Abstract
Interleukin-12 (IL-12) p70 (p40:p35) is a bioactive cytokine and its biological functions are becoming clear. On the other hand, the IL-12 p40 homodimer (p402) was considered an inactive or inhibitory molecule and its functions are poorly understood. It has been reported that increased expression of lymphotoxin-α (Lt-α) in the central nervous system as well as in peripheral immune cells is associated with multiple sclerosis and experimental allergic encephalomyelitis. Here we describe that p402 induces the expression of Lt-α in primary mouse and human microglia, BV-2 microglial cells, splenic macrophages, RAW 264.7 cells and splenic T cells. Interestingly, IL-12 p70 was either unable to induce Lt-α or was a very weak inducer of Lt-α in these cell types. Consistently, p402, but not p70, induced Lt-α promoter-driven luciferase activity in microglial cells. Among various stimuli tested, p402 emerged as the most potent followed by IL-16, lipopolyaccharide and double-stranded RNA in inducing the activation of Lt-α promoter in microglial cells. Furthermore, an increase in Lt-α messenger RNA expression by overexpression of p40, but not p35, complementary DNA and induction of Lt-α expression by p402 in microglia isolated from IL-12p35−/− mice confirm that p40, but not p35, is responsible for the induction of Lt-α. Finally, by using primary microglia from IUL-12 receptor β1 deficient (IL-12Rβ1−/−) and IL-12Rβ2−/− mice, we demonstrate that p402 induced the expression of Lt-α in microglia and macrophages via IL-12Rβ1, but not IL-12Rβ2. These studies delineate a novel biological function of p402 that is absent in IL-12.
Keywords: interleukin-12, interleukin-12 p40 homodimer, interleukin-12 receptor β1, interleukin-12 receptor β2, lymphotoxin-α
Introduction
Lymphotoxin-α [Lt-α; also known as tumour necrosis factor-β (TNF-β)] is a member of the immediate major histocompatibility complex-linked TNF family, which consists of TNF-α, Lt-α and Lt-β.1,2 It is known that Lt-α plays an important role in the development of lymphoid organs.1,2 Several studies also indicate that Lt-α promotes inflammation in autoimmune diseases and that it is present in multiple sclerosis (MS) brain lesions and cerebrospinal fluid.3–5 Accordingly, Lt-α−/− mice are quite resistant to experimental allergic encephalomyelitis (EAE), the animal model of MS, with negligible central nervous system (CNS) inflammation and demyelination compared to Lt-β−/− and TNF-α−/− mice.4,6 It has been reported that Lt-α plays a critical role in the death of oligodendrocytes during MS.7,8 Similarly, another study demonstrates that Lt-α deficiency protects C57BL/6 mice completely from developing experimental autoimmune myasthenia gravis.9 Lymphocytes, macrophages and CNS major effector cell microglia express Lt-α in active MS lesions.7 Additionally, Lt-α has been reported to have important, yet distinct, roles in various infectious disease models.10,11 According to Engwerda et al.,10 Lt-α produced by microglia or astrocytes is the principal mediator of murine cerebral malaria. Although Lt-α is an important cytokine playing many roles in physiology as well as pathophysiology, mechanisms by which this cytokine is produced in immune cells are poorly understood. Phorbol myristate acetate is known to stimulate Lt-α production in T cells. Bacterial superantigens such as, toxic shock syndrome toxin 1, staphylococcal enterotoxin B and streptococcal pyrogenic exotoxin A, are also able to upregulate Lt-α messenger RNA (mRNA) in murine splenocytes.12 Interestingly, proinflammatory cytokines [TNF-α, interleukin-1β (IL-1β) and interferon-γ (IFN-γ)] are unable to induce the expression of Lt-α in T cells.12 On the other hand, it is also not known which factor induces the expression of Lt-α in microglia and macrophages.
Interleukin-12 plays a critical role in the early inflammatory response to infection and in the generation of T helper type 1 cells, which favour cell-mediated immunity.13 It has been found that overproduction of IL-12 can be dangerous to the host because it is involved in the pathogenesis of a number of autoimmune inflammatory diseases (MS, arthritis, type 1 diabetes).14,15 Interleukin-12 consists of a heavy chain (p40) and a light chain (p35) linked covalently by disulphide bonds to give rise to a heterodimeric (p70) molecule.16,17 Recently, p40 has been shown to pair with p19 to form a newly discovered cytokine, IL-23. Either p19 or p35 is constitutively expressed in many cell types. However, dendritic cells and macrophages, cells that are able to secrete heterodimeric IL-12 or IL-23, always produce an excess of p40 as homodimer (p402).18 Again, several reports14,18,19 indicate that the level of p40 mRNA in the CNS of patients with MS is much higher than in the CNS of control subjects whereas the level of p35 mRNA is about the same or decreases compared to that of controls. Similarly, in mice with EAE, an animal model of MS, expression of p40 mRNA, but not p35 mRNA, increases in brain and spinal cord.20 These studies suggest that p40 may have a key function as a homodimer not just as part of either the p40 : p35 heterodimer forming IL-12 or the p40 : p19 heterodimer forming IL-23. However, it was thought that p402 was biologically inactive until we demonstrated the induction of nitric oxide synthase (iNOS) and TNF-α by p402 in microglia and macrophages.21,22
Here we describe how p402 is endowed with another novel biological function. Interestingly, p402, but not p70, the so-called bioactive cytokine, induces the expression of Lt-α in microglia and macrophages via IL-12Rβ1, the binding receptor of the high-affinity IL-12 receptor complex. These results further emphasize that p402 is biologically active and suggest that p402 may be considered as a separate cytokine with biological functions distinct from IL-12 p70.
Materials and methods
Reagents
Fetal bovine serum, Hanks’ balanced salt solution and Dulbecco’s modified Eagle’s minimal essential medium (DMEM)/F-12 were obtained from Mediatech, Valley Park, MO. Recombinant mouse IL-12 p70, p402 (the p40 homodimer), TNF-α, IL-1β and IFN-γ were obtained from R&D Systems, Minneapolis, MN. Antibodies against Lt-α were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Human immunodeficiency virus type 1 glycoprotein 120 (HIV-1 gp120) was obtained from US Biologicals (Swampscott, MA). Poly inosinic : cytidilic acid [poly(IC)] was purchased from Sigma (St Louis, MO). IL-12p35−/−, IL-12Rβ1−/− and IL-12Rβ2−/− mice and littermate controls were purchased from Jackson Laboratories (Bar Harbor, ME).
Isolation of mouse primary microglia
Microglial cells were isolated from mixed glial cultures according to the procedure of Giulian and Baker.23 Animal maintenance and experimental protocols were approved by the Rush University Animal Care Committee. Briefly, mixed glial cells were prepared from 7- to 9-day-old mouse pups. On day 9, the mixed glial cultures were washed three times with DMEM/F-12 and subjected to shaking for 2 hr at 37° on a rotary shaker. The floating cells were washed and seeded onto plastic tissue-culture flasks and incubated at 37° for 1 hr. The attached cells were removed by trypsinization and seeded onto new plates for further studies. To monitor purity, cells were immunostained with antibodies (BD Pharmingen, San Diego, CA) against Mac-1 surface antigen, a marker for microglia/macrophages. Ninety to ninety-five per cent of this preparation was found to be positive for Mac-1. For the induction of LT-α production, cells were stimulated with p70, p402, IL-12p23 or other stimuli in serum-free DMEM/F-12. Mouse BV-2 microglial cells (a gift from V. Bocchini of the University of Perugia, Italy) were also maintained and induced as indicated above.
Isolation of primary human microglia
Primary human microglia were isolated from mixed glial cultures according to the procedure of Jana et al.24 Briefly, 13- to 17-week-old human fetal brains were obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA). All of the experimental protocols were reviewed and approved by the Institutional Review Board of the Rush University Medical Center. The Human Embryology Laboratory of the University of Washington received consent from donors. These tissues were dissociated by trituration and trypsinization. Cells were plated on poly-d-lysine precoated 75 cm2 flasks and incubated at 37° with 5% CO2 in air. On the 9th day, the cultures were placed on a rotary shaker at 37° for 2 hr to remove microglia. The cell suspensions were placed on uncoated culture plates for 30 min followed by removal of non-adherent cells by washing. Adherent cells were cultured in DMEM/F-12 containing 10% fetal bovine serum (FBS). More than 98% of these cells stained for microglial marker CD11b.24 Twenty-four hours after plating, some of these cells showed a well-spread amoeboid morphology and the rest exhibited an elongated processed morphology.
Isolation of mouse primary macrophages
Macrophages were isolated by peritoneal lavage from mice with sterile RPMI-1640 medium containing 1% FBS and an antibiotic–antimycotic mixture (Sigma) as described earlier.21,22 Cells were washed three times with the same medium at 4° and were maintained at 37° in a humidified incubator containing 5% CO2 in air. Cells were plated in culture dishes in RPMI-1640 medium containing 1% FBS, l-glutamine and antibiotic–antimycotic mixture. After 1 hr, non-adherent cells were removed by washing, and adherent cells were 95% pure according to immunological and morphological criteria.
Isolation of splenic T cells
Specific pathogen-free female SJL/J mice (4–6 weeks old) were purchased from Harlan Sprague–Dawley, Inc. (Indianapolis, IN). Spleens were collected from these mice, and a single-cell suspension was prepared in RPMI-1640 medium containing 10% FBS, 2 mm l-glutamine, 50 μmβ1-mercaptoethanol, 100 units/ml penicillin and 100 μg/ml streptomycin. Splenocytes were cultured at a concentration of 0·5 × 106 to 1·0 × 106 cells/ml in 12-well plates. After 24 hr, non-adherent cells were passed through a mouse CD3+ T-cell enrichment column (R&D Systems) and eluted cells were more than 85% pure CD3+ T cells as observed by fluorescence-activated cell sorting (data not shown). These cells were stimulated with mouse recombinant p70 and p402.
Immunostaining of Lt-α
Immunostaining was performed as described earlier.24–26 Briefly, coverslips containing 200–300 cells/mm2 were fixed with 4% paraformaldehyde for 15 min, followed by treatment with cold ethanol (−20°) for 5 min and two rinses in phosphate-buffered saline (PBS). Samples were blocked with 3% bovine serum albumin (BSA) in PBS containing Tween-20 (PBST) for 30 min and incubated in PBST containing 1% BSA and rabbit anti-Lt-α (1 : 50). After three washes in PBST (15 min each), slides were further incubated with Cy5 and Cy2 (Jackson ImmunoResearch, West Grove, PA). For negative controls, a set of culture slides was incubated under similar conditions without the primary antibodies. The samples were mounted and observed under a Bio-Rad (Hercules, CA) MRC1024ES confocal laser-scanning microscope.
Immunoblotting assays
Cells were lysed in RIPA buffer [1×PBS, 1% nonidet-40, 0·5% sodium deoxycholate, 0·1% sodium dodecyl sulphate with freshly added 0·5% protease inhibitor cocktail (Sigma)] in ice. Protein content was estimated using Protein assay dye reagent concentrate (Bio-Rad) using the manufacturer’s protocol, and immunoblotting was performed as described previously.27 Immunoblots were probed either by chemiluminescence (Perkin-Elmer, Waltham, MA) or by fluorescence detection in the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Semi-quantitative reverse trasncription–polymerase chain reaction analysis
The expression of different proinflammatory molecules was analysed by semi-quantitative reverse trasncription polymerase chain reaction (RT-PCR) using a RT-PCR kit from BD Clontech (San Diego, CA) as described earlier.26,28 Briefly, total RNA was isolated from stimulated or unstimulated cells using the Qiagen mini kit (Valencia, CA) followed by digestion with DNase to remove contaminating genomic DNA. Briefly, 1 μg DNase-digested RNA was reverse transcribed using oligo-dT (12–18 mer) as primer and Moloney murine leukaemia virus reverse transcriptase (BD Clontech) in a 20-μl reaction mixture. The resulting complementary DNA (cDNA) was appropriately diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and the following primers. The following primers were used to amplify mouse proinflammatory molecules: iNOS [497 base pairs (bp)]: sense: 5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′, antisense: 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′; TNF-α (354 bp): sense: 5′-TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT CC-3′, antisense: 5′-GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG GG-3′; Lt-α (202 bp): sense: 5′-TGG CTG GGA ACA GGG GAA GGT TGA C-3′; antisense: 5′-CGT GCT TTC TTC TAG AAC CCC TTG G-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 276 bp): sense: 5′-GGT GAA GGT CGG TGT GAA CG-3′, antisense: 5′-TTG GCT CCA CCC TTC AAG TG-3′.
On the other hand, the following primers were used to amplify human Lt-α.
Lt-α (293 bp): sense: 5′-ACC ACG CTC TTC TGC CTG CTG CAC T-3′, antisense: 5′-GCC CTT GAA GAG GAC CTG GGA GTA-G-3′; GAPDH: sense: 5′-GGT GAA GGT CGG SGT CAA CG-3′; antisense: 5′-GTG AAG ACG CCA GTG GAC TC-3′.
Amplified products were electrophoresed on 1·8% agarose gels and visualized by ethidium bromide staining. GAPDH was used to ascertain that an equivalent amount of cDNA was synthesized from different samples. The relative expression of cytokines or Lt-α (cytokines or Lt-α/GAPDH) was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech, San Leandro, CA).
Real-time PCR analysis
Real-time PCR analysis was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) as described earlier26,28 using forward and reverse primers and FAM-labelled probes (Applied Biosystems). The mRNA expression of Lt-α was normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence detection system 1.6 software (Applied Biosystems) and analysed by analysis of variance.
Construction of mouse Lt-α promoter-driven luciferase construct
Mouse genomic DNA isolated from mouse BV-2 microglial cells was used as template during PCR. The 5′-flanking sequence of mouse Lt-α (−1180/+561) gene was isolated by PCR. Primers were designed from GenBank sequences.
Lt-α: sense: 5′-acgcgt CCC TCT GTA CAG AGC ATT GGA AGC C-3′
antisense: 5′-agatct TGG AGA CGG CCG AGC AGT GTC ATG T-3′
The sense primer was tagged with MluI restriction enzyme while the antisense primer was tagged with BglII. The PCR was performed using an Advantage-2 PCR kit (Clontech) according to the manufacturer’s instructions. The resulting fragments were gel purified and ligated into the PGEM-TEasy vector (Promega, Madison, WI). These fragments were further subcloned into the PGL-3 Enhancer vector after digestion with corresponding restriction enzymes and verification by sequencing in the automated sequencer of the University of Nebraska at Lincoln Biotechnology Center.
Assay of Lt-α promoter-driven reporter activity
Cells plated at 50–60% confluence in 12-well plates were cotransfected with 0·25 μg of pLt-α-Luc and 25 ng of pRL-TK (a plasmid encoding Renilla luciferase, used as transfection efficiency control; Promega) using Lipofectamine Plus (Invitrogen, Carlsbad, CA). After 24 hr of transfection, cells were stimulated with different stimuli under serum-free conditions for 6 hr. Firefly and Renilla luciferase activities were analysed in cell extracts using the Dual Luciferase kit (Promega) in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) as described earlier.27,29 Relative luciferase activity of cell extracts was typically represented as the ratio of firefly luciferase value : Renilla luciferase value × 10−3.
Expression of mouse p35 and p40 cDNA in BV-2 microglial cells
Cells at 50–60% confluence were transfected with different amounts of p35 and p40 cDNA expression construct22 by LipofectAMINE Plus (Invitrogen) as described in several previous studies.22,29,30 Twenty-four hours after transfection, cells were incubated with serum-free media. After 6 hr of incubation, cells were harvested and RNA was analysed by semi-quantitative and real-time PCR.
Results
IL-12 p40 homodimer (p402), but not IL-12 p70, induces the expression of Lt-α in BV-2 microglial cells
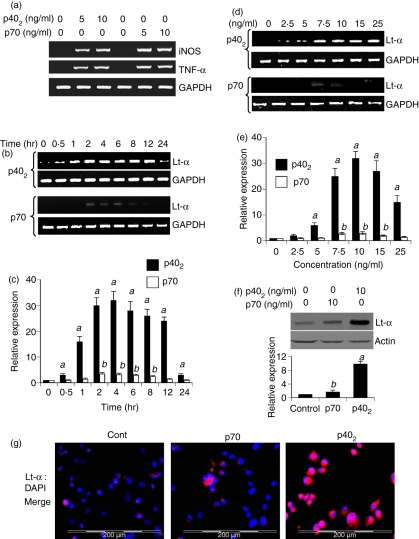
Consistent with our previous studies,21,22 both p402 and p70 induced the expression of iNOS and TNF-α in BV-2 microglial cells (Fig. 1a). Next to determine whether p402 and p70 play any role in the induction of Lt-α in microglia, we treated the mouse BV-2 microglial cells with 10 ng/ml p402 and p70 for different times. It is clearly evident from Fig. 1b that p402 markedly induced the mRNA expression of Lt-α in microglial cells at different times of stimulation. Although the mRNA level of Lt-α increased significantly within 30 min of stimulation, the maximum increase was observed within 2–4 hr of challenge followed by a gradual decrease (Fig. 1b). However, the expression of Lt-α sharply decreased at 24 hr of stimulation by p402 (Fig. 1b). On the other hand, p70 was either unable to induce or was a very weak inducer of Lt-α at different periods of stimulation (Fig. 1b). The real-time PCR results shown in Fig. 1(c) also confirm that p402 markedly induced the expression of Lt-α mRNA in BV-2 microglial cells at different stimulation times, exhibiting the maximum induction at 4 hr. In contrast, p70-mediated induction of Lt-α was very weak (Fig. 1c).
Figure 1.
Time- and dose-dependent induction of lymphotoxin-α (Lt-α) expression by interleukin-12 (IL-12) p40 homodimer (p402) and p70 in mouse BV-2 microglial cells. Cells were stimulated with different concentrations of p402 and p70 under serum-free conditions. After 6 hr of stimulation, total RNA was analysed for the expression of inducible nitric oxide synthase (iNOS) and tumour necrosis factor-α (TNF-α) by semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) (a). Cells were stimulated with p402 (10 ng/ml) and p70 (10 ng/ml) under serum-free conditions. At different time-points of stimulation, total RNA was analysed for the expression of Lt-α by semi-quantitative RT-PCR (b) and quantitative real-time PCR (c). Cells were stimulated with different concentrations of p402 and p70 under serum-free conditions. After 6 hr of stimulation, the messenger RNA expression of Lt-α was monitored by semi-quantitative RT-PCR (d) and quantitative real-time PCR (e). Results are means ± SD of three different experiments. aP< 0·001 versus control for p402; bP< 0·001 versus control for p70. (f) After 18 hr of stimulation, the expression of Lt-α protein was monitored by Western blot analysis. Actin was used as loading control. The relative expression of Lt-α (Lt-α/Actin) was measured after scanning the bands (lower panel). Results represent mean ± SD of three separate experiments. aP< 0·001 and bP< 0·05 versus control. (g) After 18 hr of stimulation, the level of Lt-α protein was monitored by immunofluorescence. DAPI was used to visualize nucleus. Results represent three independent experiments.
The dose-dependent studies illustrated in Fig. 1(d,e) also show that p402, but not p70, induced the expression of Lt-α mRNA in BV-2 microglial cells. Although the induction of Lt-α mRNA was very weak at a concentration of 2·5 ng/ml p402, the maximum increase was recorded at 10 ng/ml followed by a gradual decrease at a higher concentration of p402 (Fig. 1d,e). Similar to that observed during time-dependent studies (Fig. 1b,c), p70, at the different doses tested, was unable to induce the expression of Lt-α mRNA (Fig. 1d,e). Very weak induction of Lt-α mRNA was noted at a concentration of either 7·5 or 10 ng/ml of p70 (Fig. 1d,e). Next we examined the protein level of Lt-α by Western blot and immunofluorescence analysis. Western blot analysis (Fig. 1f; upper panel) and its quantification (Fig. 1f; lower panel) demonstrated that the protein level of LT-α was markedly increased by p402. Although p70-mediated induction of Lt-α was statistically significant, the magnitude of induction was much lower than that by p402 (Fig. 1f; lower panel). Similarly, the immunofluorescence analysis shown in Fig. 1(g) also confirmed the finding that p402, but not p70, was capable of inducing the expression of Lt-α protein in BV-2 microglial cells. Taken together, although both p402 and p70 were capable of inducing the expression of iNOS and TNF-α, only p402, but not p70, induced the expression of Lt-α in BV-2 microglial cells (Fig. 1).
IL-12p402, but not IL-12p70, induces the expression of Lt-α in primary mouse microglia
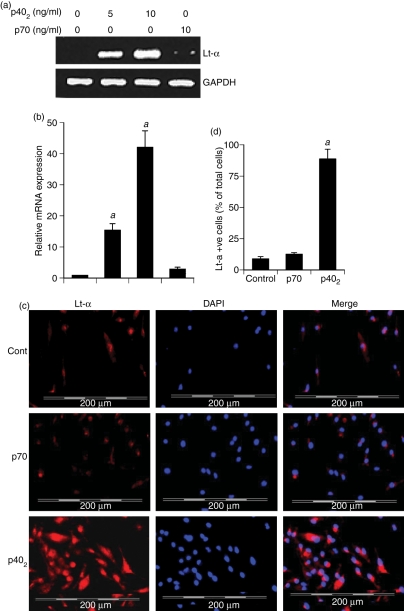
Next, to investigate whether p402 induces the expression of Lt-α in primary cells, primary microglia isolated from 7- to 9-day-old mouse pups, were stimulated with different concentrations of p402 and p70 for 6 hr and the expression of Lt-α was analysed. The semi-quantitative RT-PCR results in Fig. 2(a) clearly show that p402, at the different doses tested, markedly increased the mRNA expression of Lt-α whereas p70 was unable to stimulate the mRNA expression of Lt-α as compared with control. Immunofluorescence analysis in Fig. 3(a,d) also shows that p402 markedly increased the expression of Lt-α protein in primary microglia. On the other hand, p70 was unable to induce the level of Lt-α protein in microglia compared with untreated controls (Fig. 3a,d). These results show that p402, but not p70, is capable of inducing the expression of Lt-α in primary mouse microglia.
Figure 2.
Effect of p402 and p70 on the expression of lymphotoxin-α (Lt-α) messenger RNA in different cell types. Microglia isolated from 7- to 9-day-old mouse pups (a), microglia isolated from 13- to 17-week-old human fetal brains (b), mouse peritoneal macrophages (c), RAW 264.7 cells (d), and splenic T cells (e) were stimulated with different concentrations of p402 and p70 under serum-free condition. After 6 hr of stimulation, the messenger RNA expression of Lt-α was monitored by semi-quantitative reverse transcription–polymerase chain reaction. Results represent three independent experiments.
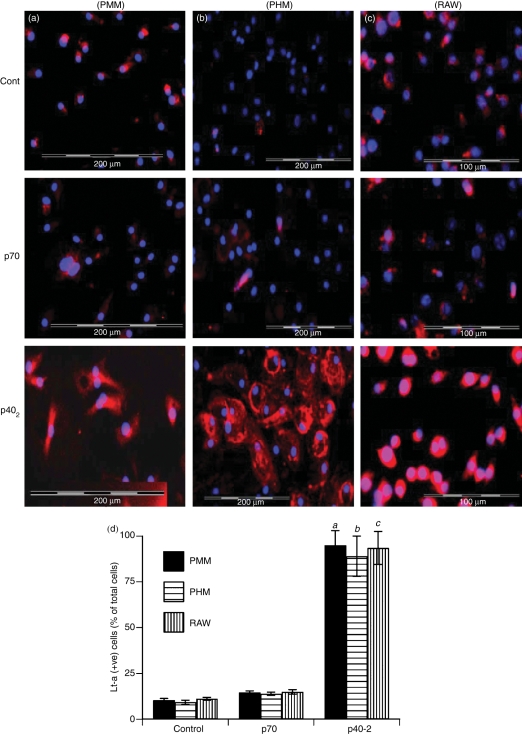
Figure 3.
Effect of p402 and p70 on the expression of lymphotoxin-α (Lt-α) protein in different cell types. Microglia (PMM) isolated from 7- to 9-day-old mouse pups (a), microglia (PHM) isolated from 13- to 17-week-old human fetal brains (b) and RAW 264.7 cells (c) were stimulated with 10 ng/ml p402 and p70 separately under the serum-free condition. After 18 hr of stimulation, the expression of Lt-α protein was monitored by immunofluorescence. DAPI was used to visualize the nucleus. Figures show merged images of DAPI and Lt-α. (d) Lt-α-positive cells were counted in three different slides of each of three different experiments in an Olympus IX81 fluorescence microscope using the microsuite imaging software and are expressed as % total DAPI-positive cells. aP < 0·001 versus control for PMM; bP< 0·001 versus control for PHM; cP < 0·001 versus control for RAW cells.
IL-12p402, but not IL-12p70, induces the expression of Lt-α in primary human microglia
Results from mouse cells are not often replicated in the human system. Therefore, next we examined the effects of p402 and p70 on the expression of Lt-α in primary human microglia. Because human recombinant p402 is not available commercially, microglia isolated from 13- to 17-week-old human fetal brains were stimulated with 10 ng/ml mouse recombinant p402 and p70 separately. As evident from the semi-quantitative RT-PCR results in Fig. 2(b), mouse p402 markedly increased the expression of Lt-α mRNA in human fetal microglia suggesting that mouse recombinant p402 was able to transduce signals in human microglia. On the other hand, under similar conditions, p70 was a very weak inducer of Lt-α (Fig. 2b). Next we examined the protein level of Lt-α by immunofluorescence analysis. Similar to that found in mouse microglia, Fig. 3(b,d) show that p402, but not p70, stimulated the expression of Lt-α protein in human microglia.
IL-12p402, but not IL-12p70, induces the expression of Lt-α in mouse macrophages and T cells
Results described above clearly indicate that p402, but not p70, induces the expression of Lt-α mRNA and protein in CNS microglia. In addition to controlling CNS inflammatory disease, Lt-α is also known to be involved in the development of several peripheral inflammatory and autoimmune disorders. Peripheral macrophages are also an important producer of Lt-α. Therefore, we investigated whether p402 was also capable of increasing the expression of Lt-α in macrophages. Mouse peritoneal macrophages and RAW 264.7 cells were treated with p402 and p70 for 6 hr under serum-free conditions and the expression of Lt-α mRNA was analysed by semi-quantitative RT-PCR. Similar to observations in microglia, the mRNA expression of Lt-α was stimulated by p402, but not p70, in mouse peritoneal macrophages (Fig. 2c) and RAW264.7 macrophages (Fig. 2d). Immunofluorescence analysis also demonstrates marked increase in Lt-α protein expression by p402, but not p70, in RAW264.7 macrophages (Fig. 3c,d).
Lymphotoxin-α was originally discovered from supernatants of T lymphocytes after either immunization by specific antigens or by stimulation with T-cell mitogens such as phytohaemagglutinin.31,32 In fact, T cells are considered as major producers of Lt-α.33,34 Therefore, we examined the effect of p402 and p70 on the expression of Lt-α in T cells. Splenic T cells were stimulated with different doses of p402 and p70 for 6 hr followed by analysis of Lt-α mRNA by semi-quantitative RT-PCR. It is clearly evident from Fig. 2(e) that p402 dose-dependently induced the expression of Lt-α in T cells. On the other hand, consistent with that observed in microglia and macrophages, very faint expression of Lt-α was observed in p70-stimulated T cells (Fig. 2e). Taken together, these results suggest that p402, but not p70, is capable of inducing the expression of Lt-α in various cell types.
Effect of different proinflammatory molecules on the activation of Lt-α promoter in BV-2 microglial cells
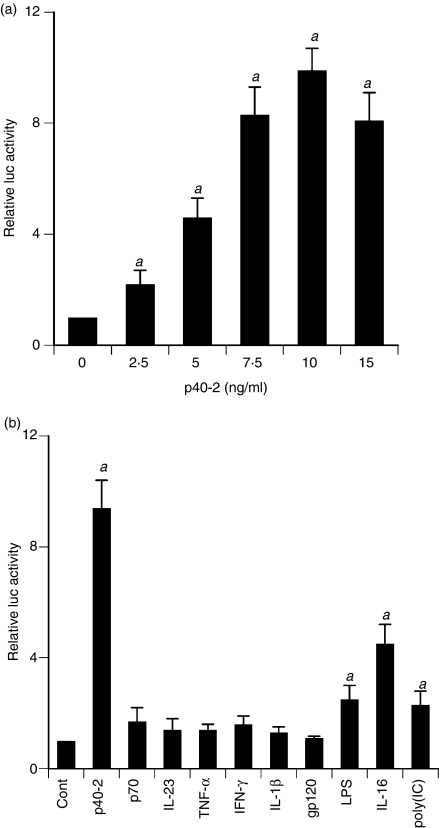
To understand the mechanism of p402-mediated stimulation of Lt-α mRNA, we examined the effect of p402 on the activation of Lt-α promoter in BV-2 microglial cells. Cells were transfected with the reporter plasmid PGL3-1641 containing Lt-α promoter fragment (−1180/+561) relative to the transcription start site. As evident from Fig. 4a, p402 dose-dependently increased Lt-α promoter-driven luciferase activity in microglial cells. Although p402 induced the activation of Lt-α promoter significantly at a concentration of 2·5 ng/ml, the activation of Lt-α promoter was maximum at a dose of 10 ng/ml p402 (Fig. 4a). Next we examined the effect of other proinflammatory stimuli on the activation of the Lt-α promoter. Similar to the regulation of Lt-α mRNA and protein, p70 did not induce Lt-α promoter-driven luciferase activity in microglial cells (Fig. 4b). Interleukin-23, another bioactive cytokine of the IL-12 family, also remained ineffective in inducing the activation of Lt-α promoter (Fig. 4b). Surprisingly, most common proinflammatory cytokines such as, TNF-α, IL-1β and IFN-γ were also unable to induce the activation of Lt-α promoter in microglial cells (Fig. 4b). HIV-1 gp120 is known to induce inflammatory reactions in many cell types including glial cells. However, this virotoxin (gp120) also had no effect on the activation of the Lt-α promoter in microglia (Fig. 4b). Double-stranded RNA (dsRNA), the active component of viral infection, is known to trigger many inflammatory processes. Out of various proinflammatory stimuli tested, only p402, lipopolysaccharide (LPS), IL-16 and double-stranded RNA [poly (IC)] were capable of inducing Lt-α promoter-driven luciferase activity (Fig. 4b). Incidentally, p402 has been found to be the strongest inducer of Lt-α promoter activation in microglia (Fig. 4b).
Figure 4.
Effect of different proinflammatory molecules on lymphotoxin-α (Lt-α) promoter-driven luciferase activity in mouse BV-2 microglial cells. Cells plated at 50–60% confluence in 12-well plates were transfected with 0·25 μg pLt-α-Luc and 25 ng pRL-TK (Renilla luciferase control) by Lipofectamine Plus (Invitrogen) as described is Materials and methods. Twenty-four hours after transfection, cells were stimulated with different concentrations of p402 (a) or with different stimuli (b) for 6 hr under serum-free conditions. Firefly and Renilla luciferase activities were determined using a Dual Luciferase Kit (Promega) following the manufacturer’s protocol. Data are mean ± SD of three separate experiments. aP< 0·001 versus control. Concentrations of different stimuli are as follows: p402, 10 ng/ml; lipopolysaccharide (LPS), 1 μg/ml; p70, 10 ng/ml; interleukin-23 (IL-23), 10 ng/ml; tumour necrosis factor-α (TNF-α), 20 ng/ml; interferon-γ (IFN-γ), 12·5 mU/ml; gp120, 200 pg/ml; IL-16, 10 ng/ml; poly(IC), 100 μg/ml; IL-1β, 10 ng/ml.
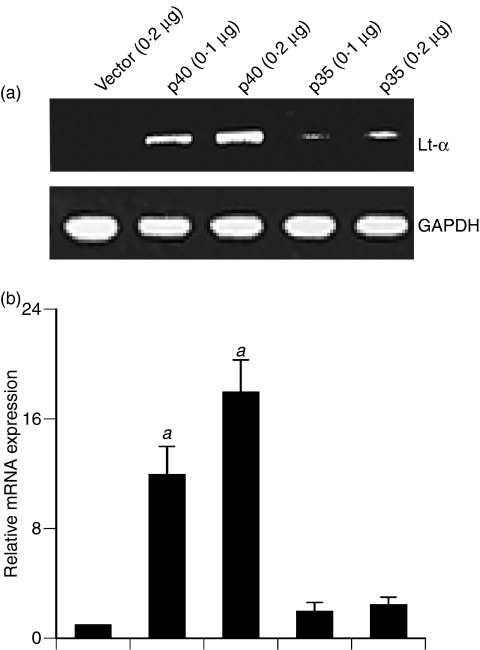
Expression of mouse p40 cDNA induces the expression of Lt-α in BV-2 microglial cells
We used recombinant mouse p402 for the stimulation of microglia and macrophages. It is possible that any contaminant in p402 preparation, but not p402 itself, is actually inducing the expression of Lt-α. To investigate the validity of this possibility, we examined the effect of transient expression of mouse p40 and p35 cDNA22 on the expression of Lt-α in BV-2 microglial cells. As evident from semi-quantitative RT-PCR analysis in Fig. 5(a) and quantitative real-time PCR analysis in Fig. 5(b), expression of p40 cDNA, but of neither p35 nor an empty vector, induced the expression of Lt-α mRNA. These results confirm that p40, but not p35, is responsible for the induction of Lt-α mRNA in microglia and that the induction of Lt-α by p402 is real, not the result of any contamination.
Figure 5.
Expression of p40, but not p35, complementary DNA (cDNA) induces the expression of lymphotoxin-α (Lt-α) in BV2 microglial cells: Microglial cells plated in 12-well plates were transfected with different amounts of either p40 or p35 cDNA by LipofectAMINE Plus (Invitrogen). Empty vector (pCIneo mammalian expression vector from Promega) was used as control. After 24 hr of transfection, cells were incubated in serum-free media. After 6 hr, the messenger RNA expression of Lt-α was monitored by semi-quantitative reverse transcription–polymerase chain reaction (PCR) (a) and quantitative real-time PCR (b). Results are means ± SD of three different experiments. aP< 0·001 versus control.
IL-12 p402 induces the expression of Lt-α in mouse primary microglia independent of p35
It is known that biologically active p70 is comprised of 35 000 molecular weight (p35) and 40 000 (p40) subunits.18 Therefore, one may envision that p40 of p402 may interact with p35 of microglia and that the resulting molecule is involved in the induction of Lt-α. To sign off on this possibility, we examined the effect of mouse p402 on the induction of Lt-α in primary microglia isolated from p35−/− mice. Cells were treated with p402 and p70 for 6 hr and mRNA expression was analysed both by semi-quantitative RT-PCR and real-time PCR. As expected, similar to observations in wild-type microglia, p70 remained ineffective in inducing the expression of Lt-α mRNA (Fig. 6a,b) and protein (Fig. 6c,d) expression in p35−/− microglia. On the other hand, marked increase in Lt-α mRNA (Fig. 6a,b) and protein (Fig. 6c,d) expression by p402 in p35−/− microglia suggest that p402 does not need any co-operation from p35 for the induction of Lt-α in microglia.
Figure 6.
Effect of p402 and p70 on the expression of lymphotoxin-α (Lt-α) in p35−/− microglia. Microglia isolated from wild-type and p35−/− mice were stimulated with different concentrations of p402 under serum-free conditions. After 6 hr of stimulation, the messenger RNA expression of Lt-α was monitored by reverse transcription–polymerase chain reaction (PCR) (a) and real-time PCR (b). Results are means ± SD of three different experiments. aP< 0·001 versus control. After 18 hr of stimulation, cells were immunostained with antibodies against Lt-α. DAPI was used to visualize nucleus (c). Lt-α-positive cells were counted in three different slides of each of three different experiments in an Olympus IX81 fluorescence microscope using the microsuite imaging software and are expressed as % total DAPI-positive cells (d). aP< 0·001 versus control.
IL-12 p402 induces the expression of Lt-α via IL-12Rβ1 but not IL-12Rβ2
Next we investigated mechanisms by which p402 induces the expression of Lt-α. Previous studies have demonstrated that p402 binds to IL-12Rβ1 whereas p70 interacts with both IL-12Rβ1 and IL-12Rβ2 in T cells,18 and microglia/macrophages express both IL-12Rβ1 and IL-12Rβ2.35 However, it is not known if p70 and p402 are utilizing any of these two receptors to induce the expression of Lt-α in microglia and macrophages.
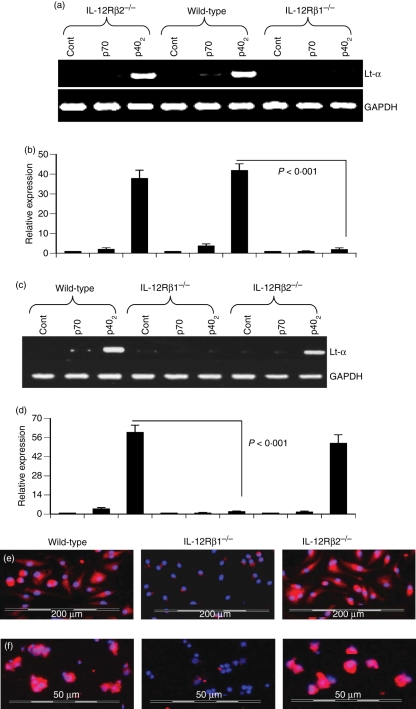
Therefore, primary microglia and peritoneal macrophages isolated from wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice were challenged with p70 and p402. The mRNA expression of Lt-α mRNA expression was examined by semi-quantitative RT-PCR and quantitative real-time PCR. As expected, p402, but not p70, induced the expression of Lt-α mRNA in microglia (Fig. 7a,b) and macrophages (Fig. 7c,d) isolated from wild-type mice. However, p402 was unable to induce the expression of Lt-α mRNA in microglia (Fig. 7a,b) and macrophages (Fig. 7c,d) isolated from IL-12Rβ1−/− mice. In contrast, p402 induced the expression of Lt-α mRNA in microglia (Fig. 7a,b) and macrophages (Fig. 7c,d) isolated from IL-12Rβ2−/− mice. To confirm these findings, we monitored the protein expression of Lt-α by immunofluorescence analysis. Consistent with mRNA results, p402 induced the expression of Lt-α protein in macrophages (Fig. 7e) and microglia (Fig. 7f) isolated from wild-type and IL-12Rβ2−/− mice, but not IL-12Rβ1−/− mice. These results clearly suggest that p402 requires IL-12Rβ1, but not IL-12Rβ2, for the expression of Lt-α. On the other hand, p70 was unable to induce the expression of Lt-α in microglia (Fig. 7a,b) and macrophages (Fig. 7c,d) isolated from wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice.
Figure 7.
Effect of p402 and p70 on the expression of lymphotoxin-α (Lt-α) in primary microglia and peritoneal macrophages isolated from wild-type, interleukin-12 receptor β1 deficient (IL-12Rβ1−/−) and IL-12Rβ2−/− mice: Microglia (a and b) and macrophages (c and d) isolated from B6.129 wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice were stimulated with 10 ng/ml of p402 and p70 under serum-free conditions. After 6 hr of stimulation, the messenger RNA expression of Lt-α was monitored by semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) (a and c) and quantitative real-time PCR (b and d). Results are means ± SD of three different experiments. Microglia (e) and macrophages (f) isolated from B6.129 wild-type, IL-12 Rβ1−/− and IL-12Rβ2−/− mice were stimulated with 10 ng/ml of p402 and p70 under serum-free conditions. After 18 hr of stimulation, cells were immunostained with antibodies against Lt-α. DAPI was used to visualize the nucleus. Figures show merged images of DAPI and Lt-α. Results represent three independent experiments.
Discussion
Several reports indicate that Lt-α plays an important role during chronic inflammation in MS. It has been shown that plaques and cerebrospinal fluid from MS patients contain increased levels of LT-α mRNA and protein compared with age-matched controls.36 Suen et al.4 have demonstrated that LT-α−/− mice, but not LT-β−/− mice, exhibit a significant reduction in symptoms upon myelin oligodendrocytes in glycoprotein-induced EAE. In another study, Plant et al.36 have shown that LT-α is detrimental during inflammation and demyelination and that Lt-α is not necessary for remyelination and repair. On the other hand, TNF-α is involved both in demyelination and remyelination processes.36 It has also been reported that mice lacking LT-α exhibit a delay in demyelination that is greater than that exhibited by TNF-α−/− mice.36 Both Lt-α and TNF-α may exert cytotoxic effects on oligodendrocytes through their receptors and cause the death of oligodendrocytes. Lymphotoxin-α is known to be extremely cytotoxic to oligodendrocytes in vitro.37,38 Mature oligodendrocytes express TNFR1 and possibly LtβR and are directly effected by LT-α.36 Taken together, LT-α plays a critical role in the development of EAE and MS.
Lt-α is mainly produced by T cells, B cells and natural killer cells.39 Additionally, antigen-presenting cells such as, macrophages and microglia, also express Lt-α. Several reports have indicated that only resident activated microglia in the CNS express LT-α.40–43 Although LPS is capable of stimulating Lt-α, it is not involved in EAE and MS. Proinflammatory cytokines (TNF-α, IL-1β and IFN-γ) which play an important role in EAE and MS do not induce Lt-α.12 Therefore, stimuli that could produce Lt-α in immune cells in MS and EAE are not well characterized and identifying such stimuli and understanding underlying mechanisms for the regulation of Lt-α are important areas of investigation. The IL-12 family of cytokines consists of six cytokines: IL-12, IL-12p40 monomer, IL-12p40 homodimer (p402), IL-23, IL-27 and IL-35.44–46 Although p40 is a component of bioactive cytokines IL-12 and IL-23, either p40 or p402 is not considered as an intrinsically functional cytokine. Earlier we have demonstrated that both p402, the so-called inactive molecule, and IL-12p70, the biologically active cytokine, are capable of inducing the expression of iNOS and TNF-α in microglia and macrophages.21,22 Several lines of evidence presented in this study clearly support the conclusion that p402, but not p70, induces the expression of Lt-α in microglia, macrophages and T cells. This conclusion was based on the following observations. First, p402 markedly induced the expression of Lt-α mRNA and protein in human and mouse microglia and mouse macrophages and T cells. On the other hand, under similar treatment conditions, p70 was either unable to induce the expression of Lt-α or was a very weak inducer of Lt-α. Second, p402 markedly induced the activation of Lt-α promoter as monitored by Lt-α promoter-driven luciferase activity. On the other hand, common inflammatory stimuli (TNF-α, IL-1β, IFN-γ, IL-12 p70, IL-23 and HIV-1 gp120) were unable to induce the activation of Lt-α promoter. Apart from p402, only three other stimuli [LPS, poly(IC) and IL-16] were capable of stimulating the activation of Lt-α promoter. However, among all inducers tested, p402 emerged as the most potent. Third, the expression of mouse p40, but not mouse p35, cDNA induced the expression of Lt-α, suggesting that the p40, but not the p35, subunit of IL-12 is involved in the induction of Lt-α in microglia. Fourth, p402 was capable of inducing the expression of Lt-α in microglia isolated from p35−/− mice, suggesting that p402 does not need any involvement from p35 for the induction of Lt-α in microglia.
Lymphotoxin-α plays a critical role in lymphoid organ development and chronic inflammation.8 For example, signalling through the Lt-β receptor (LtβR) by the Ltα/β heterotrimer is critical during lymphoid development. It has been demonstrated that Ltα−/− and Ltβr−/− mice have associated loss of lymph nodes, Peyer’s patches and changes of the lymphoid architecture of the spleen.47,48 A number of human inflammatory and autoimmune disorders are associated with the formation of ectopic lymphoid structures at the site of the inflamed organ that actually resemble secondary lymphoid organs.8 It has been suggested that Lt-α plays a crucial role in the formation of this type of secondary lymphoid organs. It is likely that immune responses to self antigens expand in these de novo lymphoid organs, as they allow colocalization of antigen-specific T cells with antigen-presenting cells.8,48 Therefore, our results suggest that p402 may facilitate the formation of secondary lymphoid organs during autoimmune disorders by increasing the production of Lt-α in microglia, macrophages and T cells.
Intracellular signalling events that lead to the expression of Lt-α have been poorly defined. IL-12 p402 has been shown to antagonize bioactive IL-12 p70 by binding to the IL-12 receptor complex.18 The high-affinity IL-12 receptor is composed of a low affinity IL-12Rβ1 combined with a low-affinity IL-12Rβ2. It appears that p402 binds to IL-12Rβ1 rather than IL-12Rβ2 whereas bioactive IL-12 binds both the receptors on T cells with high affinity.18 Therefore, it is possible that p402 may engage IL-12 receptors for the expression of Lt-α in microglia and macrophages. Here we present evidence that p402 induces the expression of Lt-α via IL-12Rβ1, but not IL-12Rβ2. While p402 was unable to induce the expression of Lt-α in primary microglia and macrophages isolated from IL-12Rβ1−/− mice, IL-12Rβ2 knockdown had no effect on p402-induced expression of Lt-α in these cells. This is an interesting observation because only the IL-12Rβ2 subunit has been reported to function as the signal transducing component of the high-affinity receptor complex.18 This has been suggested because of the absence of tyrosine residues in the cytoplasmic domain of IL-12Rβ1 and the presence of conserved tyrosine residues in the cytoplasmic domain of IL-12Rβ2.18 Therefore, the question remains: how does the non signal-transducing receptor IL-12Rβ1 engage itself for the transcription of Lt-α by p402? Probably, one could foresee the engagement of another putative signal-transducing receptor with the binding receptor IL-12Rβ1. In addition to having IL-12 and IL-23, the IL-12 family comprises of IL-27 and a recently discovered heterodimeric cytokine IL-35.49,50 While Epstein–Barr virus-induced gene 3 (EBI3) associates with a p28 protein to form IL-27, EBI3 has been shown to associate with IL-12p35 to form IL-35.49,50 Therefore, during p402 stimulation, IL-12Rβ1 may associate itself with IL-23R, IL-27R (WSX-1), putative IL-35R, or the putative p402 receptor. Studies are underway in this laboratory to investigate these possibilities.
Studies on the characterization of the Lt-α gene promoter suggest a general role for nuclear factor-κB (NF-κB) in the transcriptional upregulation of Lt-α in T cells.51–53 In contrast, the combination of phorbol myristate acetate and ionomycin markedly induced the expression of Lt-α mRNA in human peripheral blood mononuclear cells independent of NF-κB activation.54 However, in peripheral blood mononuclear cells, nuclear factor of activated T cells (NFAT) plays an important role in transcriptional upregulation of the Lt-α gene.54 Besides NF-κB and NFAT, several other functional elements have also been identified in LT-α promoter in T cells.52,55,56 Earlier Pahan et al.22 demonstrated the requirement of NF-κB activation in p402-induced expression of iNOS in microglia. Recently, we have also observed that p402 induces the activation of NF-κB in mouse microglia via IL-12Rβ1, but not IL-12Rβ2 (Jana and Pahan, unpublished observation). Therefore, it is possible that p402 is inducing the activation of NF-κB and NFAT in microglia/macrophages and/or T cells via IL-12Rβ1. However, at present, we do not know why p70, which is capable of binding both IL-12Rβ1 and IL-12Rβ2, does not induce Lt-α? We wondered if IL-12Rβ2 was transducing a negative regulatory signal for the expression of LT-α. However, p70 remained ineffective in inducing the expression of Lt-α in IL-12Rβ2−/− microglia shooting down our hypothesis. Therefore, it is likely that besides the involvement of IL-12Rβ1 in the expression of Lt-α, the engagement of both IL-12Rβ1 and IL-12 Rβ2 by p70 transduces putative negative regulatory signal(s) for the expression of LT-α.
Microglia are considered as CNS-resident professional macrophages and sensor cells that function as the principal immune effectors cells of the CNS responding to any pathological event. Activation of microglia has been implicated in the pathogenesis of a variety of neurodegenerative diseases, including MS.57,58 It has been found that activated microglia accumulate at sites of demyelination in MS patients. Although the in vitro situation of mouse and human microglia and mouse macrophages in culture and its treatment with p402 may not truly resemble the in vivo situation of microglia and macrophages in patients with MS, our results identify p402 as a possible candidate for controlling the lymphoid organ-developing factor Lt-α.
Acknowledgments
This study was supported by grants from National Institutes of Health (NS39940 and NS48923) and National Multiple Sclerosis Society (RG3422A1/1).
References
- 1.Crowe PD, VanArsdale TL, Walter BN, et al. A lymphotoxin-beta-specific receptor. Science. 1994;264:707–10. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 2.Cannella B, Sizing ID, Benjamin CD, Browning JL, Raine CS. Antibodies to lymphotoxin alpha (LT alpha) and LT beta recognize different glial cell types in the central nervous system. J Neuroimmunol. 1997;78:172–9. doi: 10.1016/s0165-5728(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 3.Matusevicius D, Navikas V, Soderstrom M, Xiao BG, Haglund M, Fredrikson S, Link H. Multiple sclerosis: the proinflammatory cytokines lymphotoxin-alpha and tumour necrosis factor-alpha are upregulated in cerebrospinal fluid mononuclear cells. J Neuroimmunol. 1996;66:115–23. doi: 10.1016/0165-5728(96)00032-x. [DOI] [PubMed] [Google Scholar]
- 4.Suen WE, Bergman CM, Hjelmstrom P, Ruddle NH. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997;186:1233–40. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navikas V, He B, Link J, et al. Augmented expression of tumour necrosis factor-alpha and lymphotoxin in mononuclear cells in multiple sclerosis and optic neuritis. Brain. 1996;119(Pt 1):213–23. doi: 10.1093/brain/119.1.213. [DOI] [PubMed] [Google Scholar]
- 6.Gommerman JL, Giza K, Perper S, Sizing I, Ngam-Ek A, Nickerson-Nutter C, Browning JL. A role for surface lymphotoxin in experimental autoimmune encephalomyelitis independent of LIGHT. J Clin Invest. 2003;112:755–67. doi: 10.1172/JCI18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raine CS, Bonetti B, Cannella B. Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev Neurol (Paris) 1998;154:577–85. [PubMed] [Google Scholar]
- 8.Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–25. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- 9.Goluszko E, Hjelmstrom P, Deng C, Poussin MA, Ruddle NH, Christadoss P. Lymphotoxin-alpha deficiency completely protects C57BL/6 mice from developing clinical experimental autoimmune myasthenia gravis. J Neuroimmunol. 2001;113:109–18. doi: 10.1016/s0165-5728(00)00420-3. [DOI] [PubMed] [Google Scholar]
- 10.Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–7. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193:239–46. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinetti M, Agyekum S, Evans T, Polak J, Cohen J. The role of lipopolysaccharide, pro-inflammatory cytokines and bacterial superantigens in the transcriptional regulation of lymphotoxin alpha and beta in mouse splenocytes. Cytokine. 1998;10:940–7. doi: 10.1006/cyto.1998.0386. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (New York, NY) 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 14.Constantinescu CS, Goodman DB, Hilliard B, Wysocka M, Cohen JA. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci Lett. 2000;287:171–4. doi: 10.1016/s0304-3940(00)01184-8. [DOI] [PubMed] [Google Scholar]
- 15.Zipris D, Greiner DL, Malkani S, Whalen B, Mordes JP, Rossini AA. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156:1315–21. [PubMed] [Google Scholar]
- 16.Schoenhaut DS, Chua AO, Wolitzky AG, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–40. [PubMed] [Google Scholar]
- 17.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 18.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, Hennerici M. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51:753–8. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- 20.Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 21.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–28. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–78. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jana M, Jana A, Pal U, Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem Res. 2007;32:2015–22. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–93. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–31. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–64. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–52. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J Biol Chem. 2002;277:45984–91. doi: 10.1074/jbc.M200250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–33. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieur AM, Pham Huu T. Lymphotoxin and chemotactic factor produced in vitro by human lymphocytes during their proliferative response in the presence of phytohaemagglutinin and Nocardia water-soluble mitogen. Ann Immunol (Paris) 1980;131D:223–32. [PubMed] [Google Scholar]
- 32.Yano K, Lucas ZJ. Cytotoxic activity of lymphocytes. VII. Cellular origin of alpha-lymphotoxin. J Immunol. 1978;120:385–94. [PubMed] [Google Scholar]
- 33.Chaplin DD, Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–97. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 34.Ohshima Y, Yang LP, Avice MN, et al. Naive human CD4+ T cells are a major source of lymphotoxin alpha. J Immunol. 1999;162:3790–4. [PubMed] [Google Scholar]
- 35.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 36.Plant SR, Arnett HA, Ting JP. Astroglial-derived lymphotoxin-alpha exacerbates inflammation and demyelination, but not remyelination. Glia. 2005;49:1–14. doi: 10.1002/glia.20089. [DOI] [PubMed] [Google Scholar]
- 37.Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol. 1991;147:1522–9. [PubMed] [Google Scholar]
- 38.Plo I, Ghandour S, Feutz AC, Clanet M, Laurent G, Bettaieb A. Involvement of de novo ceramide biosynthesis in lymphotoxin-induced oligodendrocyte death. Neuroreport. 1999;10:2373–6. doi: 10.1097/00001756-199908020-00028. [DOI] [PubMed] [Google Scholar]
- 39.Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–8. [PubMed] [Google Scholar]
- 40.Yoshida N, Arima M, Cheng G, Eda F, Hirata H, Honda K, Fukushima F, Fukuda T. Interleukin (IL)-4/IL-9 and exogenous IL-16 induce IL-16 production by BEAS-2B cells, a bronchial epithelial cell line. Cell Immunol. 2001;207:75–80. doi: 10.1006/cimm.2000.1745. [DOI] [PubMed] [Google Scholar]
- 41.Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner HD, Meyermann R. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000;100:313–22. doi: 10.1007/s004019900172. [DOI] [PubMed] [Google Scholar]
- 42.Schwab JM, Nguyen TD, Meyermann R, Schluesener HJ. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes, CD8+ T-lymphocytes and activated microglia/macrophages. J Neuroimmunol. 2001;114:232–41. doi: 10.1016/s0165-5728(00)00433-1. [DOI] [PubMed] [Google Scholar]
- 43.Schwab JM, Schluesener HJ, Seid K, Meyermann R. IL-16 is differentially expressed in the developing human fetal brain by microglial cells in zones of neuropoiesis. Int J Dev Neurosci. 2001;19:93–100. doi: 10.1016/s0736-5748(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 45.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–12. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 48.Spahn TW, Muller MK, Domschke W, Kucharzik T. Role of lymphotoxins in the development of Peyer’s patches and mesenteric lymph nodes: relevance to intestinal inflammation and treatment. Ann N Y Acad Sci. 2006;1072:187–93. doi: 10.1196/annals.1326.029. [DOI] [PubMed] [Google Scholar]
- 49.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 50.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 51.Messer G, Weiss EH, Baeuerle PA. Tumor necrosis factor beta (TNF-beta) induces binding of the NF-kappa B transcription factor to a high-affinity kappa B element in the TNF-beta promoter. Cytokine. 1990;2:389–97. doi: 10.1016/1043-4666(90)90046-v. [DOI] [PubMed] [Google Scholar]
- 52.Paul NL, Lenardo MJ, Novak KD, Sarr T, Tang WL, Ruddle NH. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990;64:5412–9. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tschachler E, Bohnlein E, Felzmann S, Reitz MS., Jr Human T-lymphotropic virus type I tax regulates the expression of the human lymphotoxin gene. Blood. 1993;81:95–100. [PubMed] [Google Scholar]
- 54.Kuprash DV, Boitchenko VE, Yarovinsky FO, Rice NR, Nordheim A, Ruhlmann A, Nedospasov SA. Cyclosporin A blocks the expression of lymphotoxin alpha, but not lymphotoxin beta, in human peripheral blood mononuclear cells. Blood. 2002;100:1721–7. [PubMed] [Google Scholar]
- 55.Suttles J, Miller RW, Tao X, Stout RD. T cells which do not express membrane tumor necrosis factor-alpha activate macrophage effector function by cell contact-dependent signaling of macrophage tumor necrosis factor-alpha production. Eur J Immunol. 1994;24:1736–42. doi: 10.1002/eji.1830240803. [DOI] [PubMed] [Google Scholar]
- 56.Worm MM, Tsytsykova A, Geha RS. CD40 ligation and IL-4 use different mechanisms of transcriptional activation of the human lymphotoxin alpha promoter in B cells. Eur J Immunol. 1998;28:901–6. doi: 10.1002/(SICI)1521-4141(199803)28:03<901::AID-IMMU901>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–40. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 58.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]