Abstract
Bile acids are powerful detergents produced by the liver to aid in the absorption of dietary lipids. We recently reported a novel role for Foxa2 in bile acid metabolism. The winged helix transcription factor Foxa2 is required to prevent intrahepatic cholestasis and liver injury in mice fed a cholic acid-enriched diet. Here, we use functional genomics to study how Foxa2 regulates its targets in a cholic acid-dependent manner. We found that multiple signaling pathways essential for the hepatic response to acute liver injury are impaired in livers of Foxa2-deficient mice, suggesting that the deletion of Foxa2 in the hepatocyte affects the liver on a large scale. We also discovered distinct feed-forward regulatory loops controlling Foxa2-dependent targets in a cholic acid-dependent or -independent manner. We show that Foxa2 interacts with different transcription factors to achieve gene expression responses appropriate for each physiologic state.
Keywords: functional genomics, metabolic and regulatory networks
bile acids are oxidized derivatives of cholesterol produced by the liver that facilitate the absorption of dietary lipids. Bile acids activate the farnesoid X receptor (FXR), a nuclear hormone receptor regulating bile acid metabolism, and influence expression of numerous genes, including their own synthesis enzymes and transporters (23). Cholestasis, either from impaired bile flow or from intrahepatic causes, can lead to hepatic fibrosis, cirrhosis, and end-stage liver disease. Biliary atresia, a pediatric cholestatic disorder, is the most common indication for liver transplantation in children. Other biliary diseases, such as primary biliary cirrhosis and primary sclerosing cholangitis, also often require liver transplants in adults. Intrahepatic cholestasis of pregnancy can lead to serious complications for the mother and grave outcomes for the fetus (1). Therefore, understanding the mechanisms leading to bile acid-induced hepatic damage is crucial to treatment of these disorders.
Foxa2, formerly known as HNF-3β, is a liver-enriched forkhead box or winged helix transcription factor initially found to bind the promoters and regulate the expression of the α1-antitrypsin and transthyretin genes (7). Binding of Foxa factors to their target sites has been shown to be essential for several nuclear receptors to access their cis-regulatory elements in multiple tissues (4, 11, 25, 36). In the liver, Foxa2 is required to facilitate chromatin access of the glucocorticoid receptor (GR) for maximal induction of target genes during fasting (36). Using genomic location analysis and tissue-specific gene ablation, we recently showed that Foxa2 controls multiple genes involved in bile acid metabolism, including those encoding conjugation and detoxification enzymes, and transporters on both the sinusoidal and canalicular surface of hepatocytes. Consequently, deletion of Foxa2 in hepatocytes in Foxa2loxP/loxP;Alfp.Cre mice leads to mild hepatic cholestasis, which is worsened when Foxa2 mutants are challenged with a diet supplemented with cholic acid (CA), a natural ligand for FXR. Under these conditions, Foxa2 mutants show intrahepatic cholestasis, a disproportionate rise in serum bile acids, and significant liver injury (3).
To investigate why Foxa2-deficient mice exhibit a more dramatic phenotype on a CA diet than on normal chow, we used a functional genomic approach to study how Foxa2 regulates its targets in a CA-dependent manner. We hypothesized that the presence of Foxa2 binding sites near bile-acid response elements bound by FXR is required to activate a set of genes critical for the hepatic response to CA. We performed gene expression profiles of Foxa2 mutants on both standard and CA-supplemented diets and a genome-wide location analysis of Foxa2 binding in the liver to determine the subset of genes directly regulated by Foxa2 in each metabolic condition. When searching for cis-regulatory motifs in CA-responsive and CA-nonresponsive direct Foxa2 targets, we discovered distinct feed-forward regulatory loops in the network of genes regulated by Foxa2 in the two conditions. Thus, Foxa2 interacts with various transcription factors to achieve gene expression appropriate for different physiologic states.
MATERIALS AND METHODS
Animals.
The derivation of the Foxa2loxP/loxP;Alfp.Cre mouse model has been reported previously (24). We also used liver-specific Ncoa2 (SRC-2 F/F) knockout mice that have been characterized previously (5). Two- to three-month-old male mice were used in all studies. Mice were genotyped by PCR of tail DNA. The bile acid feeding study was performed as described previously (3).
RNA isolation and expression analysis.
Liver RNA was isolated from Foxa2loxP/loxP;Alfp.Cre and control littermates as reported previously (36). Sample RNA quantity and quality were determined using the Agilent Bioanalyzer RNA 6000 Nano Chip Kit. We amplified and labeled 500 ng of total RNA of each sample using the Quick Amp Labeling Kit (Agilent Technologies). We hybridized 825 ng of labeled cRNA to the Agilent 4x44k Whole Mouse Genome Oligo Microarray. The experimental design involved three direct comparisons: 1) cRNA from a wild-type mouse on standard chow was hybridized against cRNA from a Foxa2 mutant on standard chow, 2) cRNA from a wild-type mouse on CA diet was hybridized against cRNA from a Foxa2 mutant on CA diet, 3) cRNA from a wild-type mouse on standard diet was hybridized against labeled from a wild-type mouse on CA diet.
Four sets of biological replicates were used in each direct comparison experiment. The data were normalized by the Loess method using the limma (Linear Models for Microarray Data) package in R (Smyth, GK 2005). Statistical analysis of the microarray data was performed using the significance of microarrays (SAM) (29) package with a false discovery rate (FDR) of 15%. The data have been deposited into ArrayExpress (accession numbers E-MEXP-2106, E-MEXP-2107, and E-MEXP-2109). Quantitative RT-PCR was performed as described previously (36).
Chromatin immunoprecipitation and genome-wide location analysis.
Chromatin immunoprecipitation (ChIP) and the following real-time PCR reactions were performed as described (22). Snap-frozen mouse liver (100 mg) from wild-type and Foxa2 mutant mice (n = 4 animals per group) was used to prepare chromatin. Immunoprecipitation was performed with rabbit anti-Foxa2 serum (2). Immunoprecipitated DNA was amplified using ligation-mediated PCR (LM-PCR) as described previously (20). Two micrograms of amplified material were labeled using BioPrime Array CGH Genomic Labeling System and hybridized to the Mouse Promoter ChIP-on-Chip Microarray (Agilent Technologies). We utilized a direct comparison design with four sets of biological replicates, where labeled DNA immunoprecipitated from a wild-type mouse was hybridized against labeled DNA immunoprecipitated from a Foxa2 mutant. The data were analyzed using ChIP Analytics software (Agilent Technologies) and deposited into ArrayExpress (accession number E-MEXP-2111).
Analysis of overrepresented functional categories.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) 2008, a bioinformatics tool provided by the National Center for Biotechnology Information (NCBI: http://david.abcc.ncifcrf.gov/), was utilized to determine functional biological categories enriched in the set of 1,516 genes bound by Foxa2 in vivo. DAVID 2008 utilizes several annotation systems, including Gene Ontology categories, PIR, SwissProt keywords, and pathway maps to summarize the functional information in a large dataset. Functional categories were ranked based on co-occurrence probability, also known as the EASE-score, a metric detecting functional categories overrepresented in the target list compared with all the genes represented on the array platform used. Ingenuity Pathways Analysis (Ingenuity Systems, http://www.ingenuity.com) for Foxa2 targets was carried out as described previously (34).
Sequence analysis.
The sequence of each oligo tile (60 bp) bound by Foxa2 on the Mouse Promoter Microarray was extended by 120 bp on each side, producing a 300 bp sequence that was used to scan for transcription factor motifs. Background sequences (also 300 bp) were obtained randomly from the overlap of genes represented on both Agilent 4x44k Whole Mouse Genome Oligo Microarray and Agilent Mouse Promoter ChIP-on-Chip Microarray. All sequences were extracted using Galaxy (27). PWMScan software (14) was used to scan the sequences with positional weight matrices (PWMs) from the Transfac database (16), and the Asap program (15) was employed to scan PWMs from the JASPAR database (31). The Fisher's exact test was utilized to assess whether a given PWM was overrepresented in the Foxa2 bound sequence compared with the background set. A permutation test (n = 1,000) was used to evaluate the difference in distances from Foxa site between the motifs in CA-responsive and CA-nonresponsive groups.
Clustering.
The clustering analysis was performed using TIGR Multiexperiment Viewer Software (MeV; The Institute for Genomic Research, Rockville, MD).
RESULTS
Foxa2 regulates distinct gene modules in different physiological conditions.
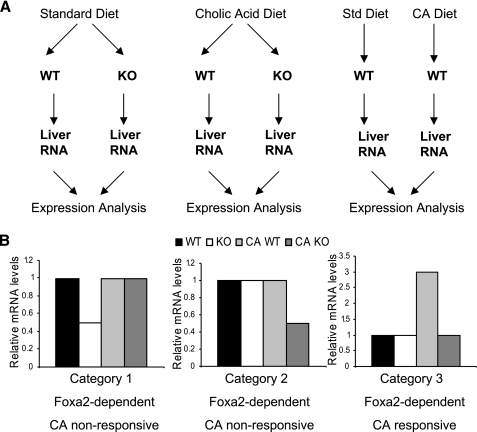
We recently reported that Foxa2 plays a significant role in bile acid metabolism, especially in mice fed a CA-containing diet (3). We hypothesized that the genes regulated by Foxa2 differ depending on the physiological condition and performed gene expression profiles of Foxa2 mutants (Foxa2loxP/loxP;Alfp.Cre) on standard chow and CA diet to investigate the molecular basis for the more severe phenotype in the latter condition (Fig. 1A).
Fig. 1.
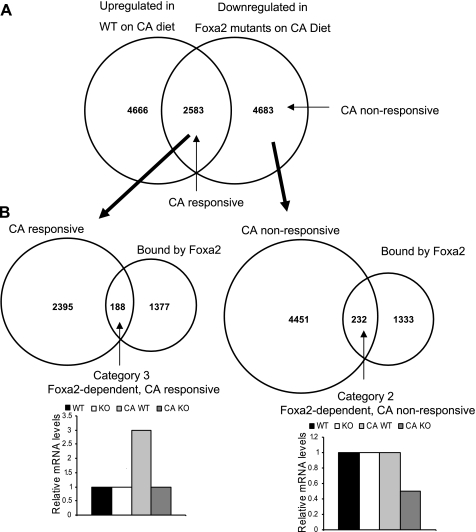
Experimental design for gene expression profiles of Foxa2 targets. A: experimental paradigms include comparing RNA from livers of wild-type (WT) and Foxa2-knockout (KO) on standard diet (left) and cholic acid (CA) diet (middle), and contrasting liver RNA from WT animals on the 2 diet treatments (right, n = 4 for each condition). B: genes classified as category 1 and category 2 are downregulated in Foxa2 knockouts on either diet treatment (Foxa2-dependent) but are not changed in WT animals on CA diet (CA nonresponsive). Foxa2-dependent and CA responsive genes (category 3) are those whose expression increased in WT mice on cholic diet compared with regular chow and was downregulated in Foxa2 mutants on CA diet compared with their control littermates on CA diet.
CA serves as a ligand of Fxr and has a massive effect on gene expression. Therefore, we also performed expression profiling of wild-type mice on CA diet (Experimental Design in Fig. 1) to identify the subset of genes activated by CA. Next we contrasted Foxa2-dependent genes that did not respond to CA (category 1 and category 2, Fig. 1B) with those that were responsive to CA (category 3, Fig. 1B). Since Foxa2 acts as a transcriptional activator (12), a gene was classified to be Foxa2-dependent if its mRNA levels were downregulated in Foxa2 mutants on either (or both) diets. If expression of a gene increased in wild type animals on the CA diet, that gene was characterized as CA-responsive. Finally, Foxa2-dependent/CA-responsive genes were classified as those whose expression increased in wild type mice on cholic acid diet compared with regular chow, and decreased in Foxa2 mutants on CA diet compared with their control littermates on the CA diet (category 3, Fig. 1B).
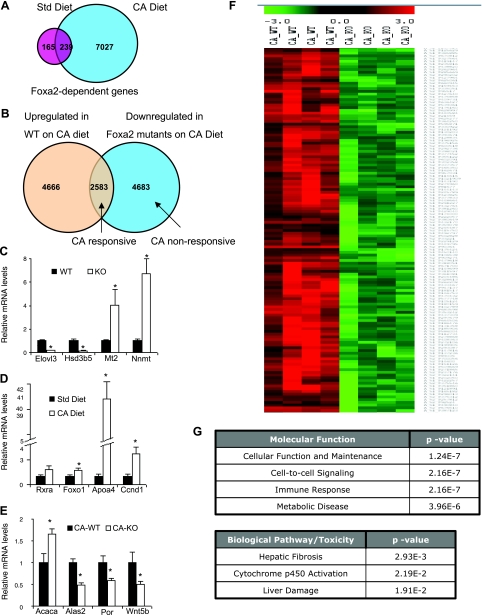
Consistent with the severe phenotype of Foxa2 mutants fed a CA-containing diet, the number of differentially expressed genes was greatly increased compared with Foxa2 mutants fed normal chow (404 genes downregulated on standard diet vs. 7066 on CA diet, Fig. 2A). Of the genes downregulated in livers of Foxa2-deficient mice fed the standard diet, 60% were also downregulated on the CA diet. Differential expression of thousands of genes in Foxa2 mutants on CA-supplemented feed is likely due to indirect effects, because these animals exhibit increased cholestatic liver injury (3), which induces proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) (28). In fact, expression of TNF-α was induced about six-fold in Foxa2-deficient mice fed the CA diet (Supplementary Table 1 1). TNF-α has been shown to decrease expression of Rxra, a nuclear receptor that heterodimerizes with Fxr, and coactivators Ppargc1a and Ppargc1b (10). Expression of these genes was indeed significantly reduced in Foxa2 mutants on the CA diet (Supplementary Table S2), explaining the magnitude of the change in gene expression in these mice.
Fig. 2.
Gene expression changes in Foxa2loxP/loxP;Alfp.Cre mice. A: the number of genes differentially expressed in Foxa2 mutant mice increases dramatically on the CA diet. B: the set of downregulated genes in Foxa2-deficient animals on CA diet is partitioned into CA responsive (those that are upregulated in WT animals fed the CA diet) and CA nonresponsive. Confirmation of Foxa2 targets on the standard diet (C), genes that were upregulated in WT mice on the CA diet (D), and Foxa2 targets fed a CA diet (E) by quantitative real-time PCR (QPCR). Values are represented as means ± SE. P values were determined by Student's t-test. *P < 0.05. F: clustering analysis of CA-responsive genes (red) that are downregulated in Foxa2 mutant mice on CA diet (green). G: analysis of functional categories overrepresented in genes summarized in F.
CA also had a considerable effect on gene expression in wild-type mice, modulating expression of >7,000 genes (Fig. 2B). Next, we intersected the set of CA-responsive genes with the set of genes downregulated in Foxa2-deficient mice on CA, dividing the set of Foxa2 targets into CA-responsive (37%) and CA nonresponsive (63%, Fig. 2B). We wanted to differentiate between the targets which are upregulated by CA as a physiological response to increased bile acid load and the damage it induces (CA-responsive) and are downregulated in absence of Foxa2 (category 3, Fig. 1B), and the targets that are downregulated in Foxa2 mutants due to other causes (category 1 and category 2). Multiple targets of Foxa2 that have been characterized previously (3) were also differentially expressed in our high-throughput experiments (5 on standard diet, 6 on the CA diet). We verified additional targets for all experiments described in Fig. 1A by quantitative real-time PCR (Fig. 2, C–E). We also performed a clustering analysis to pinpoint the genes that are CA-responsive and downregulated in Foxa2 mutants (Fig. 2F). The genes that respond to CA in wild type mice function in cellular maintenance, cell-to-cell signaling, and immune response (Fig. 2G), which is consistent with the inflammatory response associated with acute liver injury (26). The toxicity pathways overrepresented in Foxa2 mutants include hepatic fibrosis and liver damage (Fig. 2G).
Interestingly, functional analysis of the targets that respond to CA in wild-type animals but are downregulated in Foxa2loxP/loxP;Alfp.Cre mice also revealed a group of genes containing the LIM domain, a protein-protein interaction motif involved in cell fate specification, development, and cytoskeletal organization. Lhx1 (Lim1) has been shown to interact with Foxa2 during development (8), but its function in the adult liver is not known. Lhx2-null mice develop hepatic fibrosis (32). Both of these genes are induced by CA and downregulated in Foxa2 mutants on CA diet, suggesting an additional link between Foxa2 deficiency and liver injury.
Global analysis of Foxa2 occupancy.
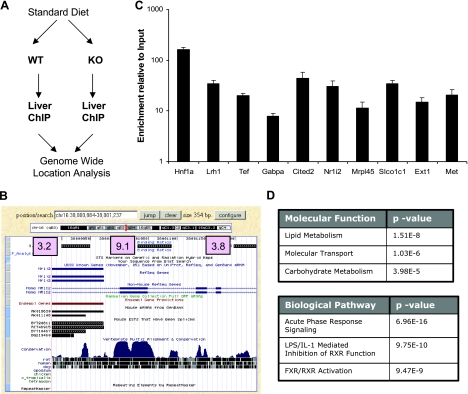
The gene expression changes in Foxa2 mutants fed a CA-containing diet were extensive. We wanted to isolate the direct transcriptional targets of Foxa2 to assess how binding of Foxa2 alters gene expression in this physiological condition. To this end we performed genome-wide location analysis to identify the set of genes directly bound by Foxa2 in vivo using the Agilent Promoter ChIP-on-Chip Microarray (Fig. 3A), with 60-mer oligos positioned every 240 bp. The design of this array simplifies the procedure of pinpointing the transcription factor binding site since the sequence space to be searched is small (Fig. 3B). This genome-wide location analysis yielded 1,516 regions as Foxa2 bound targets. The overlap between the current data set and our prior analysis utilizing a smaller promoter/enhancer array (3) was substantial (>50%), indicating the robustness of the technology. We evaluated the array results by quantitative real-time PCR (Q-PCR) of 10 randomly selected targets. All regions that were selected for verification confirmed Foxa2 binding (Fig. 3C).
Fig. 3.
Genome-wide location analysis of Foxa2 in adult liver. A: experimental paradigm for the ChIP-on-Chip assay (n = 4 for each group). B: USCS Genome Browser screenshot of Foxa2 binding at the Pxr (Nr1i2) promoter. The scores for each tile indicate that the binding site is located near the middle tile (score = 9.1), close to the evolutionarily conserved region. C: confirmation of 10 randomly chosen targets found by genome-wide location analysis by QPCR. D: analysis of functional categories overrepresented in gene set bound by Foxa2.
To distinguish which changes in the gene expression profile described above were due to direct Foxa2 regulation, we evaluated which functional categories and pathways were overrepresented in the Foxa2-bound targets compared with all genes represented on the Agilent array. The extended list of Foxa2 targets, just like the target list from previous ChIP-on-Chip analysis, contained clusters of categories with genes involved in lipid metabolism (Fig. 3D). In addition, the “molecular transport” category was found to be highly overrepresented (P value 1.03e-6), supporting our previous findings attributing the cholestatic phenotype of Foxa2 mutant mice to reduced expression of bile acid transporters. One of the top canonical pathways overrepresented among the Foxa2 targets was “FXR/RXR activation,” a pathway that is also consistent with the Foxa2 mutant phenotype, but which was not identified in our previous analysis due to the smaller number of targets represented on the BCBC Mouse Promoter array.
Foxa2 interacts with different transcription factors to achieve gene expression responses appropriate for each physiologic state.
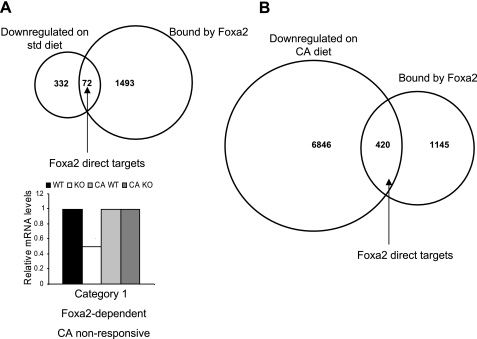
Next we identified the subset of genes changed in mRNA abundance in each physiologic condition. We intersected the sets of genes downregulated in Foxa2 mutants on both standard diet (Fig. 4A) and CA diet (Fig. 4B), with the set of regions bound by Foxa2 in liver chromatin, revealing 72 and 420 direct targets of Foxa2 in each condition, respectively (Supplemental Table S3). Direct Foxa2 targets on the standard diet (41 of which overlap with direct targets on the CA diet) function mainly in acute phase/stress response and xenobiotic metabolism, while direct Foxa2 targets on the CA diet also include genes involved in the stress response, as well as those functioning in lipid metabolism and the regulation of transcription. We further divided the set of 420 direct targets of Foxa2 on CA diet into a CA nonresponsive set of 232 genes and a CA-responsive set of 188 genes (categories 2 and 3, respectively; Fig. 5). This allowed us to distinguish between the genes that are upregulated by CA as a physiological response to increased bile acid load and those that are downregulated in Foxa2 mutants for another reason. We supplemented the set of CA nonresponsive targets with unique direct targets on standard diet (category 1, 29 targets) to complete the collection of all CA-nonresponsive genes, dependent on Foxa2 on either diet, and used scanning algorithms to detect enriched transcription factor binding motifs that were present in the regulatory elements of the CA-responsive and CA-nonresponsive genes.
Fig. 4.
Direct targets of Foxa2. A: intersecting the set of genes downregulated in Foxa2 mutant mice on standard diet (404 total) and those bound by Foxa2 in liver chromatin (1,516 total) produces 72 direct targets of Foxa2. B: the overlap between the set of genes downregulated in Foxa2loxP/loxP;Alfp.Cre animals on CA diet (7,066 total) and those bound by Foxa2 (1,516 total) consists of 420 direct targets of Foxa2.
Fig. 5.
Direct Foxa2 targets partition into CA-responsive and CA-nonresponsive sets. A: the set of downregulated genes in Foxa2-deficient animals on CA diet was partitioned into CA responsive (those that are upregulated in WT animals on CA diet) and CA nonresponsive. B: the 2 sets of genes in A were intersected with the set of genes bound by Foxa2 to produce the Foxa2-dependent and CA-responsive and Foxa2-dependent and CA-nonresponsive groups.
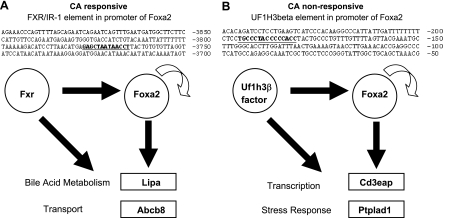
In addition to the expected numerous forkhead motifs enriched among Foxa2 targets that were identified as statistically significant, we found that motifs for ligand activated nuclear receptors (Fxr, Er, Pxr, Rora) were overrepresented exclusively in the CA-responsive targets (Table 1). The only nuclear receptor shared between the two categories was Hnf4α, which is known to co-regulate many genes in the liver with Foxa2 (18). The motifs that were present in CA-nonresponsive Foxa2-dependent targets included those of more ubiquitously expressed transcription factors (Usf, Creb), Hnf1, a motif called UF1-H3β, and a consensus sequence closely resembling that site (Egr). The element “UF1-H3β” was described previously as a sequence in the Foxa2 promoter for an ubiquitous DNA binding protein, essential to the promoter activity of Foxa2 itself (19). It is still not clear which transcription factor binds this sequence. However, in addition to appearing in the Foxa2 promoter, the UF1-H3β motif is overrepresented in regulatory elements of direct CA nonresponsive targets of Foxa2. Conversely, the IR-1 response element for FXR is present in the promoter of Foxa2 gene, as well as in regulatory elements of CA responsive direct targets of Foxa2 (Fig. 6). These distinct feed-forward regulatory loops elucidate how Foxa2 itself might be regulated.
Table 1.
Overrepresented motifs in direct targets of Foxa2
| Category | Transfac PWM | Transfac ID | P Value | Median Distance (Range) | Genes, n |
|---|---|---|---|---|---|
| CA responsive | PXR_Q2 | M00964 | 4.58E-07 | 73 (14–136) | 44 |
| CA responsive | ER_Q6_02 | M00959 | 6.87E-07 | 64 (0–180) | 48 |
| CA responsive | FXR_IR1_Q6 | M00767 | 8.46E-06 | 50 (6–133) | 31 |
| CA responsive | DR3_Q4 | M00966 | 3.97E-04 | 63 (0–193) | 45 |
| CA responsive | RORA1_01 | M00156 | 5.37E-04 | 61.5 (0–150) | 33 |
| CA nonresponsive | USF2_Q6 | M00726 | 7.60E-05 | 29 (5–238) | 24 |
| CA nonresponsive | EGR_Q6 | M00807 | 9.56E-05 | 47 (0–180) | 63 |
| CA nonresponsive | UF1H3BETA_Q6 | M01068 | 2.63E-04 | 32 (0–166) | 62 |
| CA nonresponsive | HNF1_01 | M00132 | 8.28E-04 | 35 (0–214) | 28 |
| CA nonresponsive | CREB_Q2_01 | M00916 | 1.91E-03 | 52 (8–141) | 41 |
PWM, positional weight matrix; CA, cholic acid.
Fig. 6.
Regulation of Foxa2. A: an FXR/IR-1 element is present in the promoter of the mouse Foxa2 gene. FXR/IR-1 sites are also overrepresented in cis-regulatory regions of Foxa2-dependent CA-responsive targets. B: an UF1-H3β element is located in the promoter of the Foxa2 gene. UF1-H3β-like sequences are also overrepresented in cis-regulatory regions of Foxa2-dependent CA-nonresponsive targets. The curved arrow represents the autoregulatory loop of Foxa2 regulation.
We computed the distances between the Foxa site and motifs for the other transcription factors in the regulatory elements of CA-responsive and CA-nonresponsive genes and found that, in general, the two presumed binding sites are located closer to each other in the CA nonresponsive modules (Table 1). The IR-1 response element for Fxr is the exception and is the only nuclear receptor motif that is closer to the Foxa site than any motif in the CA nonresponsive category. A permutation test revealed that this partition for the two categories was statistically significant (P value <0.016).
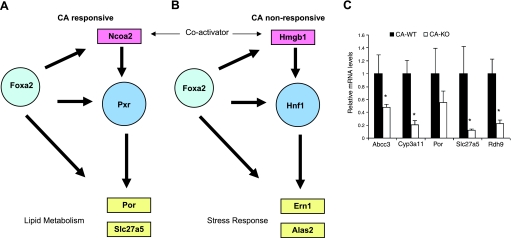
We also examined the relationship between Foxa2 and other transcription factors, whose motifs were overrepresented in regulatory elements of Foxa2 targets. We observed that Foxa2 also binds to the cis-regulatory elements of several of these transcription factors, as well as the coactivators that act on these DNA-binding proteins. In a traditional regulatory feed-forward loop, (FFL, shown in Fig. 6A) a given transcription factor, the hub in the network, regulates another transcription and its targets. We add another element to the conventional FFL, whereby Foxa2 binds also to the regulatory elements of co-activators of the transcription factor it regulates. These loops are distinct for CA-responsive and CA-nonresponsive targets (Fig. 7). In the case of CA-responsive genes, Foxa2 binds to the promoter of Pxr, as well as the promoter of Ncoa2 (SRC-2), a nuclear receptor coactivator that recruits chromatin remodeling enzymes. In the CA-nonresponsive network, Foxa2 binds the promoter of Hnf1α and a nonhistone chromosomal protein that stabilizes nucleosomes, Hmgb1, which in a protein-protein interaction helps Hnf1α bind DNA (13, 35). It has been reported previously that Foxa transcription factors can open compacted chromatin (6), but our data shows that Foxa2 also binds regulatory elements of proteins involved in chromatin remodeling, affecting the network through its targets on a larger scale.
Fig. 7.
Feed-forward loops in the hepatic gene regulatory networks dependent on Foxa2. A: in the set of CA-responsive genes, Foxa2 binds to regulatory elements of the nuclear receptor Pxr, its coactivator Ncoa2, and several target genes. B: in the set of CA-nonresponsive genes, Foxa2 binds to the regulatory elements of the homeodomain transcription factor Hnf1, its coactivator Hmgb1, and its target genes Ern1 and Alas2. C: confirmation of direct Foxa2 targets in livers of Ncoa2 (SRC-2) knockout mice fed a CA diet by QPCR. Values are represented as means ± SE. P values were determined by Student's t-test. *P < 0.05.
We found that Foxa2 regulates other transcription factors and coactivators, (Pxr and Ncoa2), which then contribute to the CA-mediated regulation of expression of a variety of target genes involved in lipid metabolism. To test our model, we evaluated whether CA regulation of these genes is altered in Ncoa2 (SRC-2) knockout mice. Expression of Slc27a5 was significantly downregulated in Ncoa2-deficient livers of mice fed a CA diet, while expression of Por showed a downward trend. Also, expression of Abcc3 and Cyp3a11, known Pxr targets, was significantly reduced in Ncoa2-deficient mice on the CA diet. We have characterized Cyp3a11 as a direct Foxa2 target and reported that expression of Abcc3 is decreased in Foxa2loxP/loxP;Alfp.Cre mice fed a CA diet (3), while others have shown that Abcc3 is also bound by Foxa2 (33). In addition, mRNA levels of Rdh9, a direct target of Foxa2 with a predicted Fxr binding site (Supplementary Table S4) were also reduced in Ncoa2-deficient livers.
In summary, we found that multiple signaling pathways necessary for the liver's response to acute liver injury are impaired in livers of Foxa2-deficient mice. We also discovered distinct feed-forward regulatory loops in the CA-responsive and CA nonresponsive direct targets of Foxa2, showing that Foxa2 interacts with different transcription factors to achieve gene expression responses appropriate for each physiological state.
DISCUSSION
Binding of Foxa2 to its targets in the adult liver has been studied previously by several groups, but none thus far has integrated occupancy data with changes in mRNA levels in Foxa2 deficient mice in an orthogonal analysis. Rada-Iglesias and colleagues (21) found 196 targets corresponding to 154 unique human genes and reported that FOXA2 often binds to distal regulatory elements. Odom and colleagues (18) performed ChIP for liver-enriched transcription factors in human hepatocytes and assigned 890 targets of FOXA2. They focused on describing the crosstalk between several hepatic transcription factors but did not carry out a functional analysis of the biological targets of Foxa2 themselves. In a subsequent study, Odom and colleagues (17) compared Foxa2 binding in mouse and human in the regions represented in both genomes (574 targets in mouse and 151 in human) and concluded that in vivo binding has diverged considerably between the two organisms.
The latest analysis of Foxa2 binding in the mouse liver, using massively parallel sequencing (ChIP-Seq), identified 5,060 genes as Foxa2 targets, 2,781 of which also contained a SAGE tag, suggesting they were expressed in adult liver (33). Binding of several of these targets was confirmed by quantitative real-time PCR (QPCR). The authors searched for other cis-regulatory motifs near the Foxa2 binding site, characterizing the distribution of Gata4, Hnf1, and Pax6 motifs relative to the Foxa2 sequence. Although the Pax6 motif was found to be overrepresented among Foxa2 targets, that transcription factor is not expressed in mature hepatocytes. Hnf1α is known to co-regulate many genes in the liver with Foxa2 (18). The authors provided a possible explanation for co-occurrence of Gata4 motif near the Foxa2 site, suggesting that both could regulate chromatin structure in general, but did not address the presence of the Pax6 motif near Foxa2 consensus sites in liver chromatin. Knocking down Foxa2 expression in Hepa1-6 hepatoma cells to test whether Foxa2 regulates the bound targets led to reduction in expression of six of the nine genes tested, suggesting that a significant portion of targets found to be bound in vivo by this study are not affected by change in Foxa2 expression, at least in the conditions studied.
We also have performed a genome-wide location analysis of Foxa2 in adult mouse liver previously using a custom array (Mouse PromoterChip BCBC-5A) and characterized the targets (107 in total) based on the strength of Foxa2 consensus motif, finding that genes with a weaker Foxa2 binding consensus are more liver-specific (30). As mentioned above, we had previously employed ChIP-on-Chip to discover a novel role for Foxa2 in bile acid metabolism (3).
To investigate why Foxa2loxP/loxP;Alfp.Cre mice exhibit a more dramatic phenotype on a CA-enriched diet, we used a comprehensive functional genomic approach to examine how Foxa2 regulates its targets in a CA-dependent manner. CA activates thousands of genes in a physiological response to increased bile acid load, many of which are downregulated in the absence of Foxa2. We found that signaling pathways necessary for the liver's response to acute liver injury are impaired in livers of Foxa2-deficient mice. We extended our analysis of the set of genes directly bound by Foxa2 in vivo by using a larger platform and detected 1,516 target regions. Unlike previous studies of transcriptional regulation by Foxa2 in the adult liver, we concentrated on direct targets of Foxa2, genes that are bound and whose expression is downregulated in Foxa2-deficient mice. Wederell and colleagues reported that only 43.5% of the genes that were found to be Foxa2-bound by massive parallel sequencing are expressed in the adult liver (33). While various binding studies can inform on all potential genes that could be affected by Foxa2, we have shown that expression of a specific subset of bound genes is changed in absence of Foxa2 in the adult liver and that the set of regulated genes depends on in the physiological condition. It is likely that other bound targets of Foxa2 could be influenced by Foxa2 status in other physiological situations, or, since tissue-specific regulation is often modular (20), that expression of several transcription factors might need to be reduced to observe a change in the expression of a given target.
In general, a transcription factor activates its targets by binding to a specific sequence in the DNA and interacting with cofactors to modify chromatin and to recruit the basal transcriptional machinery. Tissue-specific gene regulation is combinatorial, as cis-regulatory modules often comprise binding sites for multiple transcription factors. Several groups have attempted to chart the transcriptional regulatory circuits operative in hepatocytes by combining genome-wide location analysis with computational predictions [Kyrmizi et al. (11a); Odom et al. (18)], but we are the first to show that these cis-regulatory modules depend on the physiological state examined.
Naturally occurring and synthetic ligands for nuclear receptors, such as CA, are typically employed to study the function of specific nuclear receptors. Here we demonstrate that a winged helix transcription factor regulates its targets differentially depending on the presence of a ligand to a nuclear receptor, i.e., Fxr. We have shown that the number of both direct and indirect targets of Foxa2 increases dramatically in mice fed a CA diet, reinforcing the notion that Foxa2 plays an important role in bile acid metabolism. We also discovered distinct feed-forward regulatory loops controlling the CA-dependent and -independent targets of Foxa2, suggesting that Foxa2 interacts with different transcription factors to achieve gene expression appropriate for each physiological state. In addition, we validated our predicted regulatory loop in the CA condition, showing that CA regulation of several Foxa2 targets involved in lipid metabolism is blocked in Ncoa2 (SRC-2) mutant mice, as predicted by our model.
Finally, we located several motifs present in the promoter of Foxa2, which are also overrepresented among the regulatory elements of Foxa2 targets, hinting at how Foxa2 itself could be regulated in different conditions. It is possible that bile acid stimulate Fxr to activate transcription of Foxa2 in an acute condition, while its expression, like that of bile acid synthesis enzymes and certain transporters, is repressed in chronic cholestatic injury. Previous reports have demonstrated reduced expression of Foxa2 in rodent models of cholestasis (9, 37), while we showed that FOXA2 expression is decreased in livers of human patients with different cholestatic syndromes (3). Understanding the mechanisms leading to bile acid-induced hepatic damage and how Foxa2 is regulated will be helpful to the treatment of these disorders.
GRANTS
I. M. Bochkis was supported by Penn Genomics Institute Graduate Fellowship. This work was supported by National Diabetes and Digestive and Kidney Diseases Institute Grant DK-049210 to K. H. Kaestner.
Acknowledgments
We thank Sridhar Hannenhalli for valuable discussions and the use of PWMScan software, Jeffrey Whitsett (Cincinnati Children's Hospital Medical Center) for providing rabbit polyclonal antibody to Foxa2, and Elizabeth Helmbrecht for care of the mice.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Arrese M, Macias RI, Briz O, Perez MJ, Marin JJ. Molecular pathogenesis of intrahepatic cholestasis of pregnancy. Expert Rev Mol Med 10: e9, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 5: 193–208, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med 14: 828–836, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322: 1395–1399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 9: 279–289, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Costa RH, Grayson DR, Darnell JE Jr. Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol 9: 1415–1425, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foucher I, Montesinos ML, Volovitch M, Prochiantz A, Trembleau A. Joint regulation of the MAP1B promoter by HNF3beta/Foxa2 and Engrailed is the result of a highly conserved mechanism for direct interaction of homeoproteins and Fox transcription factors. Development 130: 1867–1876, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Geier A, Dietrich CG, Grote T, Beuers U, Prufer T, Fraunberger P, Matern S, Gartung C, Gerbes AL, Bilzer M. Characterization of organic anion transporter regulation, glutathione metabolism and bile formation in the obese Zucker rat. J Hepatol 43: 1021–1030, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C, Feingold KR. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism 56: 267–279, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. From the Cover: Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA 102: 11651–11656, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev 20: 2293–2305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai E, Prezioso VR, Tao WF, Chen WS, Darnell JE Jr. Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev 5: 416–427, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Landsman D, Bustin M. Assessment of the transcriptional activation potential of the HMG chromosomal proteins. Mol Cell Biol 11: 4483–4489, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy S, Hannenhalli S. Identification of transcription factor binding sites in the human genome sequence. Mamm Genome 13: 510–514, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Marstrand TT, Frellsen J, Moltke I, Thiim M, Valen E, Retelska D, Krogh A. Asap: a framework for over-representation statistics for transcription factor binding sites. PLoS ONE 3: e1623, 2008. [DOI] [PMC free article] [PubMed]
- 16.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, Voss N, Stegmaier P, Lewicki-Potapov B, Saxel H, Kel AE, Wingender E. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–D110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, Fraenkel E. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet 39: 730–732, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odom DT, Dowell RD, Jacobsen ES, Nekludova L, Rolfe PA, Danford TW, Gifford DK, Fraenkel E, Bell GI, Young RA. Core transcriptional regulatory circuitry in human hepatocytes. Mol Syst Biol 2: 0017, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pani L, Quian XB, Clevidence D, Costa RH. The restricted promoter activity of the liver transcription factor hepatocyte nuclear factor 3 beta involves a cell-specific factor and positive autoactivation. Mol Cell Biol 12: 552–562, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phuc Le P, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet 1: e16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rada-Iglesias A, Wallerman O, Koch C, Ameur A, Enroth S, Clelland G, Wester K, Wilcox S, Dovey OM, Ellis PD, Wraight VL, James K, Andrews R, Langford C, Dhami P, Carter N, Vetrie D, Ponten F, Komorowski J, Dunham I, Wadelius C. Binding sites for metabolic disease related transcription factors inferred at base pair resolution by chromatin immunoprecipitation and genomic microarrays. Hum Mol Genet 14: 3435–3447, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Rubins NE, Friedman JR, Le PP, Zhang L, Brestelli J, Kaestner KH. Transcriptional networks in the liver: hepatocyte nuclear factor 6 function is largely independent of Foxa2. Mol Cell Biol 25: 7069–7077, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Sund NJ, Ang SL, Sackett SD, Shen W, Daigle N, Magnuson MA, Kaestner KH. Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol Cell Biol 20: 5175–5183, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Yu X, Chaurand P, Araki Y, Lareyre JJ, Caprioli RM, Matusik RJ, Orgebin-Crist MC. Epididymis-specific promoter-driven gene targeting: a transcription factor which regulates epididymis-specific gene expression. Mol Cell Endocrinol 250: 184–189, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int 28: 1052–1064, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Taylor J, Schenck I, Blankenberg D, Nekrutenko A. Using galaxy to perform large-scale interactive data analyses. Curr Protoc Bioinformatics 10: Unit 10.5, 2007. [DOI] [PMC free article] [PubMed]
- 28.Tilg H Cytokines and liver diseases. Can J Gastroenterol 15: 661–668, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuteja G, Jensen ST, White P, Kaestner KH. Cis-regulatory modules in the mammalian liver: composition depends on strength of Foxa2 consensus site. Nucleic Acids Res 36: 4149–4157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlieghe D, Sandelin A, De Bleser PJ, Vleminckx K, Wasserman WW, van Roy F, Lenhard B. A new generation of JASPAR, the open-access repository for transcription factor binding site profiles. Nucleic Acids Res 34: D95–D97, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci USA 101: 16549–16554, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wederell ED, Bilenky M, Cullum R, Thiessen N, Dagpinar M, Delaney A, Varhol R, Zhao Y, Zeng T, Bernier B, Ingham M, Hirst M, Robertson G, Marra MA, Jones S, Hoodless PA. Global analysis of in vivo Foxa2-binding sites in mouse adult liver using massively parallel sequencing. Nucleic Acids Res 36: 4549–4564, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White P, May CL, Lamounier RN, Brestelli JE, Kaestner KH. Defining pancreatic endocrine precursors and their descendants. Diabetes 57: 654–668, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Wang J, Li W, Yuan YZ, Li CY, Qian XH, Xu WX, Zhan YQ, Yang XM. Proteomic screen defines the hepatocyte nuclear factor 1alpha-binding partners and identifies HMGB1 as a new cofactor of HNF1alpha. Nucleic Acids Res 36: 1209–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab 2: 141–148, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, Denk H, Trauner M. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol 289: G798–G805, 2005. [DOI] [PubMed] [Google Scholar]