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) and random primers. To amplify human Na+-K+-2Cl− cotransporter isoform 1 (NKCC1), we used the forward primer, 5′-AGATGTCCATCGATCAAGCC-3′, and reverse primer, 5′-TGAGTTGCAGTCTTGCCATC-3′, which yields a product of 460 bp. To amplify human NKCC2, we used the forward primer, 5′-TTTTGCTAATGCAGTGGCTG-3′, and the reverse primer, 5′-AGCCCGTACTGACATGGTTC-3′, which yields a product of 642 bp. Human kidney mRNA obtained from BD Biosciences Clontech (Palo Alto, CA) was used as a positive control for both NKCC1 and NKCC2. PCR amplification of cDNA was performed using GoTaq Hot Start Polymerase (Promega, Madison, WI), an annealing temperature of 58°C, an extension time of 1 min, and 50 cycles. Amplified products were separated using electrophoresis and detected on a Kodak Image Station 440CF digital imaging system.
Immunoblotting procedure.
Proteins were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated overnight with affinity-purified primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.5) with 5 g/dl nonfat dry milk. After washing, membranes were exposed to secondary antibody (goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase; Promega) at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate; Pierce Biotechnology) and a Kodak Image Station 440CF digital imaging system.
Immunohistochemistry.
Rh glycoprotein immunolocalization was performed using standard immunoperoxidase procedures. The sections were dewaxed in ethanol and rehydrated and rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 for 30 min. The sections were blocked for 30 min with 10% normal goat serum (Vector Laboratories) in PBS followed by Mouse Detective (Biocare Medical, Concord, CA) for 45 min and then incubated at 4°C overnight with primary antibody. The sections were washed in PBS and incubated for 30 min with polymer-linked peroxidase-conjugated goat anti-rabbit IgG (MACH 2; Biocare Medical, Walnut Creek, CA), again washed with PBS, and then exposed to diaminobenzidine. The sections were washed in distilled water and then dehydrated with xylene, mounted, and observed by light microscopy.
Double immunolabeling procedure.
Colocalization was accomplished using sequential immunoperoxidase procedures. The first immunoperoxidase procedure used the protocol for single labeling described above. After the diaminobenzidine reaction, the sections were washed in glass-distilled water and then in PBS and incubated in 3% H2O2 for 30 min and blocked with 10% normal goat serum and Mouse Detective. The sections were treated for 60 min with the second primary antibody, washed in PBS, incubated with the polymer-linked peroxidase-conjugated anti-rabbit secondary antibody, and then washed with PBS. Vector SG (Vector Laboratories) was used as the chromagen to produce a blue label easily distinguishable from the brown label produced by the diaminobenzidine used for detection of the first protein. The sections were washed with glass-distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
Measurement of [14C]MA transport activity.
We measured transporter activity as [14C]MA uptake (0.275 μCi/ml, 5 μM) as we (26, 27) described previously. Briefly, cells were rinsed with radiotracer-free uptake medium, followed by exposure to uptake medium with [14C]MA added. Cells were rinsed rapidly with ice-cold, radiotracer-free medium to terminate uptake. In preliminary studies, [14C]MA uptake was linear during the initial 3 min; we terminated uptake at 1 min in all subsequent studies in this report. Soluble radioactivity was extracted by precipitating proteins with 10% TCA; cell protein was then solubilized in 0.2% SDS-0.2 N NaOH and quantified using a bicinchoninic acid assay. Uptake medium, unless detailed otherwise, contained, in mM, 115 NaCl, 5 KCl, 10 HEPES, 25 choline chloride, 5 glucose, and 1.2 CaCl2 and was titrated to pH 7.50.
MA transport modeling.
We determined the relative roles of diffusive and transporter-mediated transport as described previously (26, 27). Briefly, we modeled uptake using the equation: Jtotal = (Jdiffusive × MA) + Jtransporter × [MA/(MA + Km)] where Jtotal is total uptake, Jdiffusive is the diffusive rate coefficient, MA is extracellular MA concentration, Jtransporter is the transporter-mediated rate coefficient, and Km is the affinity for transporter-mediated MA transport. Jdiffusive, Jtransporter, and Km were determined using least-squares minimization (Quattro Pro X3; Corel).
Competitive inhibition of MA transport.
We used Dixon plot analysis to quantify BEAS-2B cell affinity for ammonia using techniques we (26, 27) described previously. Briefly, we measured 5 and 10 μM [14C]MA uptake in the presence of graded concentrations of extracellular ammonia. Because BEAS-2B cells have both diffusive and transporter-mediated [14C]MA transport, we estimated the diffusive component as detailed above and then calculated transporter-mediated [14C]MA uptake at each ammonia concentration by subtracting the diffusive component from total uptake. A Dixon plot (reciprocal velocity vs. inhibitor concentration) was used to determine the inhibitor constant (Ki) for ammonia to inhibit transporter-mediated MA uptake and to determine whether ammonia was a competitive or noncompetitive inhibitor of transporter-mediated [14C]MA uptake.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using Student's t-test with P < 0.05 taken as statistical significance; n refers to number of separate animal or cell culture experiments.
RESULTS
Rhbg and Rhcg mRNA expression.
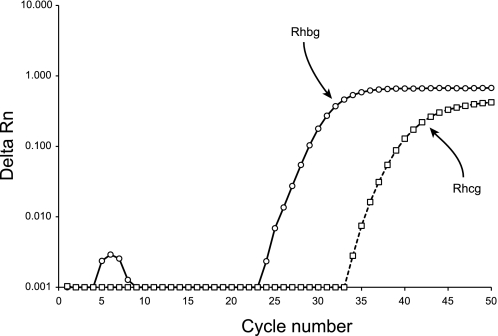
We began examining pulmonary Rhbg and Rhcg expression by using real-time RT-PCR to determine whether the murine lung expresses Rhbg and/or Rhcg mRNA. Figure 1 shows typical results. In all tissue examined, both Rhbg and Rhcg mRNA expression was identified. In the absence of reverse transcription, there was no amplification of either Rhbg or Rhcg, demonstrating genomic DNA was not amplified. We then quantified lung Rhbg and Rhcg mRNA expression relative to the kidney, another CO2 and ammonia-transporting organ. Lung Rhbg mRNA expression averaged 7.5 ± 0.4% of kidney Rhbg expression (n = 3), and lung Rhcg mRNA expression was 0.069 ± 0.058% (n = 3) of kidney Rhcg mRNA expression. Thus the murine lung expresses both Rhbg and Rhcg mRNA but at levels substantially lower than in the kidney.
Fig. 1.
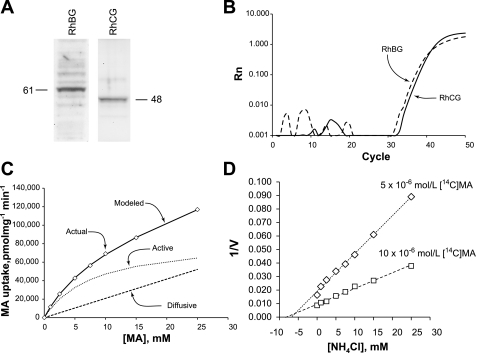
Real-time RT-PCR amplification of Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg) mRNA. RNA from murine lung was reverse transcribed and then amplified using gene-specific primers and TaqMan probe for Rhbg and for Rhcg. Amplification of both Rhbg and Rhcg was observed. Kidney mRNA was used as a positive control. No Rhbg or Rhcg amplification was seen in the absence of reverse transcription or in the absence of tissue RNA (data not shown), confirming specific expression of lung mRNA.
Rhbg and Rhcg protein expression.
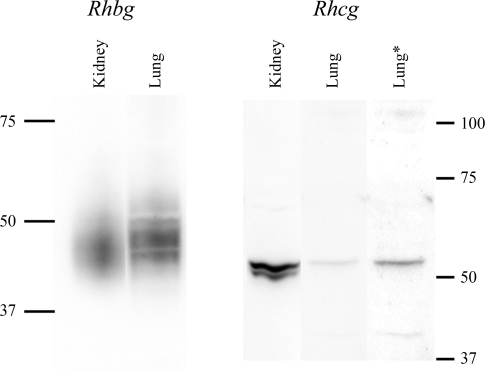
We then used immunoblot analysis to determine whether the murine lung expresses Rhbg and Rhcg protein. Figure 2 shows typical results. The murine lung expresses both Rhbg and Rhcg protein under basal conditions.
Fig. 2.
Immunoblot of kidney and lung tissue for Rhbg and Rhcg. Membrane-enriched fraction of cellular lysate from mouse lung and mouse kidney was examined by immunoblot analysis for expression of Rhbg (left) and Rhcg (right). On the left, 1.25 μg of kidney and 40 μg of lung protein were used. Lung Rhbg expression was ∼3% of renal Rhbg expression after adjustment for amount of protein loaded. On the right, 20 μg of lung tissue and 25 μg of kidney protein were used. The lanes labeled kidney and lung are from the same immunoblot and are reproduced with original intensity. The lane labeled “lung*” uses contrast-enhancement of the lung lane to more clearly show the Rhcg band. Lung Rhcg expression was ∼6% of renal Rhcg expression after adjustment for amount of protein loaded.
Cellular localization of Rhbg in the lung.
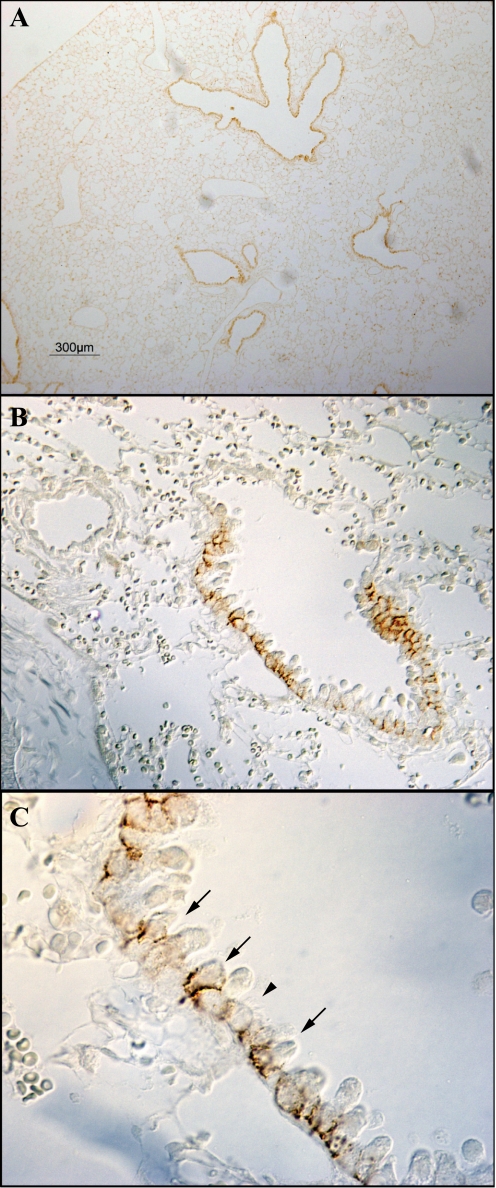
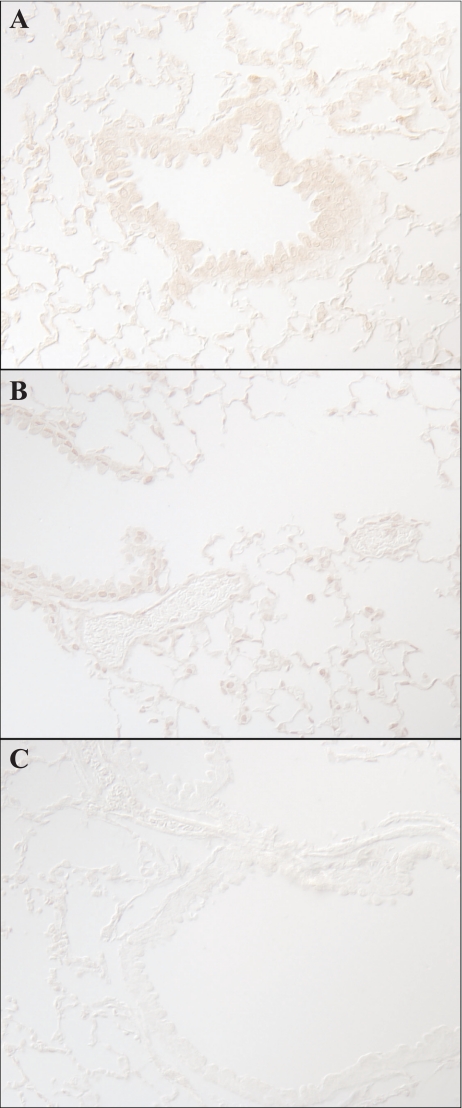
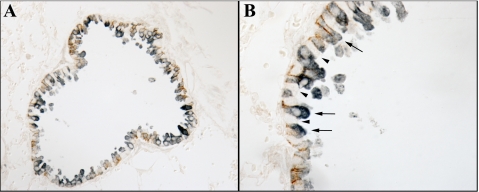
Multiple cell types are present in the lung, including two types of alveolar epithelial cells, vascular endothelial cells, bronchiolar epithelial cells, and peribronchial smooth muscle cells. To determine the Rhbg cellular localization in the mouse, lung we used immunohistochemistry. Rhbg immunoreactivity was identified exclusively in a subpopulation of bronchial and bronchiolar epithelial cells, where it exhibited exclusively basolateral immunoreactivity (Fig. 3). High-power magnification showed that the Rhbg-positive cells were nonciliated cells (Fig. 4). Detectable Rhbg immunoreactivity was not observed in alveolar tissues, vascular tissues, or peribronchial smooth muscle. Furthermore, no detectable Rhbg immunoreactivity was observed either when rabbit IgG was substituted for the primary antibody or when primary antibody was preabsorbed with the immunizing peptide (Fig. 5). The selective presence of Rhbg in nonciliated, nongoblet cells suggests cell-specific expression in Clara cells.
Fig. 3.
Immunohistochemical localization of Rhbg. A shows a low-power micrograph of Rhbg expression in the murine lung. Expression is evident only in bronchial tissue. B shows higher power micrograph showing Rhbg immunoreactivity only in bronchial epithelial cells, with no detectable expression in alveolar or vascular sites. C shows high-power magnification of bronchiolar tissue. Basolateral Rhbg expression is evident in a subset of cells (arrows). A 2nd set of cells do not express detectable Rhbg immunoreactivity (arrowhead).
Fig. 4.
High-power micrograph of Rhbg localization in bronchiolar epithelial cells. High-power micrograph of Rhbg immunoreactivity both confirms basolateral expression and, using differential interference contrast (DIC) optics, demonstrates that ciliated cells (“*”) do not express detectable Rhbg immunoreactivity, whereas nonciliated cells (arrows) demonstrate easily detectable basolateral Rhbg.
Fig. 5.
Confirmation of Rhbg and Rhcg antibody specificity. A shows immunohistochemistry when rabbit IgG was substituted for the primary antibody. No specific immunoreactivity is present. B shows results when Rhbg antibody was preincubated with immunizing peptide. No specific immunoreactivity is present. C shows immunohistochemistry using Rhcg antibody preincubated with immunizing peptide. No specific immunoreactivity is present.
To confirm Rhbg expression by Clara cells, we colocalized Rhbg with the Clara cell marker, Clara cell secretory protein. Figure 6 shows representative results. Only Clara cell secretory protein-positive cells expressed basolateral Rhbg immunoreactivity.
Fig. 6.
Colocalization of Rhbg with Clara cell secretory protein. To determine the cell-specific expression of Rhbg double immunolabeling of Rhbg (brown) with the Clara cell-specific protein, Clara cell secretory protein (blue) was used. Clara cell secretory protein is a cytoplasmic protein expressed only by Clara cells. Cells expressing basolateral Rhbg also expressed Clara cell secretory protein immunoreactivity (arrows). Cells without detectable Clara cell secretory protein expression did not express detectable Rhbg immunoreactivity (arrowheads).
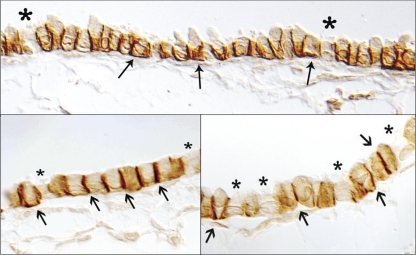
Cellular localization of Rhcg in the lung.
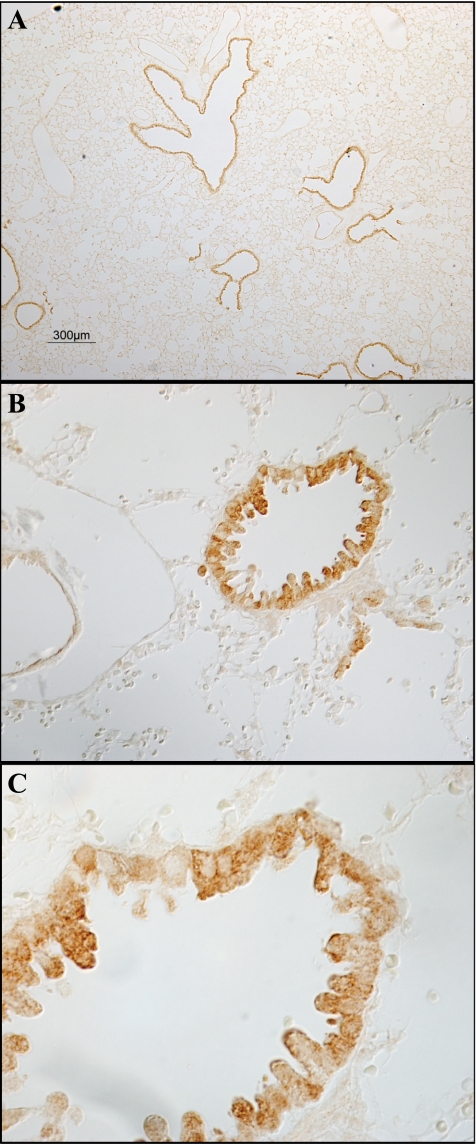
We performed a similar series of studies to determine the cell-specific expression of Rhcg in the mouse lung. Figure 7 shows representative results. Rhcg immunoreactivity was identified in bronchial and bronchiolar epithelial cells beginning in main stem bronchi and extending to terminal bronchioles. No immunoreactivity was identified in alveolar epithelial or endothelial cells, pneumocytes, or vascular endothelium. Similar to Rhbg, no immunoreactivity was observed when rabbit IgG was substituted for the primary antibody or when primary antibody was preabsorbed with the immunizing peptide (Fig. 5).
Fig. 7.
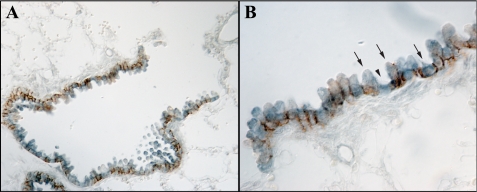
Immunohistochemical localization of Rhcg. A shows a low-power micrograph of murine lung for immunohistochemical detection of Rhcg expression. Immunoreactivity is present only in bronchial and bronchiolar tissue. No significant expression is present in alveolar tissue or in vascular sites. B shows intermediate-power micrograph further confirming expression only in bronchial or bronchiolar epithelial cells and not in alveolar or vascular tissues. C shows high-power micrograph in bronchiolar tissue. All cells express detectable Rhcg immunoreactivity, although expression is greater in a subpopulation of cells. Expression is many cells has an apical predominance, whereas in other cells Rhcg has a cytoplasmic expression pattern.
The expression of Rhcg differed from that of Rhbg. In contrast to Rhbg, Rhcg immunolabel was apical and intracellular, rather than exclusively basolateral as shown for Rhbg. In further contrast to Rhbg, all bronchial epithelial cells, with the exception of goblet cells, expressed Rhcg immunoreactivity. In particular, both ciliated and nonciliated bronchial epithelial cells expressed Rhcg immunoreactivity, suggesting that both Clara cells and non-Clara bronchial epithelial cells express Rhcg immunolabel.
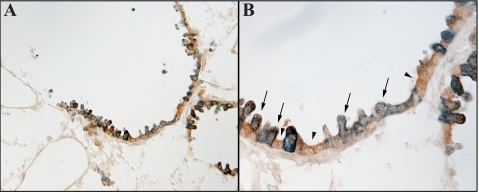
To confirm Rhcg expression by both Clara and non-Clara cells, we performed double-immunolabeling studies with Rhcg and anti-Clara cell secretory protein antibodies. These studies clearly showed that both bronchial epithelial cell types expressed Rhcg immunolabel with Clara cell secretory protein (Fig. 8). To confirm this further, we examined the colocalization of Rhcg with Rhbg, which, as shown previously, is expressed specifically in Clara cells. Double-immunolabeling of Rhbg with Rhcg clearly demonstrated colocalization of Rhbg with Rhcg in a subset of bronchial epithelial cells, i.e., Clara cells, and expression of only Rhcg without detectable Rhbg immunolabel in non-Clara cells (Fig. 9).
Fig. 8.
Colocalization of Rhcg with Clara cell secretory protein. Double labeling of Rhcg (brown) with Clara cell secretory protein (blue) shows that all bronchial epithelial cells express Rhcg immunoreactivity, both Clara cells (arrows) and non-Clara cells (arrowheads).
Fig. 9.
Colocalization of Rhbg and Rhcg in the murine lung. Double immunolabeling of Rhcg (blue) with Rhbg (brown) is shown in both low-power (A) and high-power (B) micrographs. All bronchial epithelial cells express detectable Rhcg immunoreactivity. The majority of bronchial epithelial cells also express Rhbg immunoreactivity (arrows). A subset of cells expresses only Rhcg immunoreactivity and do not have detectable basolateral Rhbg immunoreactivity (arrowhead).
Rh glycoprotein expression in cultured bronchial epithelial cells.
To begin examining the functional role of RhBG and RhCG in bronchial epithelial cells, we used the well-characterized human bronchial epithelial cell line, BEAS-2B. Immunoblot analysis demonstrated both Rhbg and Rhcg protein expression (Fig. 10A). Real-time RT-PCR confirmed RhBG and RhCG mRNA expression (Fig. 10B).
Fig. 10.
Rhbg and Rhcg expression in the BEAS-2B bronchial epithelial cell line. A shows immunoblot analysis demonstrating Rhbg and Rhcg protein expression in BEAS-2B cells. B shows real-time RT-PCR amplification of BEAS-2B mRNA for RhBG and RhCG. No amplification was observed in the absence of reverse transcription or in the absence of template RNA (data not shown). C shows BEAS-2B methylammonia (MA) transport as a function of extracellular MA. Transport can be modeled as consisting of both a saturable, carrier-mediated component with Michaelis-Menten kinetics (dotted line) and a diffusive component (dashed line). D shows Dixon plot of ammonia-inhibitable uptake of 5 μM (diamonds) and 10 μM (squares) [14C]MA. Units for ordinate axis are (pmol·mg [14C]MA uptake−1·min−1)−1. Lines were calculated using least-squares linear regression. Lines intersect at −Ki, and intersection above the abscissa indicates that ammonia competitively inhibits transport.
Ammonia transport.
In view of the evidence that the ammonia transporter family members, Rh glycoproteins, Rhbg and Rhcg, are expressed in bronchial epithelial cells, we examined whether bronchial epithelial cells exhibited transporter-mediated ammonia transport. We quantified ammonia transport as [14C]MA uptake as described previously (26, 27). In preliminary studies, the NKCC cotransporter inhibitor, furosemide, 100 μM, inhibited a minor component, 17 ± 0.5% (n = 3), of total [14C]MA uptake, whereas the Na+-K+-ATPase inhibitor, ouabain, 10 μM, did not alter transport significantly. RT-PCR amplification of BEAS-2B cell mRNA with NKCC cotransporter isoform-specific primers identified human NKCC1, but not NKCC2, mRNA expression (data not shown). Accordingly, 100 μM furosemide was utilized in all solutions in experiments assessing whether an ammonia transport activity attributable to RhBG and RhCG was present in bronchial epithelial cells.
To determine whether transport reflected diffusive uptake or transporter-mediated uptake, we examined changes in initial uptake rate as extracellular concentration increased. Diffusive transport is linearly related to extracellular concentration, whereas transporter-mediated uptake will exhibit saturable kinetics. As shown in Fig. 10C, transport activity was a nonlinear function of extracellular MA, indicating that a saturable, carrier-mediated transport mechanism contributes to BEAS-2B MA transport. Total uptake could be modeled as consisting of both a transporter-mediated component, exhibiting Michaelis-Menten kinetics, and a diffusive component. The transporter-mediated component had a mean Km of 8.1 ± 1.0 mM (n = 3). At all concentrations examined, 0.005–25 mM, the transporter-mediated component of uptake exceeded the diffusive component. At concentrations <1 mM, the transporter-mediated component accounted for >80% of total uptake. Finally, we assessed the affinity for ammonia as the Ki of ammonia on MA uptake. Using Dixon plot analysis (Fig. 10D), ammonia competitively inhibited [14C]MA uptake with a Ki of 6.3 ± 0.3 mM (n = 3).
DISCUSSION
These studies are the first to demonstrate expression of the newly recognized gas transporters, Rhbg and Rhcg, in the mammalian lung. The murine lung expresses both Rhbg and Rhcg mRNA and protein. In the lung, Rhbg and Rhcg are expressed in bronchial epithelial cells but are not detectable in alveolar epithelial cells or endothelium. In in vitro studies, cultured human bronchial epithelial cells expressed both Rhbg and Rhcg. These cells transported the ammonia analog, MA, through a saturable, carrier-mediated mechanism with a Km similar to that reported for other Rhbg and Rhcg-expressing cell lines. These results document for the first time that Rhbg and Rhcg are expressed in the mammalian lung, where they are likely to contribute to bronchial epithelial cell ammonia transport. The absence of detectable Rhbg and Rhcg expression in alveolar epithelial cells and endothelium does not support a substantial role for Rhbg and Rhcg in lung CO2 transport.
Although the lung is not typically thought of as an ammonia-transporting organ, several lines of evidence demonstrate pulmonary ammonia transport. Exhaled breath condensates exhibits significant amounts of ammonia (8, 20, 29, 31, 37), and exhaled air contains ammonia gas (11, 32, 56, 61, 63). Ammonia excreted by the lungs is ∼5-fold greater in patients with end-stage renal disease than in patients with normal renal function (11), although the amount is unlikely to contribute importantly to acid-base homeostasis. Finally, abnormalities in pulmonary ammonia excretion are present in patients with asthma (33) and with cystic fibrosis (19, 33).
The site of alveolar and exhaled breath condensate ammonia could derive from airway epithelial cells, alveolar sites, or the oropharynx. The current studies, by showing Rhcg expression in ciliated bronchial epithelial cells and both Rhbg and Rhcg expression in Clara cells, suggest that bronchial epithelial ammonia secretion involves Rhbg and Rhcg-mediated ammonia transport. This is further supported by the observation that cultured bronchial epithelial cells express both Rhbg and Rhcg and exhibit carrier-mediated ammonia/MA transport. The affinity of bronchial epithelial cell ammonia transport for ammonia/MA is similar to that which we observed examining renal epithelial cells (26, 27) and similar to that observed in studies examining heterologous expression of Rhbg and Rhcg (2, 44, 45, 51).
The critical role of Rh glycoproteins in ammonia transport is demonstrated by two recent reports examining Rhcg deletion. Both reports, one using global Rhcg deletion and the other using renal collecting duct-specific deletion, demonstrate that Rhcg deletion decreases both basal and acidosis-stimulated renal ammonia excretion, a normal renal function critical for acid-base homeostasis (4, 38). Furthermore, direct measurements of ammonia transport show that global Rhcg deletion decreases collecting duct transepithelial ammonia permeability (4). Global Rhcg deletion also appears to alter male fertility, possibly related to critical roles for Rhcg in testicular and/or epididymal function (4). However, global Rhcg deletion did not alter Pco2, consistent with a lack of a role of Rhcg in pulmonary CO2 excretion (4).
Bronchial epithelial cell ammonia transport is also likely to be mediated, at least in part, by a furosemide-inhibitable process, most likely involving NKCC1. NKCC1 is expressed in bronchial epithelial cells and is known to contribute to transcellular Cl− transport (21, 57). A second NKCC isoform, NKCC2, mediates a critical role in renal ammonia transport but is not expressed in the lung (57). We confirmed isoform-specific NKCC cotransporter mRNA expression in the human bronchial epithelial cell line, BEAS-2B cells. Thus the current studies expand on the role of NKCC1 in bronchial epithelial cells by suggesting that it contributes, along with Rhbg and Rhcg, to bronchial epithelial cell ammonia transport. However, at least as assessed in cultured bronchial epithelial cells, NKCC1 is a minor contributor to ammonia transport, accounting for <20% of total ammonia transport.
It is likely nonbronchial epithelial cells also contribute to pulmonary ammonia excretion. Specifically, the large surface area of alveolar epithelial cells makes alveoli a likely additional site for ammonia transport. However, the lack of evidence for Rhbg and Rhcg expression in alveolar endothelial cells and epithelial cells suggests that Rh glycoprotein-mediated ammonia transport is unlikely to contribute to ammonia movement in these sites. Instead, either diffusive NH3 movement or transport by other, currently unidentified proteins across plasma membranes in these sites is likely to mediate ammonia secretion.
The finding of Rhbg and Rhcg in the lung differs from results reported in the initial cloning of these important ammonia transporter family members in which Rhbg and Rhcg mRNA expression was not identified (41, 43). The most likely explanation is that the previous studies used Northern blot analysis, which is less sensitive for detecting mRNA expression than the real-time RT-PCR assay used in the current study. Indeed, the current study shows that the levels of Rhbg and Rhcg mRNA in the lung are substantially less than in the kidney. Similar observations of the ability to detect low levels of Rhbg and Rhcg mRNA by real-time RT-PCR, but not by Northern blot analysis, have been shown previously in the stomach, small intestine, and colon (28).
As discussed in the Introduction, increasing evidence indicates that Mep/Amt/Rh glycoproteins can transport CO2. However, whether Rhbg and Rhcg mediate an important role in mammalian CO2 transport is unclear. In particular, Rhbg and Rhcg are not expressed in CO2 transport sites. For example, transmembrane CO2 transport is critical for renal proximal tubule and thick ascending limb of the loop of Henle bicarbonate reabsorption (1), but studies in the human, mouse, and rat kidney have uniformly shown the absence of detectable RhAG, Rhbg, or RhCG/Rhcg expression in these sites (14, 24, 54, 64, 66). Renal intercalated cells express both Rhbg and Rhcg (14, 54, 64) and use plasma membrane CO2 transport in the process of urinary acidification (1). However, neither Rhbg nor Rhcg deletion alters renal urinary acidification (4, 9, 38). In the stomach, CO2 transport is critical for gastric parietal cell acid secretion, but there is no detectable Rhbg or Rhcg expression in gastric parietal cells (28). The current study shows no detectable Rhbg and Rhcg expression in alveolar cells or in endothelial cells, the sites of pulmonary CO2 and O2 transport. Finally, Pco2 was normal in mice with global Rhcg deletion (4), suggesting that pulmonary Rhcg expression is not necessary for normal CO2 excretion. Thus studies in multiple mammalian tissues either do not identify Rhbg and Rhcg expression in sites where CO2 transport is important or demonstrate the Rhbg and Rhcg deletion do not alter CO2-dependent events. Of course, one cannot exclude the possibility that under specific conditions or in response to other stimuli, Rhbg and Rhcg contribute to CO2 transport.
In contrast, abundant evidence demonstrates Rhbg and Rhcg expression in important sites of regulated ammonia transport. In the kidney, Rhbg and Rhcg are expressed in distal epithelial sites, particularly the collecting duct, where they appear to mediate important roles in regulated ammonia metabolism (24, 25, 34, 35, 40, 58, 59). Recent studies demonstrate that both global and collecting duct-specific Rhcg deletion inhibits renal ammonia transport (4, 38). In the stomach, Rhbg and Rhcg are expressed in gastric zymogenic cells, where they are instead likely to be involved in ammonia-mediated regulation of signal transduction mechanisms (28). In the jejunum, ileum and colon Rhbg and Rhcg are expressed in villous epithelial cells, where they are likely to contribute to vectorial ammonia transport (28). Rhcg is also expressed in skeletal and smooth muscle cells, another important site of ammonia transport (28, 66). Finally, perivenous hepatocytes, a critical site for high-affinity ammonia metabolism, express high levels of Rhbg (69). Thus the pattern of known expression of Rhbg and Rhcg is consistent with their mediating important roles in ammonia transport.
An important study identified evidence of evolutionary divergence of Rh glycoproteins from Amt proteins in archaeal species (30). This, in combination with studies discussed previously examining bacterial and C. reinhardtii Rh glycoproteins, has been interpreted as evidence that Rh glycoproteins attained differing physiological roles in this evolutionary process, most likely a primary role in CO2 transport rather than ammonia transport (30, 53). In view of the lack of evidence for mammalian Rh glycoprotein expression in CO2 transport sites, another possibility is that the evolutionary changes mediate the large differences in ammonia affinity observed between Mep and Amt proteins, ∼1–25 μM, and Rh glycoproteins, typically 1–5 mM. Rh glycoprotein affinity for ammonia in the low millimolar range is physiologically relevant for many of the tissues in which Rhbg and Rhcg are expressed, such as kidney, intestinal tract, and liver, where ammonia levels are much higher than the low micromolar concentrations typically observed in blood (12, 28, 36, 69).
One potential limitation of the transport studies reported here is that they used MA as an ammonia surrogate. Directly measuring transmembrane ammonia transport is difficult. Radiolabeled ammonia, using 15N, can be quantified only by mass spectrometry. Intracellular NH3- or NH4+-specific electrodes and fluorescent NH3- or NH4+-specific dyes are not available. As a result, direct ammonia transport measurements are difficult and generally not practical. Instead, numerous studies show that MA is transported through the same pathways as ammonia, and it is widely used as an ammonia surrogate (reviewed in Ref. 26). This basic technique, using a substitute radionuclide to characterize ion transport, is similar to the widely used technique where 86Rb+ is used as a potassium surrogate. Thus, although the current study uses MA as an ammonia surrogate, the results are likely to be highly relevant for understanding the molecular mechanisms of ammonia transport.
In summary, the nonerythroid ammonia transporter family members, Rhbg and Rhcg, are expressed in bronchial and bronchiolar epithelial cells. Furthermore, in cultured bronchial epithelial cells, the predominant ammonia/MA transport mechanism is a carrier-mediated mechanism that appears to be mediated by Rhbg and Rhcg. There was no detectable Rhbg or Rhcg expression in either alveolar epithelial cells or vascular endothelium, suggesting that Rhbg and Rhcg are unlikely to contribute to pulmonary CO2 transport under basal conditions.
GRANTS
Funds from the National Institutes of Health (DK-045788), Gatorade Research Funds, and the Research Service of the Malcom Randall Veterans Affairs Medical Center supported these studies.
Acknowledgments
We appreciate the secretarial assistance of Gina Cowsert and technical assistance from Jeanette Lynch. We thank the Malcom Randall Veterans Affairs Medical Center and the Department of Medicine at University of Florida College of Medicine in Gainesville, Florida, for the use of the Gene Expression Core Laboratory, which provided the ABI 7500 system used for real-time RT-PCR.
Footnotes
Ammonia consists of two molecular forms, NH3 and NH4+, that are in equilibrium with each other according to the reaction NH3 + H+ ↔ NH4+. We use the term ammonia to refer to the sum of these two molecular forms and refer to each molecular form as either NH3 or NH4+, respectively.
The standard abbreviation for human Rh A glycoprotein is RhAG and for nonhuman Rh A glycoprotein is Rhag. A similar pattern is used for Rh B glycoprotein and Rh C glycoprotein in this report.
REFERENCES
- 1.Alpern RJ Renal acidification mechanisms. In: Brenner and Rector's The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2000, p. 455–519.
- 2.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, Edelman A, Planelles G, Thomas SR, Cherif-Zahar B. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflügers Arch 450: 155–167, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Blank ME, Ehmke H. Aquaporin-1 and HCO3−-Cl− transporter-mediated transport of CO2 across the human erythrocyte membrane. J Physiol 550: 419–429, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boron WF, Waisbren SJ, Modlin IM, Geibel JP. Unique permeability barrier of the apical surface of parietal and chief cells in isolated perfused gastric glands. J Exp Biol 196: 347–360, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Boron WF Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brooks S, Haight R, Gordon R. Age does not affect airway pH and ammonia as determined by exhaled breath measurements. Lung 184: 195–200, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol 275: C1481–C1486, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Davies S, Spanel P, Smith D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int 52: 223–228, 1997. [DOI] [PubMed] [Google Scholar]
- 12.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol 1: 1193–1203, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Echevarria M, Munoz-Cabello AM, Sanchez-Silva R, Toledo-Aral JJ, Lopez-Barneo J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem 282: 30207–30215, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Flessner MF, Wall SM, Knepper MA. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol Renal Fluid Electrolyte Physiol 260: F264–F272, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Forster RE, Gros G, Lin L, Ono Y, Wunder M. The effect of 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate on CO2 permeability of the red blood cell membrane. Proc Natl Acad Sci USA 95: 15815–15820, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaston B, Ratjen F, Vaughan JW, Malhorta NR, Canady RG, Synder AH, Hunt JF, Gaertib S, Goldberg JB. Nitrogen redox balance in the cystic fibrosis airway. effects of antipseudomonal therapy. Am J Respir Crit Care Med 165: 387–390, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Gessner C, Hammerschmidt S, Kuhn H, Seyfarth HJ, Sack U, Engelmann L, Schauer J, Wirtz H. Exhaled breath condensate acidification in acute lung injury. Respir Med 97: 1188–1194, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2-chloride transporter. Am J Respir Cell Mol Biol 25: 14–20, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Good DW, DuBose TD. Concentrations of NH3 in cortex and medulla of rat kidney. Contrib Nephrol 63: 16–20, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest 75: 478–485, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol 293: F1342–F1354, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jöbsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H; ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26: 523–548, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci USA 102: 15512–15517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt JF, Erwin E, Palmer L, Vaughan J, Malhotra N, Platts-Mills TA, Gaston B. Expression and activity of pH-regulatory glutaminase in the human airway epithelium. Am J Respir Crit Care Med 165: 101–107, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Jacquez JA, Poppell JW, Jeltsch R. Partial pressure of ammonia in alveolar air. Science 129: 269–270, 1959. [DOI] [PubMed] [Google Scholar]
- 33.Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 7: 1–32, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member Rh C glycoprotein in the mouse kidney. Am J Physiol Renal Physiol 296: F543–F555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knepper MA NH4+ transport in the kidney. Kidney Int 40: S95–S102, 1991. [PubMed] [Google Scholar]
- 37.Larson TV, Covert DS, Frank R, Charlson RJ. Ammonia in the human airways: neutralization of inspired acid sulfate aerosols. Science 197: 161–163, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol (March 25, 2009). doi: 10.1152/ajprenal.90667.2008. [DOI] [PMC free article] [PubMed]
- 39.Li X, Jayachandran S, Nguyen HH, Chan MK. Structure of the Nitrosomonas europaea Rh protein. Proc Natl Acad Sci USA 104: 19279–19284, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Huang CH. The mouse Rhl1 and Rhag genes: sequence, organization, expression, and chromosomal mapping. Biochem Genet 37: 119–138, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Ludewig U Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol 559: 751–759, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marini AM, Boeckstaens M, Andre B. From yeast ammonium transporters to Rhesus proteins, isolation and functional characterization. Transfus Clin Biol 13: 95–96, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Marini AM, Vissers S, Urrestarazu A, Andre B. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J 13: 3456–3463, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayer M, Schaaf G, Mouro I, Lopez C, Colin Y, Neumann P, Cartron JP, Ludewig U. Different transport mechanisms in plant and human AMT/Rh-type ammonium transporters. J Gen Physiol 127: 133–144, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Ninnemann O, Jauniaux JC, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO J 13: 3464–3471, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng J, Huang CH. Rh proteins vs Amt proteins: an organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus Clin Biol 13: 85–94, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci USA 101: 17222–17227, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robin ED, Travis DM, Bromberg PA, Forkner CE Jr, Tyler JM. Ammonia excretion by mammalian lung. Science 129: 270–271, 1959. [DOI] [PubMed] [Google Scholar]
- 57.Rochelle LG, Li DC, Ye H, Lee E, Talbot CR, Boucher RC. Distribution of ion transport mRNAs throughout murine nose and lung. Am J Physiol Lung Cell Mol Physiol 279: L14–L24, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Singh SK, Binder HJ, Geibel JP, Boron WF. An apical permeability barrier to NH3/NH4+ in isolated, perfused colonic crypts. Proc Natl Acad Sci USA 92: 11573–11577, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith D, Spanel P. The novel selected-ion flow tube approach to trace gas analysis of air and breath. Rapid Commun Mass Spectrom 10: 1183–1198, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci USA 101: 7787–7792, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spanel P, Smith D. Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath. Med Biol Eng Comput 34: 409–419, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature 368: 332–335, 1994. [DOI] [PubMed] [Google Scholar]
- 66.Weiner ID The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hypertens 13: 533–540, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Weiner ID Expression of the non-erythroid Rh glycoproteins in mammalian tissues. Transfus Clin Biol 13: 159–163, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology 124: 1432–1440, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh-blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem 277: 12499–12502, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Westhoff CM, Siegel DL, Burd CG, Foskett JK. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein (RhAG). J Biol Chem 279: 17443–17448, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Yip KP, Kurtz I. NH3 permeability of principal cells and intercalated cells measured by confocal fluorescence imaging. Am J Physiol Renal Fluid Electrolyte Physiol 269: F545–F550, 1995. [DOI] [PubMed] [Google Scholar]