Abstract
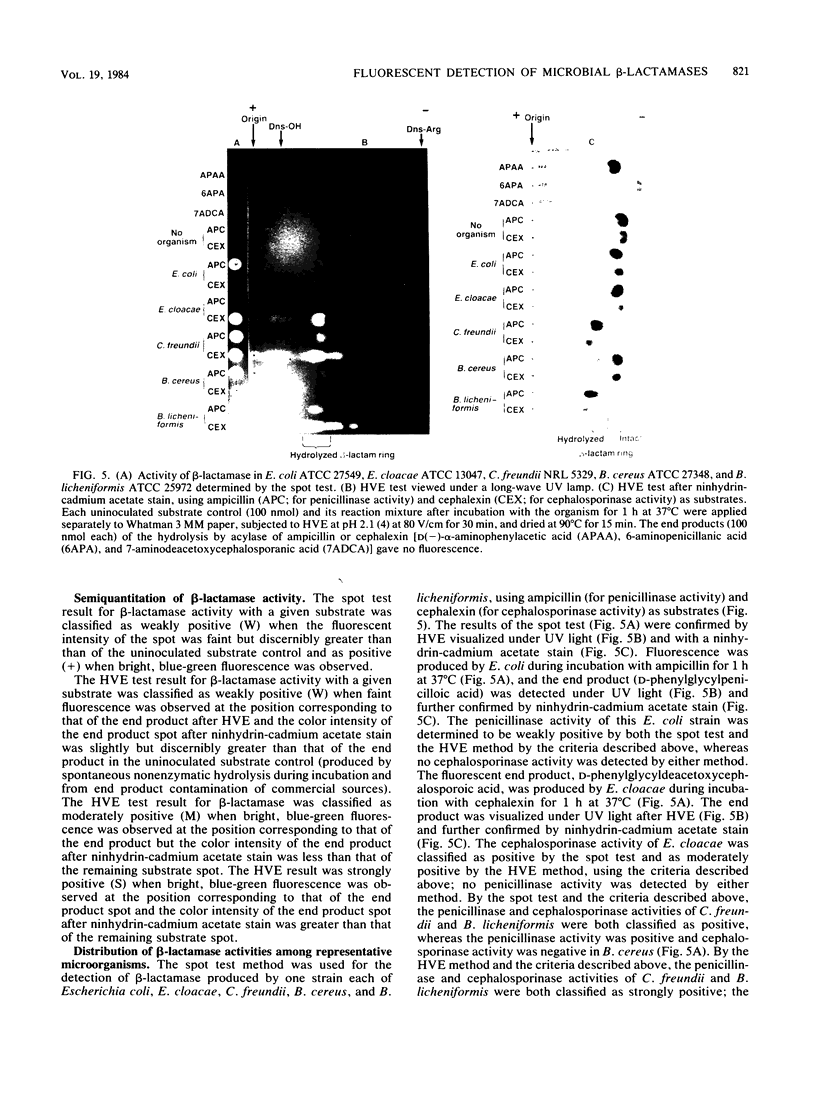
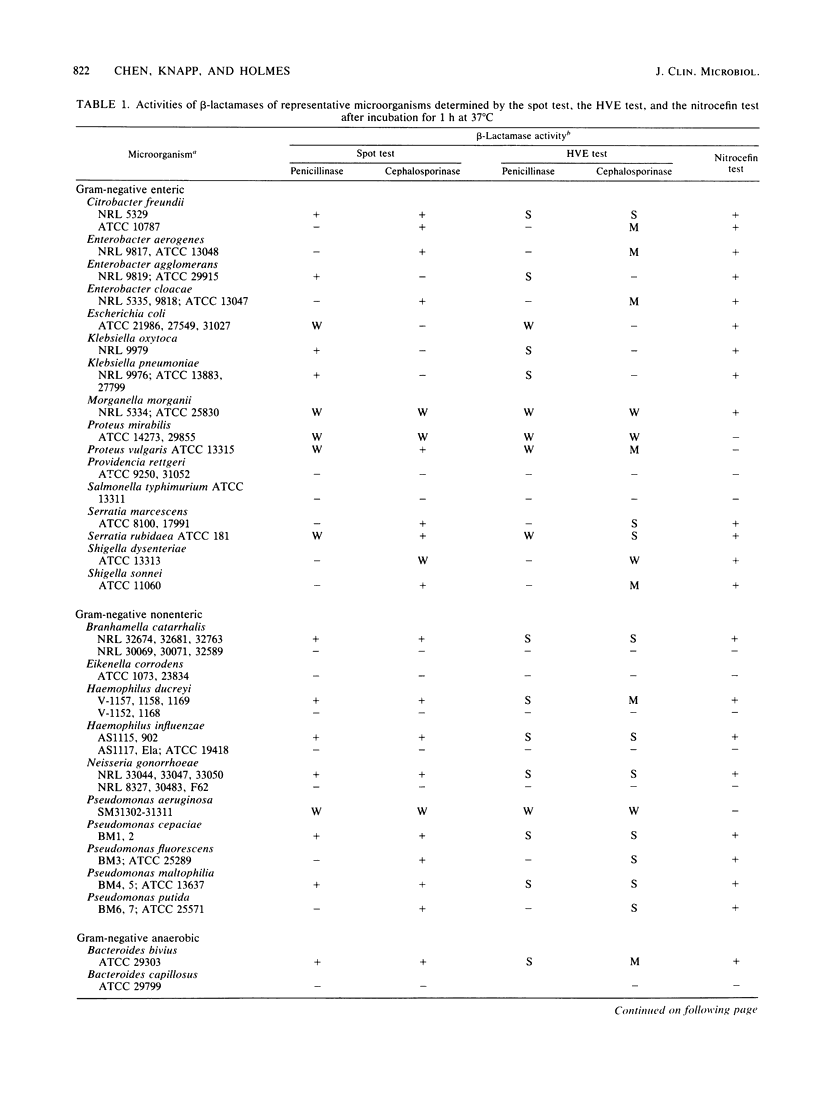
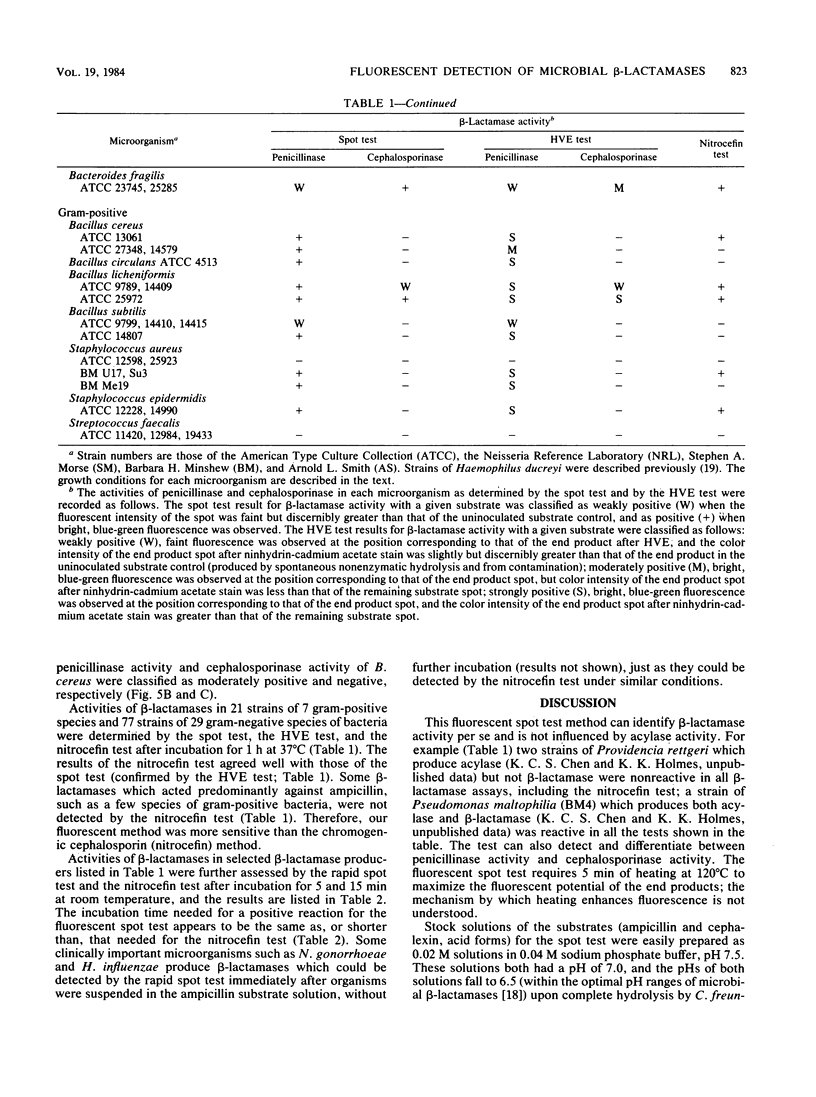
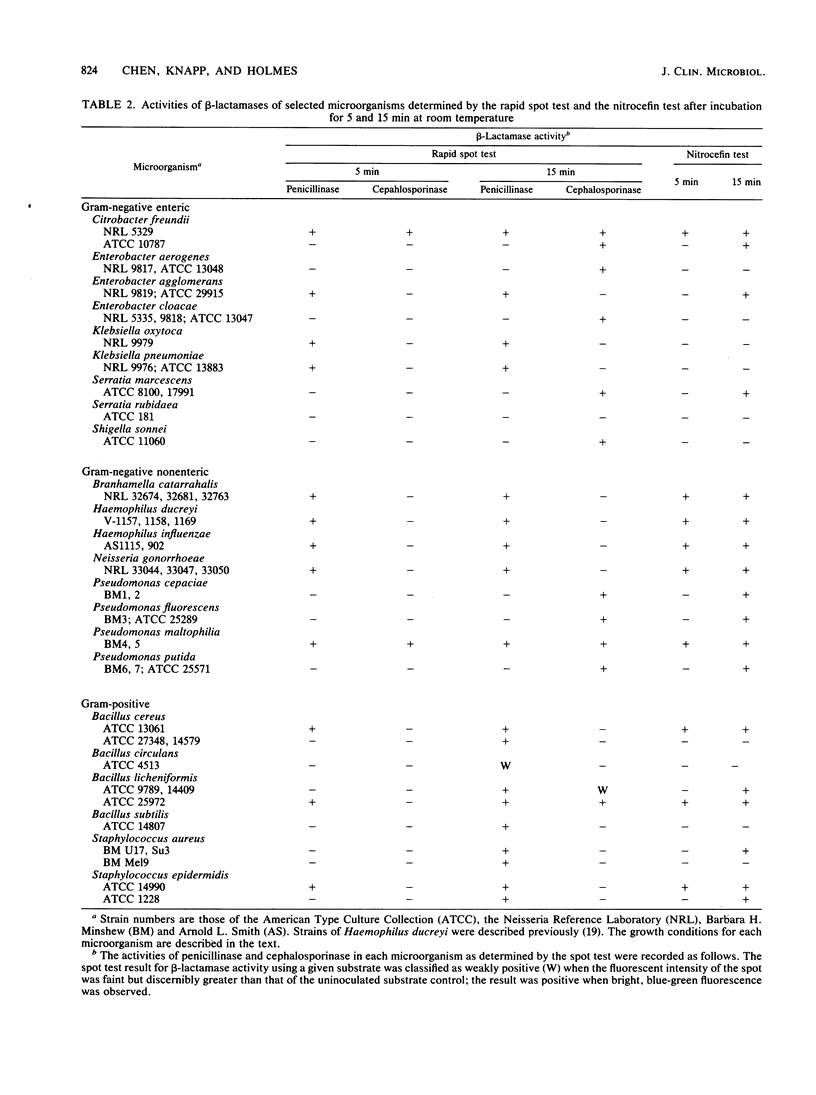
A rapid method was developed for specific detection of microbial beta-lactamases which uses ampicillin and cephalexin as substrates. The end products (open beta-lactam ring forms) generated after separately incubating either substrate with beta-lactamase-producing organisms initially were separated from the unhydrolyzed substrates by high-voltage electrophoresis at pH 2.1. The end products of both antibiotics were highly fluorescent and could be analyzed visually and semiquantitatively under a long-wave UV lamp. Application of 5 microliters of the same incubation mixture onto filter paper without subsequent electrophoretic separation also resulted in development of fluorescence after brief heating at 120 degrees C for 5 min. This spot test differentiates penicillinase activity from cephalosporinase activity and distinguishes between beta-lactamase and acylase activities, since the end products of acylase [the common side chain, D(-)-alpha-aminophenylacetic acid, and the intact beta-lactam nuclei, 6-aminopenicillanic acid and 7-aminodeacetoxycephalosporanic acid] are not fluorescent. This method was relatively rapid, inexpensive, and more sensitive than the chromogenic cephalosporin (nitrocefin) method when 21 strains of 7 gram-positive species and 77 strains of 29 gram-negative species of bacteria were tested.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anhalt J. P., Nelson R. Failure of Padac test strips to detect staphylococcal beta-lactamase. Antimicrob Agents Chemother. 1982 Jun;21(6):993–994. doi: 10.1128/aac.21.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Culbertson N. J., Knapp J. S., Kenny G. E., Holmes K. K. Rapid method for simultaneous detection of the arginine dihydrolase system and amino acid decarboxylases in microorganisms. J Clin Microbiol. 1982 Nov;16(5):909–919. doi: 10.1128/jcm.16.5.909-919.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Kindt T. J., Krause R. M. Primary structure of the L chain from a rabbit homogeneous antibody to streptococcal carbohydrate. I. Purification of antibody and sequence determination of peptides from alpha-chymo-tryptic and thermolytic digests. J Biol Chem. 1975 May 10;250(9):3280–3288. [PubMed] [Google Scholar]
- Chen K. C., Krause R. M. A peptide mapping technique--a three map system. Anal Biochem. 1975 Nov;69(1):180–186. doi: 10.1016/0003-2697(75)90579-5. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Dmitrienko G. I., Viswanatha T. N-(2-Furyl) acryloyl penicillin: a novel compound for the spectrophotometric assay of beta-lactamase I. J Antibiot (Tokyo) 1977 Oct;30(10):883–885. doi: 10.7164/antibiotics.30.883. [DOI] [PubMed] [Google Scholar]
- Ericsson H. The effect of beta-lactamases of varying origin on different types of cephalosporins. Previously announced contribution to discussion. Scand J Infect Dis Suppl. 1978;(13):33–34. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Horton K. A., Jennings B. R., Baselski V. S. Beta-lactamase testing of staphylococci in a commercial broth microdilution minimum inhibitory concentration system. J Clin Microbiol. 1982 Aug;16(2):406–407. doi: 10.1128/jcm.16.2.406-407.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Crawford S. A., Alexander G. A. Pyridinium-2-azo-p-dimethylaniline chromophore, a new chromogenic cephalosporin for rapid beta-lactamase testing. Antimicrob Agents Chemother. 1982 Jul;22(1):162–164. doi: 10.1128/aac.22.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITNER F., SWEENEY H. M., MARTIN T. F., COHEN S. INDUCTION OF STAPHYLOCOCCAL PENICILLINASE BY BENZYLPENICILLIN: EFFECT OF PH, CONCENTRATION OF FERROUS ION AND INDUCER, AND DURATION OF EXPOSURE OF CELLS TO INDUCER. J Bacteriol. 1963 Oct;86:717–727. doi: 10.1128/jb.86.4.717-727.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. T., Rosenblatt J. E. A comparison of four methods for detecting beta-lactamase in anaerobic bacteria. Diagn Microbiol Infect Dis. 1983 Jun;1(2):173–175. doi: 10.1016/0732-8893(83)90048-2. [DOI] [PubMed] [Google Scholar]
- Marshall M. J., Ross G. W., Chanter K. V., Harris A. M. Comparison of the substrate specificities of the -lactamases from Klebsiella aerogenes 1082E and Enterobacter cloacae P99. Appl Microbiol. 1972 Apr;23(4):765–769. doi: 10.1128/am.23.4.765-769.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H. Antibiotic resistance in pathogenic and producing bacteria, with special reference to beta-lactam antibiotics. Microbiol Rev. 1981 Dec;45(4):591–619. doi: 10.1128/mr.45.4.591-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., Chanter K. V., Harris A. M., Kirby S. M., Marshall M. J., O'Callaghan C. H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1973 Jul;54(1):9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Handsfield H. H., Peters D., Holmes K. K., Falkow S. Characterization of ampicillin resistance plasmids from Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Apr;21(4):622–627. doi: 10.1128/aac.21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme E. J. Enzymes involved in beta-lactam antibiotic biosynthesis. Adv Appl Microbiol. 1977;21:89–123. doi: 10.1016/s0065-2164(08)70039-x. [DOI] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Osterberg S. K., Jorgensen J. Subgingival microflora of periodontal patients on tetracycline therapy. J Clin Periodontol. 1979 Aug;6(4):210–221. doi: 10.1111/j.1600-051x.1979.tb01923.x. [DOI] [PubMed] [Google Scholar]