Abstract
Cardioplegia is used to partially alleviate the effects of surgically induced global ischemia injury; however, the molecular mechanisms involved in this cardioprotection remain to be elucidated. To improve the understanding of the molecular processes modulating the effects of global ischemia and the cardioprotection afforded by cardioplegia, we constructed rabbit heart cDNA libraries and isolated, sequenced, and identified a compendium of nonredundant cDNAs for use in transcriptomic and proteomic analyses. New Zealand White rabbits were used to compare the effects of global ischemia and cardioplegia compared with control (nonischemic) hearts. The effects of RNA and protein synthesis on the cardioprotection afforded by cardioplegia were investigated separately by preperfusion with either α-amanitin or cycloheximide. Our results demonstrate that cardioplegia partially ameliorates the effects of global ischemia and that the cardioprotection is modulated by RNA- and protein-dependent mechanisms. Transcriptomic and proteomic enrichment analyses indicated that global ischemia downregulated genes/proteins associated with mitochondrial function and energy production, cofactor catabolism, and the generation of precursor metabolites of energy. In contrast, cardioplegia significantly increased differentially expressed genes/proteins associated with the mitochondrion and mitochondrial function and significantly upregulated the biological processes of muscle contraction, involuntary muscle contraction, carboxylic acid and fatty acid catabolic processes, fatty acid β-oxidation, and fatty acid metabolic processes.
Keywords: mitochondrion, ischemia-reperfusion
myocardial ischemia-reperfusion injury occurs as the result of the attenuation or cessation of coronary blood flow such that oxygen delivery to the myocardium is insufficient to meet energy demands. The cessation of myocardial coronary blood flow induces a cascade of cellular events that rapidly alter myocardial cellular homeostasis, leading to cellular dysfunction and/or death and postischemic functional impairment (23).
To alleviate the effects of surgically induced ischemia/reperfusion injury, surgeons use cardioplegia (CP) solutions that allow for the rapid electromechanical arrest of the myocardium through the alteration of cellular electrochemical gradients (40). In a series of studies, we have shown that magnesium-supplemented potassium CP with the addition of diazoxide significantly decreases myocardial cell death and significantly enhances postischemic functional recovery (22, 40, 43).
The mechanisms through which CP affords cardioprotection are complex and cannot be investigated as a single entity; rather, they must be investigated as a system. In previous reports, we and others have used a variety of methods to identify the RNAs and proteins associated with global ischemia (GI) and with cardioprotection (11, 20, 21, 33, 47). These studies, while providing valuable information, used a targeted approach involving one or a few genes. The mostly static data garnered by these studies, while providing valuable information, did not allow for the interactive analysis of collateral, cognate gene and protein expression or the identification of relevant processes and pathways involved in GI and in cardioprotection. To improve the understanding of the molecular processes modulating the effects of GI and the cardioprotection afforded by CP, we constructed rabbit heart cDNA libraries and isolated, sequenced, and identified a compendium of nonredundant cDNAs for use in microarray analysis.
In this report, we used microarray analysis and high-throughput matrix-assisted laser desorption/ionization and ion trap time of flight (MALDI TOF/TOF) proteomic analysis for the parallel determination of relative abundance levels of the multiple transcriptomic/proteomic products associated with GI and with the cardioprotection afforded by CP. The use of these two technologies provided results that allowed for the identification of associated processes and pathways that will allow for the enhancement of current therapeutic interventions.
METHODS
Animals.
New Zealand White rabbits (n = 93, 15–20 wk, 3–4 kg) were obtained from Millbrook Farm (Amherst, MA). All experiments were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Committee and the Harvard Medical Area Standing Committee on Animals and conformed with National Institutes of Health guidelines regulating the care and use of laboratory animals (NIH Pub. No. 5377-3, 1996). All research was performed in accordance with the American physiological Society's “Guiding Principles in the Care and Use of Animals.”
Langendorff perfusion.
All rabbits were sedated with acepromazine (0.5 mg/kg im) and anesthetized with pentobarbital (50 mg/kg iv) and received heparin (200 U/kg iv) via a marginal ear vein. Langendorff retrograde perfusion was performed as previously described (12). Hearts were paced at 180 ± 3 beats/min throughout the experiment (model 5330 stimulator, Medtronic, Minneapolis, MN). Hemodynamic variables were acquired using the PO-NE-MAH digital data-acquisition system with an Acquire Plus processor board and left ventricular (LV) pressure analysis software (Gould, Valley View, OH) (23).
Myocardial function.
Myocardial function was assessed by sonomicrometry (Sonomicrometrics Digital Ultrasonic Measurement System, Sonometrics, London, ON, Canada) using five digital piezoelectric ultrasonic probes (1 mm). The probes were implanted in the subepicardial layer ∼7–10 mm apart in the LV, with one probe placed on the major axis of the heart perpendicular to the four probes placed parallel to the minor axis of the heart (including anterior and posterior positions), and secured to the epicardium with Z-suture using polypropylene stitches (5-0 Prolene, 8580H, Ethicon, Somerville, NJ). The probes were left in place until the end of the experiment. LV function was assessed as segment shortening (SS) (23). Time course changes in SS are expressed as a percentage of equilibrium values to minimize variability among individual animals (23).
Experimental protocol.
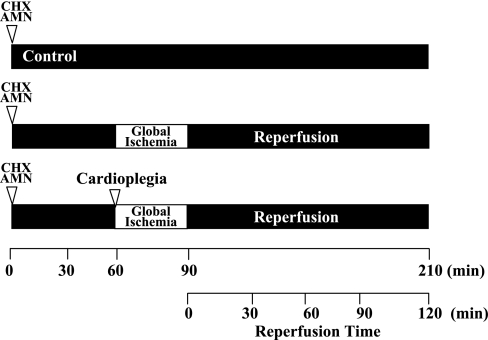
The experimental protocol is shown in Fig. 1. Control hearts (n = 6) were perfused with of Krebs-Ringer solution (containing 100 mM NaCl, 4.7 mM KCl, 1.1 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 1.7 mM CaCl2, 11.5 mM glucose, 4.9 mM pyruvic acid, and 5.4 mM fumaric acid) equilibrated with 95% O2 and 5% CO2 (pH 7.4 at 37°C) at 37°C for 210 min (12). GI hearts (n = 6) were perfused for 60 min and then subjected to 30 min of GI and 120 min of reperfusion. GI was achieved by cross-clamping the perfusion line. Reperfusion was achieved by release of the cross-clamp. CP hearts (n = 6) received normothermic (37°C) K+/Mg2+ CP solution (20 mmol/l K+ and 20 mmol/l Mg2+ containing 50 μM diazoxide in Krebs-Ringer solution) and were perfused for 5 min before GI (40). Diazoxide was dissolved in DMSO (Fisher Scientific, Fair Lawn, NJ) before being added into the Krebs-Ringer solution. The final concentration of DMSO was <0.1%. DMSO was added to control and GI hearts at the same concentration.
Fig. 1.
Experimental protocol. All experiments consisted of 60 min of equilibrium followed by 30 min of global ischemia (GI) and 120 min of reperfusion. Control hearts were perfused without GI at 37°C for 210 min. GI hearts were subjected to 30 min of GI and 120 min of reperfusion. Cardioplegia (CP) hearts received magnesium-supplemented potassium CP solution containing diazoxide (50 μM) for 5 min before GI and 120 min of reperfusion. To determine the role of RNA or protein synthesis in the cardioprotection afforded by CP, a separate group of control and CP hearts was preperfused for 55 min with Krebs-Ringer solution containing either α-amanitin (AMN; 2.5 μg/ml) to inhibit RNA synthesis or cycloheximide (CHX; 50 μg/ml) to inhibit protein synthesis. Control + AMN and control + CHX hearts were then perfused for a further 155 min. CP + AMN and CP + CHX hearts received CP for 5 min before 30 min of GI and 120 min of reperfusion.
Inhibition of RNA and protein synthesis.
To determine the role of RNA synthesis and protein synthesis in the cardioprotection afforded by CP, separate groups of hearts were preperfused for 55 min with Krebs-Ringer solution containing either the RNA polymerase II inhibitor α-amanitin (AMN; 2.5 μg/ml, Sigma Chemical, St. Louis, MO, n = 6 each for control and CP) or with Krebs-Ringer solution containing cycloheximide (CHX; a protein synthesis inhibitor, 50 μg/ml, Sigma Chemical, n = 6 each for control and CP). Control hearts (control + AMN and control + CHX) were then perfused for 155 min. CP hearts (CP + AMN and CP + CHX) received CP for 5 min before 30 min of GI and 120 min of reperfusion (16, 18, 45). Control hearts were preperfused for 55 min with Krebs-Ringer solution containing either AMN or CHX and then were perfused for 150 min with Krebs-Ringer solution alone (n = 4 each). The concentrations of AMN and CHX were determined by preliminary investigation and were required to have no effect on control (nonischemic) hearts but to reduce postischemic functional recovery and increase infarct size in CP hearts. Separate hearts (control + AMN, n = 4; control + CHX, n = 4; CP + AMN, n = 4; and CP + CHX, n = 4) were used to show that AMN decreased the myocardial cell nuclear RNA-to-DNA ratio (results not shown). Functional data were analyzed using ANOVA for repeated measures, and Dunnett's test was used for comparisons between groups.
Measurement of infarct size.
Infarct sizes were determined using 1% triphenyltetrazolium chloride (Sigma Chemical) as previously described (23). The determination of wet weight-to-dry weight ratios was performed as previously described (23).
cDNA library.
cDNA libraries were constructed from total rabbit heart tissue using the SuperScript Plasmid System (Invitrogen, Carlsbad, CA) and the plasmid vector pSPORT 1 (Invitrogen) transformed into DH5α-competent cells (Invitrogen) according to the manufacturer's instructions.
Rabbit heart cDNA libraries were constructed, and 8,647 cDNAs were isolated using a Qiagen Bio Robot 8000 automated nucleic acid purification and liquid handling workstation (Valencia, CA) and 5′ sequenced using the Applied Biosystems Taq DyeDeoxy Terminator cycle sequencing kit (Foster City, CA) and a Perkin-Elmer BIOSYS 3700 automated sequencer (Waltham, MA). From these, 3,592 nonredundant rabbit heart cDNAs were identified. Sequence identity was determined using National Center for Biotechnology Information (NCBI) blastx (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence quality was evaluated using the accuracy assessment program “phred” of the “phred/phrap/consed” assembly package (9, 10). Putative identity was assigned only to cDNAs exhibiting matches to Homo sapiens genes with E = 1e−25 (minimum P = 10−10) and a nucleotide sequence identity of >95%. Where sequences were identified as Oryctolagus cuniculus (rabbit), they were described as such. For those sequences of alternative identity, H. sapiens was used as the default identity. For all gene ontology (GO) analyses, H. sapiens nucleotide identities were used. This approach allows for ultimate usage in humans without the need for reisolation and sequence of the specific gene. All cDNA sequences have been deposited at NCBI's GenBank and are available at Accession No. EC618425–EC626095 and dbEST40150263–40157933 and at www.mccullylab.org.
Tissue samples for transcriptomic and proteomic analysis.
A separate group of control, GI, and CP hearts (n = 4 each) was perfused as described above for use in transcriptomic and proteomic analyses (Supplemental Fig. S1).1 After 120 min of reperfusion, hearts were quick frozen separately in liquid nitrogen and stored in liquid nitrogen. Whole hearts containing atria and ventricular tissue were pulverized in liquid nitrogen and then subdivided for the isolation of total RNA and total myocardial protein. Total RNA was isolated separately from each heart by homogenization in TRIzol reagent (Invitrogen) according to the manufacturer's protocol and then treated with RNase-free DNase I (New England Biolabs). RNA quality was determined by gel electrophoresis and spectrophotometry as previously described (20). Total myocardial proteins were isolated separately from each heart under nondenaturing conditions using T-Per Protein Extraction Reagent according to the manufacturer's instructions (Pierce, Rockford, IL). Isolated RNA and protein were used for transcriptomic and proteomic analyses, respectively (Supplemental Fig. S1). Transcriptomic and proteomic analyses were performed on matched samples.
Microarray analysis.
Microarray slide preparation/fabrication, print array, blocking, probe labeling and hybridization, scanning quality control, scanning and feature extraction, raw data analysis, and computational biology were performed at the Harvard Faculty of Arts and Sciences Center for Systems Biology.
cDNAs were amplified using the Takara Taq DNA Polymerase kit (TaKaRa Shuzo, Otsu, Japan) according to the manufacturer's instructions. Reaction product samples were resolved by 1.0% agarose gel electrophoresis followed by ethidium bromide staining to determine quality before being spotted on microarrays and confirming the insert size. cDNAs were spotted onto preprepared poly-l-lysine slides using an OmniGrid 100 microarrayer (GeneMachines, BST Scientific, Singapore). Two arrays were printed onto the same slide and used as technical replicates. Each cDNA in each array was printed in triplicate. Ten slides were used for each assay. Blocking was performed as described (Brown laboratory, http://cmgm.stanford.edu/pbrown/mguide/). Total RNA, isolated as described above, was reverse transcribed and labeled with Cy3 or Cy5 (New England Nuclear, Perkin-Elmer) using Superscript II reverse transcriptase (Invitrogen). Unincorporated fluorescent nucleotides were removed by glass fiber filter filtration using standard techniques. Prehybridization and hybridization were performed using standard techniques. Normalization was performed through replicate arrays with dye swapping and self:self hybridization for each sample (1).
Control RNA was included as a comparison to account for constitutive gene expression. GI was compared with the control to determine the effects of GI on RNA expression. CP was compared with the control to determine the effects of CP on RNA expression. The comparison between GI and CP was used to determine changes in RNA expression levels in CP.
Raw scanned images of Cy3 and Cy5 fluorescence were processed using an Axon 4000A microarray scanner with GenePix analysis software using Rosetta Resolver (Molecular Devices, Sunnyvale CA.). Dye swap was treated as a technical replicate to account for dye effect errors. Fold change criteria were used together with P values to identify gene expression changes. P values were calculated based on the dye swap results. The fold change was set to ≥1.2-fold to reflect the short reperfusion time after GI of only 2 h. Computational biology was performed using Spotfire Decision Site 8.1 (Spotfire, Somerville, MA). Functional annotation cluster analysis was performed using DAVID 2007 functional annotation bioinformatics microarray analysis (http://david.abcc.ncifcrf.gov/).
All microarray data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) (6) and are accessible through GEO Series Accession No. GSE14576 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14576).
Quantitative real-time RT-PCR.
Quantitative real-time RT-PCR (QRT-PCR) analysis was performed to validate changes in gene expression across experimental conditions using a Chromo4 Continuous Fluorescence Detector and Opticon Monitor 3 software (MJ Research, Waltham, MA). The iScript One-Step RT-PCR Kit with SYBR green solution (Bio-Rad, Hercules, CA) was used for the QRT-PCRs according to the manufacturer's instructions. In brief, 100 ng of total RNA and 600 nM of both forward and reverse primers were added to each reaction. Control, GI, and CP reactions were run for each primer set in duplicate. Control reactions without reverse transcriptase were also performed for each reaction.
Oligonucleotide primers (Table 1) were designed based on the laboratory's rabbit nonredundant cDNA library sequence data using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and were synthesized by Oligos ETC (Wilsonville, OR). Reaction kinetics were optimized for each primer set. The RT of RNA template occurred at 48°C for 1 h with inactivation at 94°C for 10 min. Amplification and detection occurred over 41 cycles (denaturing: 94°C for 15 s, annealing: 58–63°C for 30 s, and extension: 68–70°C for 90 s, with the plate read at 78°C). Melting curves were performed for each reaction at the conclusion of the cycling parameters from 60 to 95°C. Fold changes in gene expression were calculated using the Pfaffl method (25, 27).
Table 1.
Oligonucleotide primers used for real-time RT-PCR
| Gene | GenBank Accession Number | Oligonucleotide Primers | Product Size, bp |
|---|---|---|---|
| basic transcription factor 3 | EC618736 | Forward: 5′-GCGCTTAATCTCAGCTGGTC-3′ Reverse: 5′-TTTCTGCGAGCAGTTCCTTT-3′ | 169 |
| Rabbit cardiac muscle Ca2+ release channel (ryanodine receptor) | EC620836 | Forward: 5′-CCAGTGTCATCCACCAACAA-3′ Reverse: 5′-GCAACTGATGAAATCCCTGAA-3′ | 170 |
| Succinate dehydrogenase complex, subunit A, flavoprotein | EC621662 | Forward: 5′-CAGACAGGAACCCGTGATTT-3′ Reverse: 5′-AGCTTTGTGATGCATGCTGT-3′ | 200 |
| Ventricular myosin light chain 1 | EC618466 | Forward: 5′-GCCATCAGTTTCTCCACCTC-3′ Reverse: 5′-ATCGCCAAGAACAAGGACAC-3′ | 161 |
| Cysteine- and glycine-rich protein 3 | EC620325 | Forward: 5′-GCTCATGACAGCACGACAGT-3′ Reverse: 5′-GGGACTGTTGGAACTGGAGA-3′ | 160 |
| Mitochondrial ribosomal protein S15 | EC619691 | Forward: 5′-TCGAATCCGGGCAGTGACTC-3′ Reverse: 5′-CAGCGTAGAAGGAGGCAGGTCA-3′ | 182 |
Quantitative Real-time RT-PCR genes analyzed, oligonucleotide primers and product size.
Validation and identification of QRT-PCR products.
QRT-PCR product sizes were verified by agarose gel and electrophoresis, and the products were purified using the QIAquick PCR purification kit (Qiagen) according to the manufacturer's instructions. The purified products were sequenced using BigDye Terminator version 1.1 and version 3.1 Cycle Sequencing Kits (Applied Biosystems) using the 3130 xl Genetic Analyzer (Applied Biosystems).
Proteomics.
High-throughput profiling of protein samples was performed at the Mass Spectrometry Core Facility of the Beth Israel Deaconess Medical Center using the Applied Biosystems (ABI) 4-plex iTRAQ labeling kit with the 4700 MALDI TOF/TOF analyzer. iTRAQ (Applied Biosystems) technology uses four isobaric tags of different molecular weights that are linked to free lysine residues at the peptide level and enables the simultaneous comparison of four different samples in the same mass spectroscopy (MS) experiment. The tag consists of two parts: the tagging molecule and a reporter ion. Both are connected to one another but are dissociated upon laser excitation. Once dissociated, a reporter ion is detected for each of the four different tags: 114, 115, 116, and 117, allowing for the calculation of the relative abundance of specific peptides by comparing the amount of their respective tags.
The matrix-assisted laser desorption (MALDI) coupled to ionization TOF-MS is one of the most sensitive, high-resolution, and high-throughput approaches for biomarker discovery. Briefly, sample fractions are applied to the metal MALDI plate, and fractionations are performed with strong cation exchange and separation by reverse-phase chromatography using a Dionex Ultimate NanoLC system. The energy from a nitrogen laser source is used to mobilize the proteins off the chip. The TOF of each protein is proportional to its molecular weight and charge. The final readout graph is a multipeak spectrum with peak intensity corresponding to the relative protein amount and peak location corresponding to the precise protein or peptide molecular weight. All samples (100 μg each) were run in duplicate. The comparison between GI and CP was used to determine changes in protein expression levels in CP (Supplemental Fig. S1).
Data analysis was performed using Protein Pilot 2.0 software (ABI) on SWISS-PROT, TrEMBL (www.ebi.ac.uk/swissprot), and NCBI (www.ncbi.nlm.nih.gov/) nonredundant protein databases. Proteins that were altered significantly between GI and CP were identified on the basis of a significant P value and fold change. The P value was calculated using the Protein Pilot Paragon Algorithm, which allows results to be evaluated based on the certainty of changes in the expression, not just by the magnitude of change (32).
Comparison of transcriptomic and proteomic data.
A comparison of transcriptomic and proteomic data was performed using two strategies: 1) direct comparison on the basis of uniform identifiers (IDs) and 2) indirect comparison on the basis of affected biological processes and pathways.
For direct comparison, all transcriptomic and proteomic data were converted to uniform SWISS-PROT/TrEMBL IDs. The conversion of the cDNA Genbank Accession Nos. to proteins SWISSPROT IDs was performed using a PERL script that uses the NCBI “Gene Information Database” to perform mapping of GenBank IDs to protein IDs.
Enrichment analysis.
To investigate whether the transcriptomic and proteomic data affected different biological processes, enrichment analysis was performed using GO categories, pathways, disease sets, and subcellular localizations. GO analysis was performed using the MetaCore tool in the GeneGO package (http://www.genego.com/). For all GO analyses, H. sapiens nucleotide identities were used. Analysis was performed on the transcripts/proteins differentially expressed in GI versus CP. The significantly enriched pathways/GO categories were identified and ranked on the basis of hypergeometric P values (P < 0.05), representing the probability of mapping arising by chance, based on the number of genes/proteins identified in a particular canonical pathway/GO category compared with the total number of genes in the pathway/GO category. Categories with P < 0.05 were considered significant. The P value was calculated using the MetaCore Tool in the GeneGO package (http://www.genego.com/).
Western blot analysis.
Total protein was isolated from pooled tissue samples obtained after 120 min of reperfusion, and protein samples (25 μg) were fractionated on 10% Novex Tris-glycine gels (Invitrogen) and then electroblotted to nitrocellulose membranes (Invitrogen) as previously described. Protein equivalency, transfer efficiency, and membrane blocking were performed as previously described (36). Immunoblot analysis was performed using the mouse monoclonal cysteine- and glycine-rich protein 3 (CSRP3) antibody (1:2,000 dilution, Abcam, Cambridge, MA). Blots were detected using ECL-Plus (Amersham Pharmacia Biotech, Piscataway, NJ) with species-appropriate secondary antibodies. Densitometry analysis was performed using SigmaGel gel analysis software (Jandel Scientific, San Rafael, CA).
Statistical analysis.
Statistical analysis was performed using the SAS (version 6.12) software package (SAS Institute, Cary, NC). Means ± SE for all data were calculated for all variables. Statistical significance for all continuous physiological measurements was assessed using repeated-measures ANOVA with group as a between-subjects factor and time as a within-subjects factor. Statistical differences between groups in infarct size and Western blot analysis were evaluated by one-way ANOVA. Dunnett's test was used for comparisons between the control group and other groups to adjust for the multiplicity of tests.
RESULTS AND DISCUSSION
A common approach to the investigation of human disease and therapeutics is through preliminary investigation using animal models. In the investigation of cardiac disease and cardioprotective modalities, the rabbit heart has been extensively used, and results from these studies have shown that alterations of myocardial mRNA and protein levels occur in ischemia-reperfusion and with the cardioprotection afforded by CP. The methods used for these investigations have included Northern blot hybridization, subtraction hybridization, real-time PCR analysis, and differential display and used a targeted approach involving one or a few genes, thus limiting the information regarding the differential expression of mRNA species. In our investigation of the mechanisms modulating ischemia and cardioprotection, we used the rabbit heart model. The rabbit is an established genetic model with the advantage of being a close evolutionary reference species to humans and provides a reproducible experimental model with documented relevance to human disease, not represented in rodent models (24). The rabbit heart parallels the human heart, having a similar myosin heavy chain phenotype and metabolic rate and lacking significant myocardial xanthine oxidase (5). The isolated perfused rabbit heart model allows for surgically relevant experimentation of the whole heart (atria and ventricles) in which the effects of biological systems, pharmacological agents, and surgical methodologies can be studied interactively in a cost-effective and rational manner. Our previous results using this model recapitulate the observations seen in human cardiac surgical patients (15).
cDNA sequences.
Herein, we describe the identification, characterization, and analysis of a compendium of nonredundant rabbit heart cDNAs using microarray and proteomic analyses. The mean cDNA insert size was 1.67 kb, as verified by MluI restriction enzyme digestion of random-selected cDNAs and by the resolution of PCR products by 1.0% agarose gel electrophoresis followed by ethidium bromide staining before being spotted on microarrays (results not shown).
Myocardial function and the effects of inhibition of RNA and protein synthesis.
Our experiments have shown that GI is associated with significantly decreased postischemic functional recovery and significantly increased myocardial cell death. CP partially alleviated the effects of GI by significantly increasing LV peak developed pressure (P < 0.0001) and SS (percentage of the equilibrium value, P < 0.0001) and significantly decreasing LV end-diastolic pressure (P < 0.001) and infarct size compared with GI (Table 2).
Table 2.
LVPDP, LVEDP, systolic shortening, and infarct size in control, GI, and CP in control and CP hearts preperfused with AMN or CHX
| Group |
Perfusion Time |
Probability Versus Control | Probability Versus GI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equilibrium (55 min) |
Reperfusion |
|||||||||||||||||
| 100 min | 110 min | 120 min | 150 min | 180 min | 210 min | |||||||||||||
| LVPDP, mmHg | ||||||||||||||||||
| Control | 108.1±2.1 | 105.1±2.5 | 105.4±2.4 | 104.6±2.1 | 101.6±2.6 | 98.6±2.1 | 97.1±2.6 | <0.0001 | ||||||||||
| Control + AMN | 109.3±2.3 | 105.1±1.9 | 104.3±2.7 | 104.7±2.7 | 102.2±2.9 | 99.6±2.5 | 98.7±2.9 | |||||||||||
| Control + CHX | 108.3±2.7 | 104.7±2.6 | 106.6±2.7 | 104.4±2.5 | 101.6±2.3 | 100.3±2.6 | 99.4±2.6 | |||||||||||
| GI | 107.1±1.9 | 48.0±4.4 | 63.1±4.1 | 74.7±3.8 | 78.1±4.4 | 71.9±4.8 | 65.3±4.6 | <0.0001 | ||||||||||
| CP | 104.9±3.2 | 82.4±4.1 | 96.1±4.3 | 101.6±3.6 | 101.1±3.0 | 99.6±2.9 | 99.5±3.2 | <0.0001 | ||||||||||
| CP + AMN | 106.5±1.9 | 81.5±2.7 | 87.5±2.8 | 86.0±2.4 | 87.8±2.8 | 85.7±2.2 | 86.7±2.1 | 0.0012 | ||||||||||
| CP + CHX | 107.8±3.6 | 84.0±4.1 | 85.0±3.2 | 84.3±2.7 | 82.3±3.3 | 83.5±2.8 | 84.5±2.7 | 0.0039 | ||||||||||
| LVEDP, mmHg | ||||||||||||||||||
| Control | 7.3±0.5 | 5.6±0.7 | 5.6±0.9 | 5.7±0.7 | 5.9±0.7 | 5.9±0.6 | 6.0±0.6 | <0.0001 | ||||||||||
| Control + AMN | 7.1±0.6 | 5.7±0.9 | 5.7±1.1 | 5.7±1.3 | 5.7±0.9 | 5.7±0.9 | 5.9±0.7 | |||||||||||
| Control + CHX | 7.3±0.5 | 5.9±0.7 | 5.9±1.3 | 5.7±0.9 | 5.8±0.7 | 5.8±0.7 | 5.9±0.7 | |||||||||||
| GI | 7.3±0.6 | 12.0±2.1 | 11.0±1.7 | 9.0±1.2 | 8.9±0.8 | 8.4±0.8 | 8.9±0.9 | <0.0001 | ||||||||||
| CP | 6.3±0.6 | 6.5±1.2 | 6.0±1.3 | 5.5±1.2 | 6.0±1.1 | 6.4±1.4 | 6.9±1.5 | <0.0001 | ||||||||||
| CP + AMN | 7.7±0.4 | 7.7±0.3 | 6.8±0.3 | 6.8±0.5 | 7.7±0.4 | 8.0±0.6 | 8.5±0.8 | <0.0001 | ||||||||||
| CP + CHX | 7.7±0.2 | 7.3±0.6 | 7.3±0.8 | 7.5±1.0 | 7.8±1.1 | 7.8±1.1 | 7.8±0.9 | <0.0001 | ||||||||||
| Systolic shortening, percentage of equilibrium | ||||||||||||||||||
| Control | 100.0±0.0 | 103.8±1.8 | 100.4±2.1 | 100.0±2.0 | 97.3±2.0 | 98.5±3.5 | 96.4±3.6 | 0.0376 | ||||||||||
| Control + AMN | 100.0±0.0 | 104.7±1.4 | 101.8±2.3 | 99.8±3.1 | 103.7±2.4 | 100.8±3.3 | 96.5±3.6 | |||||||||||
| Control + CHX | 100.0±0.0 | 103.3±1.7 | 103.8±3.8 | 101.6±3.3 | 102.6±2.6 | 99.4±2.8 | 97.9±3.3 | |||||||||||
| GI | 100.0±0.0 | 54.0±9.0 | 68.0±5.6 | 71.6±7.7 | 77.0±5.1 | 74.2±5.2 | 64.5±5.3 | 0.0376 | ||||||||||
| CP | 100.0±0.0 | 78.2±3.1 | 88.1±4.6 | 92.2±5.6 | 99.1±2.6 | 95.1±5.5 | 92.7±4.5 | |||||||||||
| CP + AMN | 100.0±0.0 | 71.2±5.7 | 82.3±5.1 | 88.5±3.3 | 93.1±3.2 | 90.2±3.5 | 87.7±3.5 | |||||||||||
| CP + CHX | 100.0±0.0 | 68.6±5.8 | 86.2±5.9 | 94.2±4.9 | 90.5±5.8 | 89.0±6.3 | 86.1±6.7 | |||||||||||
| Infarct size, percent LV mass | ||||||||||||||||||
| Control | 1.73±0.15 | <0.0001 | ||||||||||||||||
| Control + AMN | 1.95±0.21 | 0.0002 | ||||||||||||||||
| Control + CHX | 1.88±0.17 | <0.0001 | ||||||||||||||||
| GI | 24.18±3.70 | <0.0001 | ||||||||||||||||
| CP | 1.75±0.035 | <0.0001 | ||||||||||||||||
| CP + AMN | 5.75±1.59 | <0.0001 | 0.0006 | |||||||||||||||
| CP + CHX | 6.06±1.61 | <0.0001 | 0.0007 | |||||||||||||||
Values are means ± SE. Left ventricular (LV) peak developed pressure (LVPDP), LV end-diastolic pressure (LVEDP), systolic shortening, and infarct size were determined at the end of preperfusion and during global ischemia (GI) and reperfusion in control, GI, and cardioplegia (CP) hearts and in control, GI, and CP hearts preperfused for 55 min with Krebs-Ringer solution containing α-amanitin (AMN; 2.5 μg/ml) to inhibit RNA synthesis or cycloheximide (CHX; 50 μg/ml) to inhibit protein synthesis. Control, control + AMN, and control + CHX hearts received sham ischemia. There were no significant differences observed within or between for any variable for control, control + AMN, and control + CHX hearts. Statistical comparisons are shown. Significant differences at P < 0.05 are also shown.
To demonstrate the requirement for RNA- and protein-dependent mechanisms in the cardioprotection afforded by CP, we used AMN, an irreversible inhibitor of nuclear and mitochondrial RNA transcription that acts by binding to RNA polymerase II, and CHX, an irreversible protein synthesis inhibitor that halts mRNA translation by blocking the translocation of tRNA at the ribosome (16, 18, 45). Our results showed that the inhibition of RNA or protein synthesis decreases the cardioprotection provided by CP (Table 2).
Microarray analysis.
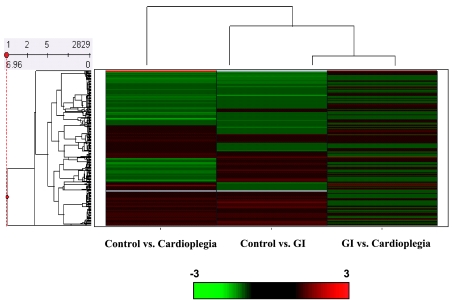
Hierarchical clustering was performed on 2,982 genes using Spotfire Decision Site 8.1 (Spotfire, Somerville, MA). This allowed for the identification of four major clusters consisting of two large upregulated clusters separated by two large downregulated clusters in control versus CP (Fig. 2). Our results showed that there were 148 downregulated cDNAs and 76 upregulated cDNAs (>1.2-fold and P < 0.05) in GI compared with the control (Fig. 2) and 158 upregulated cDNAs and 125 downregulated cDNAs (>1.2-fold and P < 0.05) in CP compared with the control (Fig. 2). The comparison between CP and GI hearts demonstrated that there were 42 upregulated cDNAs and 54 downregulated cDNAs (>1.2-fold and P < 0.05; Fig. 2).
Fig. 2.
Right: hierarchical cluster analysis for CI and GI compared with control to account for constitutively expressed RNAs and CP versus GI to show RNAs up- and downregulated in CP compared with GI. Dendograms are shown on the left, with a scale bar. Cluster numbers for control versus CP are shown on the left. The red color indicates upregulated RNAs, and the green color indicates downregulated RNAs.
In this study, we used a reperfusion time of 2 h, where control hemodynamics are stable (12, 23). Perfusion for >3 h has been shown to significantly decrease hemodynamics (12, 23). The limited reperfusion time of 2 h was considered to reflect changes in early reperfusion and early RNA level changes, and thus a fold increase of >1.2 was used to identify up- and downregulated cDNAs.
Functional annotation clustering.
Functional annotation clusterings for GI versus the control, CP versus the control, and CP versus GI (enrichment score >2.0) are shown in Tables 3, 4, and 5. P values were determined by a modified Fisher exact P value, where the smaller the P value the more enriched the functional group. A P value of <0.05 was used as a cutoff as it is considered to demonstrate strong enrichment in the annotation category.
Table 3.
Functional annotation clustering downregulated in GI versus control
| Number of cDNAs | P Value | |
|---|---|---|
| Annotation cluster 1 (enrichment score: 4.72) | ||
| Cellular respiration | 9 | 1.60 e−11 |
| Aerobic respiration | 8 | 4.80 e−10 |
| Tricarboxylic acid cycle | 6 | 3.00 e−7 |
| Acetyl-CoA catabolism | 6 | 3.00 e−7 |
| Coenzyme catabolism | 6 | 4.40 e−7 |
| Acetyl-CoA metabolism | 6 | 8.40 e−7 |
| Cofactor catabolism | 6 | 8.40 e−7 |
| Oxidoreductase activity | 16 | 5.20 e−6 |
| Generation of precursor metabolites and energy | 12 | 1.30 e−3 |
| Coenzyme metabolism | 6 | 5.70 e−3 |
| Catabolism | 10 | 6.10 e−3 |
| Cellular catabolism | 9 | 6.80 e−3 |
| Cofactor metabolism | 6 | 8.60 e−3 |
| Cellular carbohydrate metabolism | 6 | 4.00 e−2 |
| Annotation cluster 2 (enrichment score: 2.56) | ||
| Cytoplasm | 46 | 4.80 e−9 |
| Intracellular | 65 | 2.60 e−5 |
| Intracellular membrane-bound organelle | 47 | 1.90 e−3 |
| Membrane-bound organelle | 47 | 2.00 e−3 |
| Intracellular organelle | 53 | 2.30 e−3 |
| Organelle | 53 | 2.40 e−3 |
Table 4.
Functional annotation clustering upregulated and downregulated in CP versus the control
| Number of cDNAs | P Value | |||
|---|---|---|---|---|
| Functional annotation clustering upregulated in CP versus the control | ||||
| Annotation cluster 1 (enrichment score: 2.11) | ||||
| Fatty acid β-oxidation | 5 | 2.00 e−6 | ||
| Fatty acid oxidation | 5 | 2.60 e−5 | ||
| 3-Hydroxyacyl-CoA dehydrogenase activity | 3 | 3.10 e−4 | ||
| Mitochondrion | 12 | 7.50 e−4 | ||
| Fatty acid metabolism | 6 | 1.30 e−3 | ||
| Acyl-CoA dehydrogenase activity | 3 | 2.10 e−3 | ||
| Carboxylic acid metabolism | 8 | 1.20 e−2 | ||
| Organic acid metabolism | 8 | 1.20 e−2 | ||
| Oxidoreductase activity, acting on the CH-CH group of donors, with NAD or NADP as acceptors | 3 | 1.80 e−2 | ||
| Cellular lipid metabolism | 7 | 3.60 e−2 | ||
| Functional annotation clustering downregulated in CP versus the control | ||||
| Annotation cluster 1 (enrichment score: 5.59) | ||||
| Serine-type endopeptidase activity | 13 | 1.90 e−11 | ||
| Serine-type peptidase activity | 13 | 7.10 e−11 | ||
| Endopeptidase activity | 16 | 2.40 e−9 | ||
| Proteolysis | 18 | 3.20 e−8 | ||
| Peptidase activity | 16 | 2.40 e−7 | ||
| Hydrolase activity | 24 | 8.20 e−5 | ||
| Macromolecule metabolism | 39 | 1.40 e−4 | ||
| Cellular protein metabolism | 29 | 6.90 e−4 | ||
| Cellular macromolecule metabolism | 29 | 8.90 e−4 | ||
| Protein metabolism | 29 | 2.40 e−3 | ||
| Annotation cluster 2 (enrichment score: 4.4) | ||||
| Cellular respiration | 7 | 2.30 e−8 | ||
| Acetyl-CoA catabolism | 6 | 1.90 e−7 | ||
| Coenzyme catabolism | 6 | 2.70 e−7 | ||
| Acetyl-CoA metabolism | 6 | 5.20 e−7 | ||
| Cofactor catabolism | 6 | 5.20 e−7 | ||
| Coenzyme metabolism | 10 | 8.90 e−7 | ||
| Cofactor metabolism | 10 | 2.00 e−6 | ||
| Cellular catabolism | 10 | 9.60 e−4 | ||
| Cellular carbohydrate metabolism | 8 | 1.60 e−3 | ||
| Catabolism | 10 | 3.20 e−3 | ||
| Annotation cluster 3 (enrichment score: 2.16) | ||||
| Catalytic activity | 43 | 9.10 e−5 | ||
| Macromolecule metabolism | 39 | 1.40 e−4 | ||
| Cellular protein metabolism | 29 | 6.90 e−4 | ||
| Cellular macromolecule metabolism | 29 | 8.90 e−4 | ||
| Protein metabolism | 29 | 2.40 e−3 | ||
| Primary metabolism | 46 | 3.20 e−2 | ||
| Cellular metabolism | 47 | 3.70 e−2 | ||
| Cellular physiological process | 60 | 4.50 e−2 | ||
Table 5.
Functional annotation clustering upregulated and downregulated in CP versus GI
| Number of cDNAs | P Value | |||
|---|---|---|---|---|
| Functional annotation clustering upregulated in CP versus GI | ||||
| Annotation cluster 1 (enrichment score: 2.66) | ||||
| Mitochondrion | 9 | 6.10 e−7 | ||
| Fatty acid β-oxidation | 3 | 1.60 e−4 | ||
| Fatty acid metabolism | 4 | 5.40 e−4 | ||
| Fatty acid oxidation | 3 | 5.50 e−4 | ||
| Functional annotation clustering downregulated in CP versus GI | ||||
| Annotation cluster 1 (enrichment score: 5.11) | ||||
| Serine-type endopeptidase activity | 13 | 2.00 e−16 | ||
| Serine-type peptidase activity | 13 | 7.90 e−16 | ||
| Endopeptidase activity | 13 | 2.70 e−11 | ||
| Proteolysis | 14 | 4.00 e−10 | ||
| Peptidase activity | 13 | 1.50 e−9 | ||
| Cellular protein metabolism | 18 | 3.90 e−5 | ||
| Cellular macromolecule metabolism | 18 | 4.90 e−5 | ||
| Hydrolase activity | 14 | 1.10 e−4 | ||
| Protein metabolism | 18 | 1.10 e−4 | ||
| Macromolecule metabolism | 20 | 4.10 e−4 | ||
| Primary metabolism | 24 | 4.70 e−3 | ||
| Catalytic activity | 19 | 7.30 e−3 | ||
| Cellular metabolism | 24 | 8.10 e−3 | ||
| Extracellular region | 8 | 8.20 e−3 | ||
| Metabolism | 25 | 8.60 e−3 | ||
Functional annotation clustering analysis of GI compared with the control revealed the downregulation of two annotation clusters with enrichment scores >2.0, namely, that of mitochondrion function and energy production and the downregulation of cofactor catabolism, generation of precursor metabolites of energy, cellular carbohydrate metabolism, regulation of biosynthesis, regulation of transcription, and mitochondrial structure and function (Table 3). There were no annotation clusters upregulated in GI versus the control.
In contrast, CP significantly upregulated of mitochondrial function and energy production with an involvement in β-oxidation, fatty acid, organic acid, and cellular lipid metabolism, oxidoreductase activity, acting on the CH-CH group of donors with NAD or NADP as acceptors, and GTPase and enzyme regulator activity (Table 4).
Functional annotation clusters downregulated in CP compared with the control were associated with peptidase activity proteolysis, macromolecular, cellular, and protein metabolism, and energy production, including aerobic respiration, cellular respiration, coenzyme and cofactor catabolism, carbohydrate catabolism and catalytic activity, and cellular and macromolecular metabolism (Table 4).
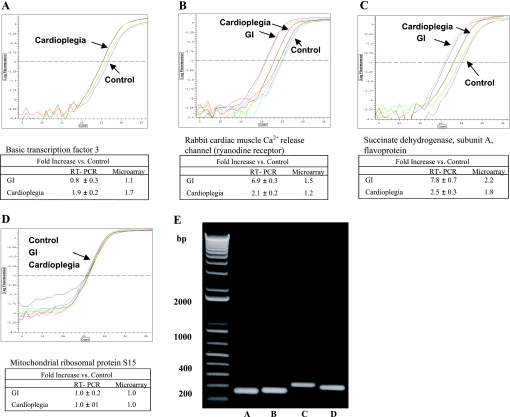
The functional annotation clustering analysis comparison between CP and GI revealed an upregulation of mitochondria, fatty acid β-oxidation, and metabolism and a downregulation of serine-type endopeptidase and peptidase activity, proteolysis, and cellular macromolecular metabolism (Table 5). All results were confirmed by QRT-PCR analysis and sequence analysis of QRT-PCR products (Fig. 3 and Table 1).
Fig. 3.
Representative real-time RT-PCR analysis of transcripts up- and downregulated in cDNA microarray analysis. Real-time RT-PCR graphs are shown for the following: basic transcription factor 3 (A); rabbit cardiac muscle Ca2+ release channel (the ryanodine receptor; B); succinate dehydrogenase, subunit A, flavoprotein (C); and mitochondrial ribosomal protein S15 (D), which was used as a control gene. All results are shown as means ± SE for 6 assays each. Real-time RT-PCR levels tended to be increased compared with microarray results. E: real-time RT-PCR products for the transcripts in A–D (lanes A–D, respectively) were confirmed by gel electrophoresis and by sequence analysis (results not shown).
Proteomic analysis.
Proteomic analysis yielded ∼4,000 peptides belonging to ∼950 different proteins. After false positive results were filtered on the basis of the protscore (>2), 474 proteins were identified with high confidence. These high confidence proteins were further used to identify differentially expressed proteins between CP versus GI. The class comparison between CP versus GI was performed on the basis of fold change (>1.4) and P value (P < 0.05) using a PERL script. The class comparison identified 73 upregulated proteins and 12 downregulated proteins in CP compared with GI (differentially expressed; Fig. 4). Functional analysis on the differentially expressed proteins using DAVID identified the enrichment of mitochondrial proteins involved in acetylation, fatty acid and glucose metabolism, ATP biosynthesis, and oxidoreductase activity. Supplemental Table S1 lists all significantly enriched GO terms along with P values, numbers of proteins, and their SWISS-Prot IDs.
Fig. 4.
Hierarchical cluster analysis for proteins having >1.4-fold change between CP and GI. The proteins are identified with short descriptions obtained from the SWISS-PROT database. Protein expression is shown with a pseudocolor scale (from −3 to 3) with the red color indicating a high expression level and the green color indicating low expression in CP compared with GI.
Comparison of microarray and proteomic data.
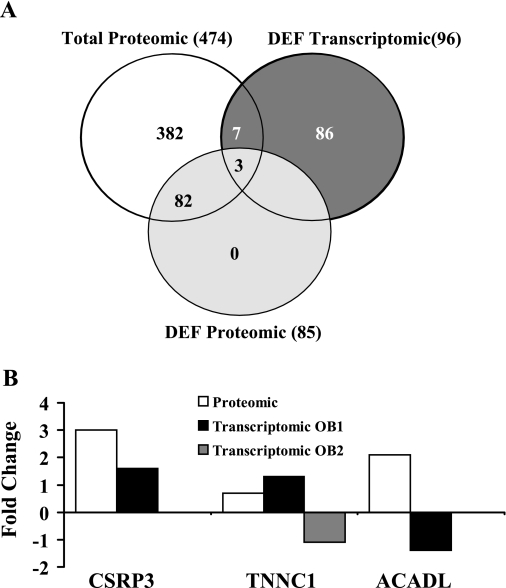
The direct comparison of microarray and proteomic data identified 10 proteins that were common in transcriptomic and proteomic data, of which 3 protiens were significantly differentially detected (differentially expressed) in the proteomic data (Fig. 5A). Expression changes for the three proteins are shown in Fig. 5B.
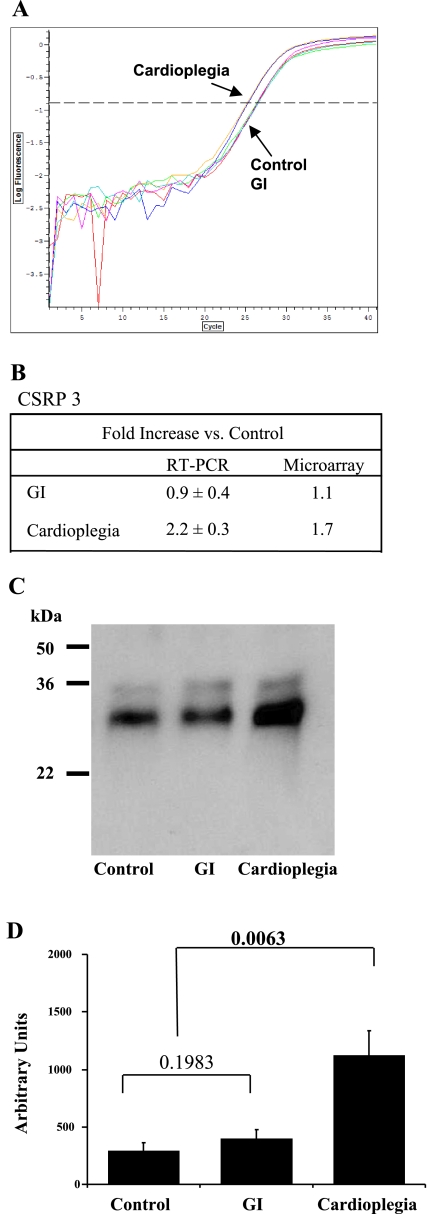
Fig. 5.
Direct comparison of microarray and proteomic data. A: Venn diagrams for the direct comparison of microarray and proteomic data. Ten proteins were common between differentially expressed (DEF) transcriptomic and proteomic data, of which three proteins were significantly differentially expressed in the proteomic data. B: expression changes for the three proteins. CSRP3, cysteine- and glycine-rich protein 3; TNNC1, troponin C; ACADL, acyl-CoA dehydrogenase. TNNC1 was compared using two different probes (OB1 and OB2) in the transcriptomic data.
CSRP3 was upregulated (2.9-fold) in the proteomic data and upregulated by 1.7-fold in the transcriptomic data. These changes were confirmed by QRT-PCR (2.2-fold increase compared with GI) and Western blot analysis (3.88-fold increase compared with GI; Fig. 6). Troponin C (TNNC1) was downregulated in GI in the proteomic data but upregulated/downregulated by two different probes (OB1 and OB2) in the transcriptomic data. This anticorrelation was observed with acyl-coA dehydrogenase, which was upregulated in the proteomic data but downregulated in the transcriptomic data. There was less correlation using the indirect comparison approach.
Fig. 6.
A: representative real-time RT-PCR analysis of CSRP3. B: fold changes for CSRP3 compared with the control. C: representative Western blot for CSRP3. Molecular masses (in kDa) are shown. D: densimetric analysis for n = 3 blots. Significant differences between groups, determined by one-way ANOVA, are shown in bold type.
The upregulation by cardioplegia of CSRP3 [also known as muscle LIM protein (MLP)] is of some importance. Previous studies have shown that CSRP3 (MLP) is involved in the regulatory processes modulating cellular development and differentiation. While previously thought to be a cytoskeletal protein associated with sarcomere structures, new analysis has shown that CSRP3 (MLP) is located in the nucleus and cytoplasm and is mainly located in the cytosol of cardiomyocytes (13). The role of CSRP3 (MLP) has been shown to enhance myogenesis and preserve structural integrity in a variety of cell types via an interaction with the basic helix-loop helix protein MyoD (8). This would agree with our previous study (36) showing significant alterations in the structural integrity of the myocardium as well as gap and adherens junction protein expression levels with increasing GI time. These alterations in the structural integrity of the myocardium were ameliorated by CP (43).
It is possible to speculate that CSRP3 (MLP) may aid in the maintenance of cell structure. Indeed, there are data to show that a missense mutation of CSRP3 plays an important role in the genesis of hypertrophic cardiomyopathy. Geier et al. (13) have shown that CSRP3 missense mutations destabilize the heart, leading to impaired mechanosensory stress signaling.
Indirect comparison using biological categories enrichment.
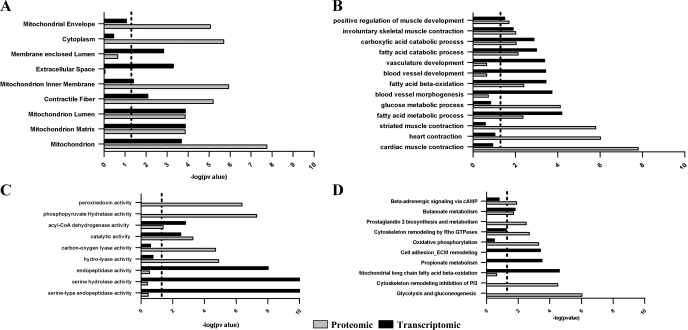
To investigate whether the transcriptomic and proteomic data affected different biological processes, enrichment analysis was performed using GO categories, pathways, disease sets, and subcellular localizations. This analysis identified the cellular components in which the differentially expressed transcripts/proteins were overrepresented. Our analysis identified that differentially expressed proteomics and transcriptomics are increased in the mitochondrion, the mitochondrial matrix, lumen, and inner membrane and in contractile fiber categories in CP compared with GI (Fig. 7A).
Fig. 7.
Gene ontology (GO)-based comparison of microarray and proteomic data. A: GO cellular components; B: GO biological processes; C: GO metabolic functions; and D: GO canonical pathways. Proteomics data are shown by the shaded bars; transcriptomic data are shown by the solid bars. The dotted lines in A–D show the significance threshold (P < 0.05). P values were calculated using the MetaCore Tool in the GeneGO package (http://www.genego.com/).
Mitochondrial envelope- and cytoplasm-related proteins were significantly overexpressed in the proteomic data, whereas extracellular space and membrane-enclosed lumen categories were significantly overexpressed in the transcriptomic data in CP compared with GI (Fig. 7A).
Enrichment analysis using GO processes.
The biological processes commonly affected in both transcriptomic and proteomic data for CP compared with GI included positive regulation of muscle contraction, involuntary muscle contraction, carboxylic acid and fatty acid catabolic processes, fatty acid β-oxidation, and fatty acid metabolic processes (Fig. 7B). Genes related to vascular development, blood vessel morphogenesis, and development were significantly overexpressed in the transcriptomic data, whereas the proteomic data showed significant overexpression of proteins involved in heart contraction (cardiac and smooth muscle contraction) and glucose metabolism in CP compared with GI.
Enrichment analysis using metabolic functions.
Metabolic function-based enrichment analysis identified acyl-CoA dehydrogenase activity and catalytic activity as being affected in both transcriptomic and proteomic data in CP compared with GI (Fig. 7C). Serine-type endopeptidase activity, serine hydrolase activity, and endopeptidase activity were significantly overexpressed in the transcriptomic data, whereas phosphopyruvate hydrolase activity (enolase), peroxiredoxin activity, carbon oxygen lyase activity, and hydrolase activity were significantly overexpressed in the proteomic data for CP compared with GI (Fig. 7C).
Enrichment analysis using canonical pathways.
Enrichment analysis using conical pathways identified the significantly upregulated pathways of glycolysis and gluconeogenesis, oxidative phosphorylation, prostaglandin 2 biosynthesis and metabolism, β-adrenergic signaling via cAMP, and cytoskeleton remodeling by inhibition of phosphoinositide 3-kinase (PI3K) in the proteomic data for CP compared with GI (Fig. 7D).
Mitochondrial long-chain fatty acid β-oxidation, propionate metabolism, cell adhesion, and extracellular matrix modeling were significantly upregulated in the transcriptomic data for CP compared with GI. Cytoskeleton remodeling by Rho GTPases and butonate metabolism were significantly upregulated in both the transcriptomic and proteomic data for CP compared with GI (Fig. 7D). The number of transcripts/proteins enriched in GO categories and pathways is shown in Supplemental Table S2.
We found a better overlap between the transcriptomic and proteomic data using the indirect comparison approach compared with the direct comparison approach. This observation may be due to the effect of the transcriptomic and proteomic data on the different transcipts/proteins of same pathways/GO categories. In the case of fatty acid metabolism, the transcriptomics data (e.g., long-chain acyl-CoA dehydrogenase, 3-oxoacyl-synthase, and hydroxyacyl-CoA dehydrogenase) and proteomics data (acetyl-CoA acyltransferase 2, peroxisomal enoyl CoA hydratase 1, and C-4 to C-12 straight-chain acyl-CoA dehydrogenase) affected different transcripts/proteins. The poor correlation between the transcriptomic and proteomic data might be due to 1) the complexities of protein expression, which includes posttranslational modifications; 2) the difficulty of detecting hydrophobic/membrane peptides using whole organelle extracts; and 3) degradation of the mRNA before translation. An alternative explanation may be that multiple genes/transcripts map to single GO terms and, therefore, overlap between GO terms is more likely than single genes/transcripts across transcriptomic- and proteomic-based technologies.
The transcriptomic and proteomic data demonstrate that the mitochondrion plays a significant role in both GI and the cardioprotection provided by CP.
Previously, we have shown that GI significantly alters mitochondrial structure and function and that these changes are associated with significantly decreased high-energy phosphates preservation and resynthesis and significantly decreased postischemic functional recovery and myocardial cell viability (23, 36, 38, 39, 43). These effects are ameliorated by CP. In this report and in previous studies, we have shown that CP significantly preserves mitochondrial structure and function and significantly enhances the preservation and resynthesis of ATP levels (11, 22, 29, 41). These effects resulted is a significant enhancement of postischemic functional recovery and myocardial cellular and sarcomere structural preservation (22, 29).
Glycolysis, gluconeogenesis, and oxidative phosphorylation and redox regulation.
The modulation of ATP preservation and resynthesis is complex. Under homeostatic conditions, it has been shown that the energy demands of the myocardium are predominantly supplied by the β-oxidation of free fatty acids that are required to maintain translational control of protein synthesis in the heart (4). Lopaschuk (17) suggested that after ischemia, high rates of fatty acid β-oxidation would decrease cardiac function and efficiency during reperfusion and that, therefore, there is a delay in the return to fatty acid β-oxidation until the heart returns to a stabilized condition. In the interim, after ischemia, there is a continuation of glycolysis, gluconeogenesis, and increased flux via the tricarboxylic acid cycle, which has been shown to enhance postischemic functional recovery.
Our data agree with these observations as we showed that CP, in contrast to GI, significantly upregulated the pathways of glycolysis, gluconeogenesis, and oxidative phosphorylation as well as long-chain fatty acid β-oxidation and significantly upregulated acyl-CoA dehydrogenase activity and catalytic activity. Acyl-CoA dehydrogenase is the initial enzyme allowing for the mitochondrial catalysis of fatty acid β-oxidation. The increase in glycolysis, gluconeogenesis, and oxidative phosphorylation would be expected to increase the production of protons and ROS and reduce cardiac performance during reperfusion.
However, the detrimental effects of increased glycolysis, gluconeogenesis, and oxidative phosphorylation may be tempered with CP through redox regulation. Our results showed that CP upregulates both the enolase complex (enolase-1, -2, and -3) and redox regulation/peroxide reduction genes (peroxiredoxin-1, -2, and -3). In previous reports, it has been shown that the enolase complex plays a critical role in hypoxia tolerance (enolase-1), muscle contraction (enolase-2), and neurotrophic and neuroprotection (enolase-3), whereas redox regulation/peroxide reduction genes (peroxiredoxin-1, -2, and -3) have been shown to be involved in antioxidation and the regulation of hydrogen peroxide-mediated signal transduction (46). The combined upregulation of the pathways of glycolysis, gluconeogenesis, and oxidative phosphorylation as well as long-chain fatty acid β-oxidation together with the upregulation of redox regulation genes would therefore act in synergy and allow for enhanced cardioprotection.
α-Adrenergic signaling via cAMP.
In cardiac surgery, the recovery of the myocardium is monitored primarily by functional parameters, and inotropic agents are frequently used during weaning from cardiopulmonary bypass. In an experimental study (34), it has been shown that the use of inotropic agents, while allowing for enhanced performance, also increases cell death through apoptosis. Although the induction of cell death is not intended, these agents are needed to enhance postischemic cardiac function. At present, no alternative to the use of inotropes is available; however, our results indicated that CP induces an endogenous inotropic response. Our results showed that CP upregulates β-adrenergic signaling via cAMP, which has been previously shown to mediate the positive inotropic effect of catecholamines on heart cells. The extent and level of induction of this endogenous response have not been investigated, and the differences between endogenous compared with exogenous supplementation remain to be elucidated. However, our data suggest that enhanced cardiac function without the complimentary induction of the apoptotic pathway may be possible.
The inflammatory response.
The inflammatory response after ischemia-reperfusion injury has been shown to significantly contribute to myocardial cell death (28). Our results show that prostaglandin 2 biosynthesis and metabolism are upregulated by CP. The upregulation and release of prostaglandins (autacoids released in inflammatory states or by the occurrence of inflammatory processes) would be expected to improve coronary flow and the response to inflammation.
Cytoskeleton remodeling.
Previous studies have shown that the actin cytoskeleton plays an active role in a large number of cellular functions, such as cell shape change and cell-to-cell adhesion (14).Our data showed that CP upregulates cytoskeleton remodeling through the inhibition of PI3K and upregulates cell adhesion and extracellular matrix modeling and cytoskeleton remodeling by Rho GTPases. Our results also showed that CP upregulates genes associated with GTPase regulator activity. GTPase regulator activity has been shown to be involved in myocardial cellular viability. GTPase activity protein has been shown to bind to Akt, a member of the serine/threonine protein kinase family involved in cellular survival pathways (48). The binding of GTPase activity to Akt is dependent on an upstream integrin-related kinase and, once bound to Akt, protects the cell from apoptosis through an Akt-dependent pathway (48). These observations agree with our data showing a significant decrease in apoptosis with CP (22).
Our data also agree with the proteomic data of others who have shown changes in the sarcomere and cytoskeleton and energy metabolism after ischemia and reperfusion in the New Zealand White rabbit (44) and with the data of Schomisch et al. (31), who showed that genes associated with energy metabolism, calcium regulation, the immune inflammatory response, and transcription were decreased in ischemia but preserved by CP in the rat heart.
Our data show some divergence with Ruel et al. (30), who showed that in human atrial tissue samples, there was an upregulation of inflammation/transcription activators, apoptotic genes, and stress genes and a downregulation of immunoglobulin genes associated with cardiopulmonary bypass and CP. These differences may be attributable to the tissue sources used for analysis. In our study and that of Schomisch et al. (31), total myocardial tissue, including the atria and ventricles, was used for the isolation of RNA and protein for microarray and proteomic investigations.
The ability to obtain viable ventricular tissue was not ethically feasible in the study by Ruel et al. (30), and the differences seen between studies may therefore due to chamber-specific gene expression. Previous reports have shown that atrial and ventricular gene expression patterns are varied and showed specific expression patterns in response to stress and disease (2, 3, 7, 35). However, both our data and those of Ruel et al. (30) indicated a role for altered transcription and suggested possible modes for enhanced cardioprotection. It should be noted that our data do not describe possible posttranscriptional alterations in proteins, including phosphorylation, changes in protein conformation, and protein translocation, which may play an essential role in conferring cardioprotection (49).
In conclusion, our results indicated that the cardioprotection afforded by CP is modulated by RNA- and protein-dependent mechanisms. Trancriptomic and proteomic enrichment analyses indicated that GI downregulates transcripts/proteins associated with mitochondrial function and energy production and cofactor catabolism (the generation of precursor metabolites of energy). In contrast, CP significantly increased differentially expressed transcripts/proteins associated with the mitochondrion and mitochondrial function and significantly upregulated the biological processes of muscle contraction, involuntary muscle contraction, carboxylic acid and fatty acid catabolic processes, fatty acid β-oxidation, and fatty acid metabolic processes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-29077, HL-068915, and HL-066186.
Supplementary Material
Footnotes
Supplemental material for this article is available online at the Physiological Genomics website.
REFERENCES
- 1.Altman N Replication, variation and normalization in microarray experiments. Appl Bioinformatics 4: 33–44, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Pfeufer A, Uberfuhr P, Dugas M, Steinbeck G, Nabauer M. Functional profiling of human atrial and ventricular gene expression. Pflügers Arch 50: 201–208, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Cardin S, Pelletier P, Libby E, Le Bouter S, Xiao L, Kääb S, Demolombe S, Glass L, Nattel S. Marked differences between atrial and ventricular gene-expression remodeling in dogs with experimental heart failure. J Mol Cell Cardiol 45: 821–831, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Crozier SJ, Bolster DR, Reiter AK, Kimball SR, Jefferson LS. β-Oxidation of free fatty acids is required to maintain translational control of protein synthesis in the heart. Am J Physiol Endocrinol Metab 283: E1144–E1150, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Eddy LJ, Stewart JR, Jones HP, Engerson TD, McCord JM, Downey JM. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am J Physiol Heart Circ Physiol 253: H709–H711, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellinghaus P, Scheubel RJ, Dobrev D, Ravens U, Holtz J, Huetter J, Nielsch U, Morawietz H. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J Thorac Cardiovasc Surg 129: 1383–1390, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Etzion S, Barbash IM, Feinberg MS, Zarin P, Miller L, Guetta E, Holbova R, Kloner RA, Kedes LH, Leor J. Cellular cardiomyoplasty of cardiac fibroblasts by adenoviral delivery of MyoD ex vivo: an unlimited source of cells for myocardial repair. Circulation 106: I125–I130, 2002. [PubMed] [Google Scholar]
- 9.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8: 186–194, 1998. [PubMed] [Google Scholar]
- 10.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8: 175–185, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Faulk EA, McCully JD, Hadlow NC, Tsukube T, Krukenkamp IB, Federman M, Levitsky S. Magnesium cardioplegia enhances mRNA levels and the maximal velocity of cytochrome oxidase I in the senescent myocardium during global ischemia. Circulation 92: 405–412, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg H, Levitsky S, Lee SL. The quiescent heart: excitability, compliance,and vascular resistance. Am J Physiol Heart Circ Physiol 251: H1085–H1089, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Geier C, Gehmlich K, Ehler E, Hassfeld S, Perrot A, Hayess K, Cardim N, Wenzel K, Erdmann B, Krackhardt F, Posch MG, Bublak A, Nägele H, Scheffold T, Dietz R, Chien KR, Spuler S, Fürst DO, Nürnberg P, Ozcelik C. Beyond the sarcomere: CSRP3 mutations cause hypertrophic cardiomyopathy. Hum Mol Genet 17: 2753–2765, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Hall A Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kim C, Redberg RF, Pavlic T, Eagle KA. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin Cardiol 30: 491–495, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science 170: 447–449, 1970. [DOI] [PubMed] [Google Scholar]
- 17.Lopaschuk G Regulation of carbohydrate metabolism in ischemia and reperfusion. Am Heart J 139: S115–S119, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H, McCully JD, Levitsky S. Inhibition of RNA transcription modulates magnesium supplemented potassium cardioplegia protection. Ann Thorac Surg 70: 2107–2112, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Mayr M, Zhang J, Greene AS, Gutterman D, Perloff J, Ping P. Proteomics-based development of biomarkers in cardiovascular disease: mechanistic, clinical, and therapeutic insights. Mol Cell Proteomics 5: 1853–1864, 2006. [DOI] [PubMed] [Google Scholar]
- 20.McCully JD, Lotz MM, Krukenkamp IB, Levitsky S. A brief period of retrograde hyperthermic perfusion enhances myocardial protection from global ischemia: association with accumulation of Hsp 70 mRNA and protein. J Mol Cell Cardiol 28: 231–241, 1996. [DOI] [PubMed] [Google Scholar]
- 21.McCully JD, Myrmel T, Lotz MM, Krukenkamp IB, Levitsky S. The rapid expression of myocardial Hsp 70 mRNA and the heat shock 70 kD protein can be achieved after only a brief period of retrograde hyperthermic perfusion. J Mol Cell Cardiol 27: 873–882, 1995. [DOI] [PubMed] [Google Scholar]
- 22.McCully JD, Wakiyama H, Cowan DB, Federman M, Levitsky S. Diazoxide amelioration of myocardial injury and mitochondrial damage during cardiac surgery. Ann Thorac Surg 74: 2138–2146, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol 286: H1923–H1935, 2004. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien SJ, Eizirik E, Murphy WJ. On choosing mammalian genomes for sequencing. Science 292: 2264–2266, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podgoreanu MV, Michelotti GA, Sato Y, Smith MP, Lin S, Morris RW, Grocott HP, Mathew JP, Schwinn DA. Differential cardiac gene expression during cardiopulmonary bypass: ischemia-independent upregulation of proinflammatory genes. J Thorac Cardiovasc Surg 130: 330–339, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Ramakers C, Ruijeter JM, Duprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ren G, Dewald O, Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy 2: 242–256, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Diazoxide and cardioplegia ameliorate ischemia/reperfusion cell death through the modulation of mitochondrial volume and calcium accumulation and mitochondrial respiratory control index. Am J Physiol Heart Circ Physiol 287: H1967–H1976, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Ruel M, Bianchi C, Khan TA, Xu S, Liddacott JR, Voisine P, Araujo E, Lyon H, Kohane IS, Libermann TA, Sellke FW. Gene expression profile after cardiopulmonary bypass and cardioplegic arrest. J Thorac Cardiovasc Surg 126: 1521–1530, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Schomisch SJ, Murdock DG, Hedayati N, Carino JL, Lesnefsky EJ, Cmolik BL. Cardioplegia prevents ischemia-induced transcriptional alterations of cytoprotective genes in rat hearts: a DNA microarray study. J Thorac Cardiovasc Surg 130: 1151–1158, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics 6: 1638–1655, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104: 826–831, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Stamm C, Friehs I, Cowan DB, Cao-Danh H, Choi YH, Duebener LF, McGowan FX, del Nido PJ. Dopamine treatment of postischemic contractile dysfunction rapidly induces calcium-dependent pro-apoptotic signaling. Circulation 106, Suppl 1: I290–I298, 2003. [PubMed] [Google Scholar]
- 35.Tabibiazar R, Wagner RA, Liao A, Quertermous T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circ Res 93: 1193–120, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Tansey EE, Kwaku KF, Hammer PE, Cowan DB, Federman M, Levitsky S, McCully JD. Reduction and Redistribution of gap and adherens junction proteins following ischemia/reperfusion. Ann Thorac Surg 82: 1472–1479, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol Heart Circ Physiol 259: H1822–H1825, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Ton C, Hwang DM, Dempsey AA, Tang HC, Yoon J, Lim M, Mably JD, Fishman MC, Liew CC. Identification, characterization, and mapping of expressed sequence tags from an embryonic zebrafish heart cDNA library. Genome Res 10: 1915–1927, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda Y, Di Gregorio V, Parker RA, Levitsky S, McCully JD. Anti-stunning and anti-infarct effects of adenosine enhanced ischemic preconditioning. Circulation 102: 326–331, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda Y, Levitsky S, McCully JD. Opening of mitochondrial ATP-sensitive potassium channels enhances cardioplegic protection. Ann Thorac Surg 71: 1281–1289, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Tsukube T, McCully JD, Metz RM, Cook CU, Levitsky S. Amelioration of ischemic calcium overload correlates with high energy phosphates in the senescent myocardium. Am J Physiol Heart Circ Physiol 273: H418–H427, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Vondriska TM, Pass JM, Ping P. Scaffold proteins and assembly of multiprotein signaling complexes. J Mol Cell Cardiol 37: 391–397, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP-sensitive potassium channels during cardiopulmonary bypass decreases apoptosis and necrosis in a model of acute myocardial infarction. Eur J Cardiothorac Surg 21: 424–433, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White MY, Cordwell SJ, McCarron HC, Prasan AM, Craft G, Hambly BD, Jeremy RW. Proteomics of ischemia/reperfusion injury in rabbit myocardium reveals alterations to proteins of essential functional systems. Proteomics 5: 1395–1410, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Wieland T The toxic peptides from Amanita mushrooms. Int J Peptide Protein Res 22: 257–276, 1983. [DOI] [PubMed] [Google Scholar]
- 46.Wood ZA, Poole LB, Karplus A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Li J, Simons M, Li J, Laham RJ, Sellke FW. Expression of vascular endothelial growth factor and its receptors is increased, but microvascular relaxation is impaired in patients after acute myocardial ischemia. J Thorac Cardiovasc Surg 121: 735–742, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Yue Y, Lypony J, Hedhli N, Abdellatief M. Ras GTPase activity binds to Akt and is required for it's activation. J Biol Chem 279: 12883–12889, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Zong C, Young GW, Wang Y, Lu H, Deng N, Drews O, Ping P. Two-dimensional electrophoresis-based characterization of post-translational modifications of mammalian 20S proteasome complexes. Proteomics 8: 5025–5037, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.