Abstract
Task-dependent modulation of primary afferent depolarization (PAD) was studied in the cervical spinal cord of two monkeys performing a wrist flexion and extension task with an instructed delay period. We implanted two nerve cuff electrodes on proximal and distal parts of the superficial radial nerve (SR) and a recording chamber over a hemi-laminectomy in the lower cervical vertebrae. Antidromic volleys (ADVs) in the SR were evoked by intraspinal microstimuli (ISMS, 3–10 Hz, 3–30 μA) applied through a tungsten microelectrode, and the area of each ADV was measured. In total, 434 ADVs were evoked by ISMS in two monkeys, with onset latency consistently shorter in the proximal than distal cuffs. Estimated conduction velocity suggest that most ADVs were caused by action potentials in cutaneous fibers originating from low-threshold tactile receptors. Modulation of the size of ADVs as a function of the task was examined in 281 ADVs induced by ISMS applied at 78 different intraspinal sites. The ADVs were significantly facilitated during active movement in both flexion and extension (P < 0.05), suggesting an epoch-dependent modulation of PAD. This facilitation started 400–900 ms before the onset of EMG activity. Such pre-EMG modulation is hard to explain by movement-induced reafference and probably is associated with descending motor commands.
INTRODUCTION
Presynaptic inhibition regulates transmission at synapses in the central nervous systems of vertebrates and invertebrates (Rudomin and Schmidt 1999). By modulating the release of transmitter from presynaptic terminals, it controls the effectiveness of specific inputs to postsynaptic neurons. Several neurotransmitters mediate presynaptic inhibition (Miller 1998); the most extensively studied mechanism involves the GABAA receptor (Curtis and Lodge 1982; Curtis et al. 1995; Eccles et al. 1963a). In the spinal cord, activation of GABAA receptors on the intraspinal terminals of afferent axons produces so-called primary afferent depolarization (PAD) by opening chloride channels and allowing the efflux of Cl− ions from the terminals (Alvarez-Leefmans et al. 1988; Gallagher et al. 1978). This depolarization increases the terminal membrane conductance (Curtis et al. 1995) and inactivates sodium channels (Graham and Redman 1994) and voltage-gated Ca2+ channels (Graham and Redman 1994; Walmsley et al. 1995), all of which may reduce neurotransmitter release. PAD has been shown in the intraspinal terminals of afferents from Ia (Eccles et al. 1962b), Ib (Eccles et al. 1963b), group II (Harrison and Jankowska 1989), and cutaneous (Eccles et al. 1962a) receptors of cat hindlimb.
The GABAergic neurons that induce PAD in afferent terminals are activated mainly by peripheral afferents and descending systems. PAD of cutaneous afferent terminals is induced by stimulating other cutaneous afferents (Eccles et al. 1963c), descending tracts from motor cortex (Andersen et al. 1964; Carpenter et al. 1962; Eguibar et al. 1994), the nucleus raphe magnus, and reticular formation (Martin et al. 1979; Quevedo et al. 1995). The central control of PAD suggests that presynaptic inhibition plays a significant role in regulating peripheral sensory input during volitional behavior, a function that remains to be experimentally studied. Studies using combined reflex tests in human subjects (Hultborn et al. 1987) suggested the modulation of presynaptic inhibition of Ia afferents during voluntary movement, but PAD itself cannot be directly evaluated by such noninvasive techniques. Presynaptic inhibition of cutaneous afferents has never been examined in these studies.
Several lines of evidence suggest that the PAD system may be involved in the control of dynamic motor behaviors. During fictive and real locomotion, intra-axonal recordings of cutaneous (Gossard et al. 1990) and muscle afferents (Duenas and Rudomin 1988) in cat showed that the size of PAD changed as a function of the locomotor cycle. Similar modulation has also been reported in cat lumbar segments during fictive scratching (Baev et al. 1978; Cote and Gossard 2003). These results suggest that central pattern generators for autonomous rhythmic movement can modulate the size of PAD in a task-dependent manner (Cote and Gossard 2003).
In 1958, Wall first proposed that PAD could be documented by “excitability testing” (Wall 1958). In Wall's test, the terminals of primary afferents are electrically stimulated via an extracellular microelectrode in the spinal cord. The number of axons antidromically activated, and consequently the size of the antidromic volley (ADV) measured in a peripheral nerve, is dependent on the level of depolarization in the terminals. This method has shown that PAD in group I afferents is modulated during fictive locomotion in the cat (Duenas and Rudomin 1988). Ghez and Pisa (1972) reported that, during reaching in the awake cat, the afferent volley in the medial lemniscus was reduced and the ADVs in cutaneous nerves were increased, showing for the first time that PAD is involved in presynaptic inhibition in the dorsal column nuclei during voluntary movements.
Using methods to record and stimulate the cervical spinal cord in awake, behaving monkeys (Perlmutter et al. 1998), we showed that the monosynaptic responses of first-order cutaneous interneurons were suppressed during active wrist movement (Seki et al. 2003). This suppression was associated with facilitation of ADVs. In this study, we used excitability testing to evaluate the size of spinal PAD of different peripheral afferents during performance of an instructed delay task. Results indicate that the PAD in cutaneous afferent terminals is modulated during voluntary movement in a functionally relevant manner. Preliminary results were presented in abstract form (Seki et al. 2004).
METHODS
Subjects
We obtained data from two male Macaca nemestrina (monkeys K and M). The technique for recording ADVs in cutaneous afferents was optimized in the first animal (monkey K), and the basic characteristics and behavioral modulation of ADVs were obtained using that technique in the second monkey (monkey M). Experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington. During training and recording sessions, the monkeys sat upright in a primate chair with the right arm restrained and elbow bent at 90°. The hand was held in a cast with the fingers extended and the wrist in the mid-supination/pronation position. The cast holding the monkey's hand was attached to a servomotor-driven manipulandum that measured flexion-extension torques about the wrist. The left arm was loosely restrained to the chair.
Behavioral paradigm
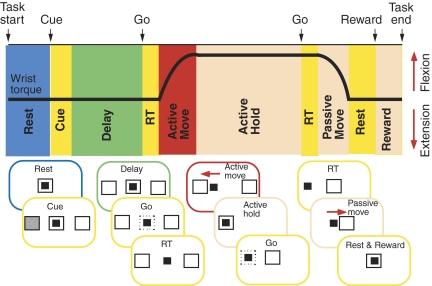
The monkeys performed a wrist flexion-extension task with an instructed delay period (Fig. 1). The position of a cursor displayed on a video monitor in front of the monkey was controlled by flexion-extension torque about the wrist. Trials began with the monkey holding the cursor in a center target window, corresponding to zero torque, for 1.3–1.6 s (rest). Next, flexion and extension targets were shown to the left and right of the center target. One target was filled transiently for 0.3 s (cue), indicating the correct movement to be performed at the end of the instructed delay period (delay), which was signaled by the disappearance of the center target (go). Trials were accepted only if no wrist movement occurred during the delay period (1.5–2 s). Following a brief reaction time (RT) after the go signal, the monkey moved (active move) the cursor to the desired target quickly (<1.5 s including RT) and held the cursor in the target window for a period of 1.5 s (active hold). The movements were made against an elastic load applied by the servo motor. At the end of the active hold period, the torque target disappeared and the center target reappeared (2nd go). After a second reaction time (RT), the monkey relaxed the forearm muscles, allowing the servo-spring to passively return the wrist (passive move) to the zero torque position (rest). After keeping the cursor within the center target for 0.8 s, the monkey was rewarded with applesauce (reward) for successful trials.
FIG. 1.
Wrist flexion-extension task. Typical torque trace during a single flexion trial is shown with task epochs. Diagrams below depict the cursor controlled by the monkey (small filled square) and targets (larger squares) on video screen for the 10 epochs: 1st rest, cue, delay, 1st reaction time (RT), active move, active hold, 2nd RT, passive move, 2nd rest, reward.
Surgical implants
After training, surgeries were performed aseptically with the animals under 1–1.5% isoflurane anesthesia. Head stabilization lugs were cemented to the skull with dental acrylic, anchored to the bone via screws. A stainless steel recording chamber was implanted over a hemi-laminectomy in the lower cervical vertebrae (C4–T1; Perlmutter et al. 1998). Pairs of stainless steel wire (AS632, Cooner Wire) were implanted subcutaneously in 10–12 muscles [extensor carpi ulnaris (ECU), extensor carpi radialis (ECR), extensor digitorum communis (EDC), extensor digitorum-2,3 (ED-2,3), extensor digitorum-4,5 (ED-4,5), flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), flexor digitorum profundus (FDP), flexor digitorum superficialis (FDS), palmaris longus (PL), pronator teres (PT), abductor pollicis longus (APL), supinator (SUP), and brachioradialis (BR)] that were active in one or both directions. Each muscle was identified on the basis of its anatomic location and characteristic movements elicited by trains of low-intensity intramuscular stimuli.
Nerve cuffs
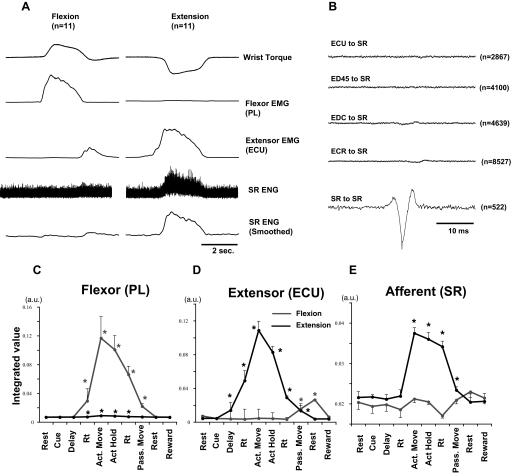
Two cuff electrodes (Haugland 1996) were implanted on the superficial radial nerve (SR; see Fig. 3): a distal bipolar cuff for stimulation (approximately midway between elbow and wrist) and a tripolar cuff for recording antidromic volleys [evoked by intraspinal microstimulation (ISMS)] and orthodromic volleys (evoked by stimulation through distal cuff). The proximal cuff was implanted ∼4–5 cm proximal to the distal cuff. Movement-induced activity and evoked potentials of the SR were recorded in the proximal cuff by three circumferential electrodes (platinum foil, 25-μm thickness, 1-mm width, 6-mm spacing) embedded in the silicone rubber tubing. The differential recording was obtained from the central electrode referenced to the two outer electrodes, which were connected together. This “center versus tied ends” recording geometry proved optimal for reducing pickup of EMG and other noise generated by sources external to the nerve cuff (Hoffer 1990). Typical examples of SR activity recorded by this electrode are shown in Fig. 2 A. Clear bursts of activity were recorded during extension movements. Nerve recordings may be susceptible to remote pick up of surrounding muscle activity. To test this possibility, EMG-triggered averages of the SR electroneurogram (ENG) were aligned on the positive motor unit potentials in the EMG from four extensor muscles. As shown in Fig. 2B, there was no significant peak in the average ENG, suggesting that burst activity recorded differentially by the nerve cuff during active extension arose from activated cutaneous receptors, not from surrounding muscle.
FIG. 2.
Activities of superficial radial nerve (SR) and muscles during wrist flexion and extension. A: typical records of average wrist torque, activity in superficial radial nerve (SR) and muscles (flexor, extensor) during flexion (left) and extension (right) trials (11 trials). Low-pass filter (3 Hz) was used to smooth EMG and electroneurogram (ENG) profiles. Flexor [palmaris longus (PL)] and extensor [extensor carpi ulnaris (ECU)] muscles generally showed reciprocal pattern of activity during task, and SR was active during extensor torque. B: test for possible cross-talk between EMG and ENGs. Averages of unrectified ENG were triggered from wrist extensor muscle or nerve activity when EMG exceeded a threshold level, just above the baseline noise of unrectified EMG. Note that there were no significant peaks in any EMG-to-SR average (i.e., <10% of the SR-to-SR peak). C--E: mean ± SE EMG (arbitrary units) of PL, ECU, and ENG of SR during each behavioral epoch (14 flexion, 13 extension trials were summed). *Significantly different from activity during pretrial rest, P < 0.05.
ISMS
During recording sessions, the head and vertebral implants were secured to the primate chair and a microdrive was attached to the chamber via an X-Y positioning stage. Tungsten microelectrodes were advanced into the C6–T1 segment while the monkey performed wrist flexion and extension movements in an instructed delay task. To find intraspinal sites where SR afferent terminals were located, SR was stimulated through the distal cuff (Fig. 3A), evoked volleys were recorded in the proximal cuff and cord dorsum, and synaptic responses of spinal neurons were recorded as single unit activity and local field potentials (LFPs) by the tungsten electrode. The intraspinal locations of SR terminal afferents were identified as sites where monosynaptic responses of single units (Seki et al. 2003) and/or LFPs were found. We applied ISMS (0.1-ms bipolar pulses, 3–10 Hz, 3–30 μA) through the microelectrode during task performance and recorded and averaged the antidromic action potentials in the proximal SR cuff electrode (Fig. 3B). One hundred fifteen (monkey K) and 112 (monkey M) tracks were made during a period of 5 mo. None of the animals exhibited observable behavioral deficits at any time.
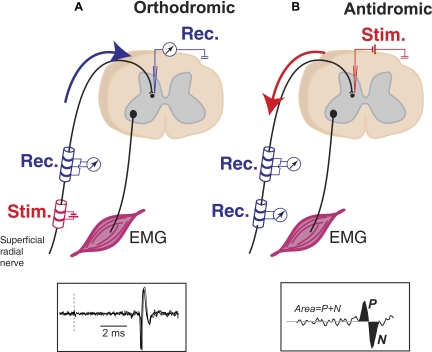
FIG. 3.
Orthodromic and antidromic responses from SR. A: before the excitability testing, intraspinal terminals of SR were localized by finding interneurons that responded monosynaptically to electrical stimulation of SR. Single-unit or field potentials were recorded using a microelectrode inserted into the spinal gray matter. Bottom inset: successive monosynaptic responses of interneuron to stimulation at dashed line. B: microstimuli were applied to the electrodes at the recording sites to activate the terminal of SR afferents. Antidromic volleys (ADVs) evoked by the stimulation were recorded by the cuff electrodes. Inset: size of the ADVs was measured by area of positive and negative peaks.
Excitability testing during voluntary movement
Stimulus-triggered averages (StTAs) of SR ADVs elicited from individual spinal sites were compiled separately for the 10 behavioral epochs (Fig. 1). The magnitude of an ADV was defined as its area (Fig. 3B, inset). The bins with maximal (peak) and minimal (trough) amplitudes were first identified from a comprehensive average of the ADV compiled for all stimuli and all behavioral epochs. This average was also used to identify onset and offset times and the time of the inflection between the peak and trough of the volley. The inflection was defined as the time that the average waveform crossed the mean of the baseline, taken as the period from 10 to 5 ms before the intraspinal stimulation. The area of individual nonaveraged ADVs was measured by summing the values of each bin from onset to inflection and from inflection to offset and subtracting the latter from the former. This method is better than adding absolute values because it avoids contribution from noise. For each behavioral epoch, the mean areas of individual ADVs were statistically compared relative to the control period (rest; see Fig. 1) using Student's t-test or ANOVA.
RESULTS
Task-related activity of SR nerve (ENG) and forearm muscles (EMG)
We first examined the task-related activity of SR afferents and forearm muscles by ENG and EMG recordings, respectively (Fig. 2). EMG activity generally started toward the end of the delay period or during the reaction time, reached its maximum during active movement, and decreased during the hold period (Fig. 2, C and D). In contrast, SR activity started after the onset of active extension movements and was maintained until the onset of passive flexion (Fig. 2E). This pattern is consistent with stimulation of SR receptive fields on the dorsum of the hand, which was pressed against the cast holding the monkey's hand during extension movements. The onset of SR activity after EMG onset suggests that it is a movement-induced reafferent signal.
Antidromic volleys evoked in the primate SR nerve
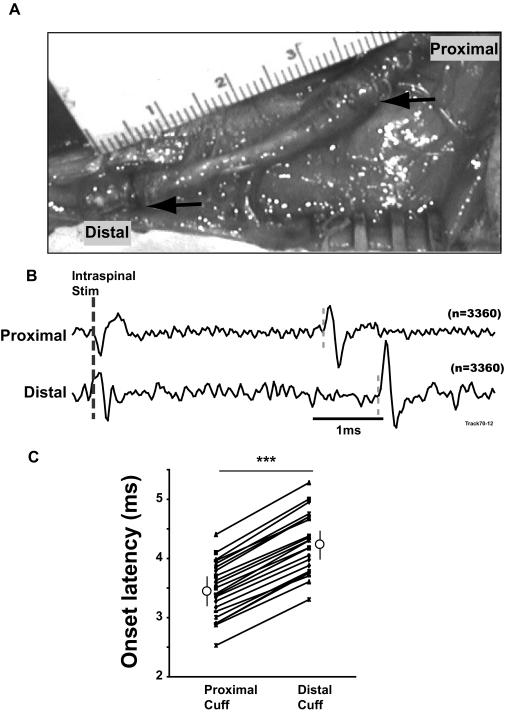
Stimuli were applied at intraspinal sites and evoked responses were recorded with two cuff electrodes separated by 45 mm (center-to-center) on SR (Fig. 4). The timing of the responses recorded by the proximal and distal cuffs confirmed that the volleys were conducted antidromically. For the representative stimulus-triggered averages shown in Fig. 4B, the onset latency of the response in the proximal cuff (3.5 ms) was earlier than that in the distal cuff (4.3 ms). StTAs of EMG activity (data not shown) did not show any stimulus-locked features that could contribute to the volleys. ISMS at all 22 tested sites evoked responses with statistically significantly shorter latencies in the proximal than distal cuffs (Fig. 4C).
FIG. 4.
Antidromically conducted responses in SR induced by intraspinal microstimulation (ISMS). A: Tri-polar (proximal) and bipolar (distal) cuff electrodes implanted on SR. B: Responses in the proximal and distal cuffs evoked by intraspinal stimulation (4 μA). C: comparison of the onset latencies of the responses recorded in the 2 cuff electrodes (n = 22). Open circles and vertical lines show mean ± SD of the data. ***P < 0.001.
ADVs appearing in StTAs (e.g., Fig. 4B) can be generated by two possible mechanisms. The volley could be 1) the antidromic response of a single axon, in which case its amplitude would reflect the axon's response probability to the stimulation, or 2) a compound action potential representing the summed action potentials evoked in different axons with similar conduction velocity (CV). One example that may represent the former mechanism is shown in Fig. 5. This example shows sizable ADVs that could be seen without averaging (Fig. 5A). Using a dual time-amplitude window discriminator to detect these potentials (Fig. 5A, blue lines), we compiled raster plots and peristimulus time histograms aligned with the stimulus (Fig. 5B). These ADVs showed negligible jitter in latency. When ISMS was delivered with increasing current intensity, the ADVs appeared in an all-or-none fashion (Fig. 5, C and D), as would be expected for the firing probability of a single afferent. Further examples shown in Fig. 6 may represent both mechanisms. ISMS with large enough stimulus intensity often recruited multiple volleys at separate latencies (Fig. 6A). For some individual volleys, plotting the size of the volley as a function of stimulus current (Fig. 6, B and C) showed sharp recruitment curves (Fig. 6C) that saturated at the highest current strengths. These properties resemble the response probability of a single SR axon. On the other hand, the area of some antidromic volleys increase linearly with increasing stimulus current. This property suggests the recruitment of multiple SR axons with similar CV were recruited at higher currents.
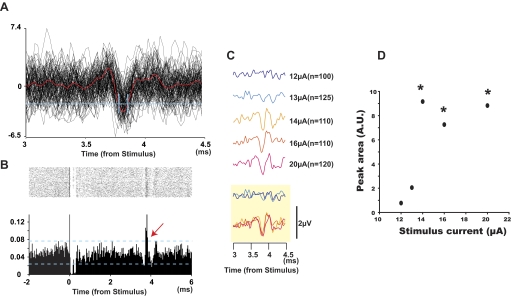
FIG. 5.
Antidromic responses in single afferent. A: superposition of 100 raw traces recorded from SR aligned with ISMS; mean waveform is shown in red. A dual time-amplitude window discriminator was used to discriminate individual antidromic responses (threshold level and 2 time windows shown in blue). B: dot raster and peristimulus time histogram (PSTH) of all discriminated events in A show time-locked responses around 3.8 ms (red arrow). C: responses evoked by different stimulus currents: 12–13 μA evoked no volley; 14–20 μA evoked volleys with a fixed amplitude. Overlapping plots of traces shown at bottom (yellow box). D: peak area of the ADVs as a function of stimulus current. A.U. means arbitrary units. This “all-or-none” recruitment pattern suggests that responses were from a single SR afferent. *Significantly different from the background noise measured 2–3 ms after each stimulus, P < 0.05.
FIG. 6.
Recruitment curves of ADVs. A: increasing stimulus currents usually recruited multiple volleys with different latencies. B and C: sizes of ADVs as a function of the stimulus currents for 2 sites. B refers to sweeps in A. Note that the amplitudes of volleys 3 and 4 in B and 2 and 5 in C saturated at the higher currents tested.
Afferent fibers conveying information of specific modalities have preferential ranges of CV. The CV of ADVs was estimated as the distance from the proximal cuff to the dorsal root entry zone of the cervical spinal cord (measured postmortem) divided by the latency of the volley recorded in the proximal cuff. Figure 7 shows that the CVs from the two monkeys were distributed between 3.8 and 91.6 m/s. The mean CV was 55.2 m/s in monkey M (Fig. 7A) and 66.5 m/s in monkey K (Fig. 7B).
FIG. 7.
Conduction velocity of recorded antidromic responses. Distribution of conduction velocity of ADVs in monkey M (A) and monkey K (B). Second axis also shows onset latency from ISMS. A: mean ± SD (open circles and lines) was 55.2 ± 13.9 m/s (4.95 ± 4.99 ms from stimulus). B: mean ± SD was 66.5 ± 9.9 m/s (5.58 ± 1.27 ms).
Task-dependent modulation of afferent fiber excitability
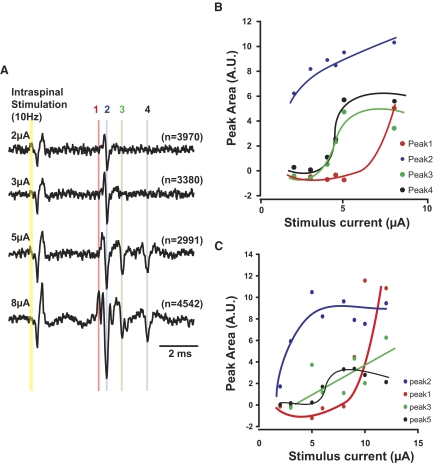
At 78 intraspinal sites in monkey M, we applied ISMS while the monkey performed the wrist flexion-extension task and compared the size of ADVs across behavioral epochs. Typical results of such excitability testing are shown in Fig. 8. In these averages computed for different epochs, the first volley was not modulated significantly during the phases of flexion trials (Fig. 8B). The second volley showed significant facilitation (P < 0.05) during active flexion movement (Fig. 8C). This result suggests that the excitability of the terminals of two SR afferents near the electrode tip were modulated differently during active flexion (but see Fig. 10 for an alternative interpretation of this result).
FIG. 8.
Excitability testing during wrist flexion-extension task. A: ADVs in SR induced by ISMS of a single intraspinal site in each behavioral epoch during flexion trials; stimulus time is at the left of the traces. Two distinct volleys (gray lines) were apparent in all behavioral epochs. B and C: mean ± SE of the amplitude of ADVs with earlier (B) and later (C) latencies in each behavioral epoch. *Significant difference relative to rest (P < 0.05). Number of responses averaged for each trace is shown in parentheses.
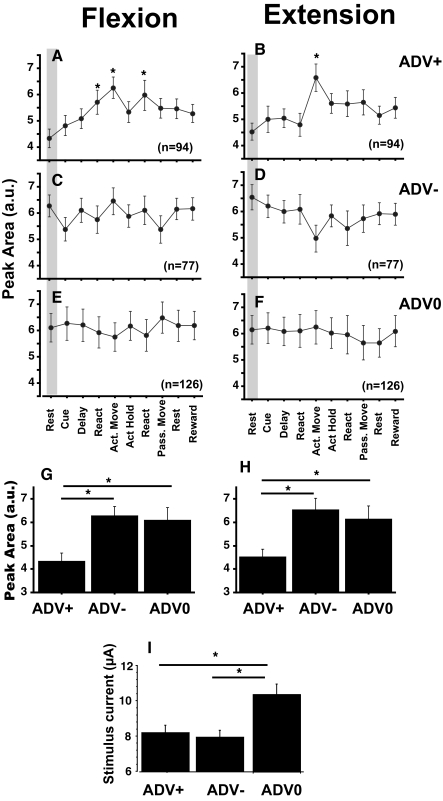
Similar task dependence was analyzed for all ADVs recorded (n = 281). The results of the excitability testing were subdivided into three groups according to the modulation pattern during the task. The volleys that showed facilitation or suppression in at least one behavioral epoch were categorized as ADV+ and ADV−, respectively, and the remaining nonmodulated volleys were categorized as ADV0. Sixteen ADVs were facilitated during one epoch and suppressed during another; these were included in both ADV+ and ADV− categories. Figure 9 shows the average size of ADVs evoked from all spinal sites, separated into category, movement direction, and behavioral epoch. Significant facilitation of the volley was found during active movement in both flexion and extension and during the reaction time before active flexion and passive extension (P < 0.05; Fig. 9, A and B). In both ADV− and ADV0 groups, no significant modulation was found for the population (P < 0.05; Fig. 9, C–F). These results suggest that ADV was frequently facilitated during active movement in both flexion and extension but that there were no preferred epoch for ADV suppression.
FIG. 9.
Modulation of the size of average ADVs. A–F: mean ± SE area of ADVs evoked from all spinal sites during the 10 behavioral epochs in flexion (left) and extension (right) trials. Volleys were separated into groups that exhibited facilitation (ADV+), suppression (ADV−), or no modulation (ADV0) in at least 1 behavioral epoch relative to the rest period. Numbers of ADVs in each group are shown in each panel. *Significantly different from volley during rest (shaded), P < 0.05. G and H: comparison of the area of ADVs during rest. I: stimulus current used to evoke volleys in each category.
Specificity of afferents involved in the task-dependent modulation
To determine the target specificity of ADV modulation, we compared the size of ADVs compiled during rest (control) in the ADV+, ADV−, and ADV0 groups (Fig. 9, G and H). The size of the control volleys in the ADV+ group was smaller than that in the ADV− and ADV0 groups. This result suggests that ADV facilitation during active movement (Fig. 9, A and B) occurs preferentially in afferent terminals responding with lower probablility at rest.
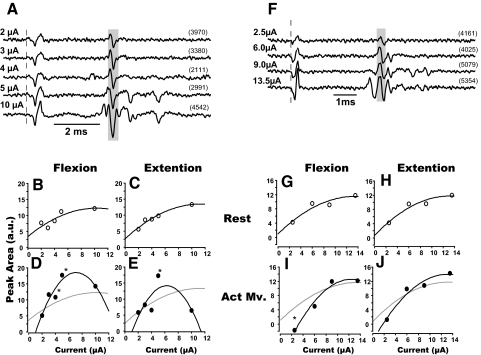
In addition, Fig. 9I shows that the modulation of ADV size was preferentially detected when we used a lower stimulus current. It is possible that high currents applied at rest activated afferents with a firing probability near 100%, masking any modulation of ADV during the task. To select a stimulus current that is optimal to evaluate ADV modulation, it is necessary to document the recruitment curve of each ADV (as shown in Figs. 5 and 6). However, it was usually impossible to maintain recording stability long enough to elicit ADVs with a number of different stimulus currents, so we used a single fixed current randomly selected between 5 and 15 μA for many of the excitability tests. Therefore in some cases, ADV modulation was probably evaluated using stimulus current outside of the recruitment range. Two examples for which both ADV modulation and recruitment range were measured (Fig. 10, A–E and F–J) support this interpretation. The behavioral modulation of these two volleys was tested with different stimulus currents. The recruitment range of one ADV (Fig. 10A) seemed to be <8 μA (Fig. 10, B and C). Facilitation during active movement occurred only when the volley was evoked using 4- to 5 μA current and not at 10 μA. In this case, we would not have been able to observe behavioral modulation had we tested excitability with currents of 2 or 10 μA. The dynamic range of the volley shown in Fig. 10, F–H, seemed to be <6 μA (Fig. 10, G and H), and significant reduction of its size during active movement was only observed for 2 μA currents. These examples suggest that our results underestimate the extent of behavioral modulation of ADV. Some ADVs in the ADV0 (e.g., the 1st component of Fig. 8, A and B) group might have shown modulation had we used lower currents within their dynamic range.
FIG. 10.
Recruitment properties of ADVs during active movements. A and F: ADVs induced by ISMS (broken line) at 2 different intraspinal sites with various current strengths (all epochs combined). B–E and G–J: changes of the amplitude of ADVs as a function of the stimulus current during rest (B, C, G, and H) and active movement (D, E, I, and J) for flexion (B, D, G, and I) and extension (C, E, H, and J). *Significantly larger volley during active movement vs. rest period for the same stimulus current, P < 0.05. Note that ADV facilitation was observed only when current of 4–5 μA was used. Gray lines in D, E, I, and J are fits for data at rest for comparison.
We also compared the estimated CV for the ADVs that showed significant facilitation, suppression, or no modulation (Fig. 11). As shown in Fig. 11, A–C, the distribution of CVs for the ADV0 group was shifted to slower speeds. The difference between the mean velocities for the groups was statistically significant (Fig. 11D). This suggests that faster-conducting afferents may be a preferential target for behavioral modulation.
FIG. 11.
Conduction velocities. Histograms of conduction velocities (CVs) of ADVs with task-dependent facilitation (A, ADV+), suppression (B, ADV−), and no modulation (C, ADV0). The mean ± SE CVs of these groups were statistically different (D, P < 0.05). ADVs with CVs of <20 m/s (open bars in A–C) were not included in the statistical test.
Potential source of the ADV modulation
In an awake animal performing voluntary movement, the spinal cord receives inputs from descending tracts (e.g., corticospinal tract) that convey motor commands and from peripheral afferents that carry feedback signals from sensory receptors that are stimulated by self-induced movement (reafference). Contributions from these two sources of modulation can be distinguished by comparing the onset latency of the modulation to the earliest onset latencies of muscle activity during wrist movements. Any modulation before the onset of earliest muscle activity should be associated with the motor command because there is no reafference signal during this period. Using this criterion, we examined the potential source of the significant modulation of the ADVs during active movement and reaction time (Fig. 9). This analysis concerned only volleys that were facilitated or suppressed during the reaction or active movement periods.
Figure 12 shows the size of ADVs relative to the onset time of the activity of PL (flexor) and ED45 (extensor) muscles, separated into ADV± groups and flexion and extension trials. None of the other agonist muscles had earlier onset times. ADV enhancement started before EMG, and the size of the volleys continued to increase after EMG onset. In contrast, ADV suppression was only evident after EMG onset. There is also a difference between ADV modulation before flexion and extension movements. Increases in ADV area start significantly earlier in flexion (900 ms) than extension (400 ms) trials. These results suggest that reafference can facilitate or suppress the size of ADV, but motor commands exclusively facilitate the ADV during preparation and initiation of movements.
FIG. 12.
Premovement onset of PAD modulation. Mean area of antidromic potentials that showed facilitation (top) or suppression (bottom) during active movement and/or reaction time were plotted relative to EMG onset (left: PL; right: ED45). Open circle at left of each panel shows ADV area during rest period. *Mean amplitude significantly different from rest, P < 0.05. Vertical axis, area of ADV (au); horizontal axis, time from onset of EMG (ms).
DISCUSSION
ISMS induced antidromic volleys in cutaneous afferents of awake, behaving monkeys
This study showed that ADVs induced by ISMS can be recorded in the awake behaving monkey using implanted nerve cuff electrodes. Previous studies with anesthetized or decerebrated animals documented ADVs evoked by ISMS through intra-axonal recordings in the spinal cord (Eccles and Krnjevic 1958; Gossard et al. 1989; Jimenez et al. 1988) or the proximal stump of transected dorsal rootlets (Beloozerova and Rossignol 1999, 2004; Cote and Gossard 2003). Ghez and Pisa (1972) first used cuff electrodes to document modulation of ADVs evoked by stimulation of cuneate nucleus in cats performing forelimb movements. Similarly, we used pairs of cuff electrodes and stimulus-triggered averaging to document ADVs in the intact SR.
What determines the size of the antidromic volley?
As described above, the size of an averaged ADV can be changed during voluntary movement or by applying different strengths of electrical stimuli. Amplitude changes could reflect 1) changes in firing probability of a single SR axon, 2) variable recruitment of multiple axons with nearly identical CVs, or 3) both.
Changes in the firing probability would pertain if ADVs represent action potentials of single cutaneous afferents, as suggested by the evidence in Figs. 5 and 6. Threshold current to recruit these volleys was usually <10 μA (Figs. 6 and 10), which is comparable to the intraspinal threshold for antidromic activation of single muscle afferents by glass microelectrodes placed near the afferent terminals (Duenas and Rudomin 1988). The areas of ADVs increased in a sigmoid manner with increasing stimulus current (Figs. 5 and 6, B and C), probably representing the enhancement of firing probability of these afferent terminals in response to the ISMS. These ADVs saturated at currents (e.g., 5–10 μA in Fig. 6, B and C) well below the intensities needed to maximize the area of compound antidromic action potentials of cat lateral gastrocnemius nerve (160 μA) (Enriquez-Denton et al. 2004).
Alternatively, ADVs may reflect multiple recruited axons that have essentially the same conduction velocity. In that case, a change in the amplitude of ADV could be ascribed to changes in the number of recruited axons, not only the modulation of firing probability of each terminal. In this study, activation of separate afferents can be detected if their CVs were different, as shown in Fig. 6A, consistent with a broad range of CVs (Fig. 7). Peak-to-peak time of a typical ADV is ∼0.1–0.3 ms. Differences in axon latencies of this duration could significantly reduce the size of averaged ADVs and make them difficult to detect among the noisy background. In monkey M, the ADVs of axons with CVs of 50 and 55 m/s would have a latency difference of 0.4 ms. Therefore to create a significant ADV by summation of multiple action potentials, the axons must have essentially identical conduction velocities. It is known that the effective radius of current spread for a 10 μA stimulus is ∼80 μm for cortical PT cells (Stoney et al. 1968) or 200–300 μm for intraspinal axons (Gustafsson and Jankowska 1976). Considering the highly divergent pattern of intraspinal branching of primary afferents (Willis and Coggeshall 1991), it is possible that the terminals of SR axons with similar CVs (i.e., differing by <5 m/s) are localized within this range. Therefore the modulation of the size of ADV could be ascribed, at least in part, to changes in the recruitment of SR axons with similar CVs.
These two mechanisms are hard to dissociate definitively in our experimental conditions, but fortunately in both cases a change in PAD leads to a covarying change in the ADV. If the ADV is the action potential of a single afferent, increased PAD would increase its firing probability, leading to increased size of the average ADV. If the ADV represents a superposition of multiple action potentials with the same CV, increased PAD could, in addition, lead to an increase in the number of axons recruited. Because our goal is to detect changes in PAD, which underlies both mechanisms, we will not attempt to distinguish between them in the rest of the discussion.
Antidromic volley and conduction velocity
The range of CVs for ADVs in this study agrees well with those reported previously for the SR in squirrel monkeys (4–88 m/s) (Perl 1968). The CVs of >97% of ADVs were larger than that of nociceptors (28 m/s) (Perl 1968). This result is consistent with the fact that most ADVs were evoked from intraspinal sites at which cells showed monosynaptic responses to low levels of SR stimulation (<2 times threshold for dorsal root afferent volley). Therefore most ADVs were probably caused by action potentials in cutaneous fibers originating from low-threshold tactile receptors.
Another mechanism that could conceivably contribute to the ADVs is the dorsal root reflex, namely recurrent potentials in peripheral nerve triggered by sufficiently intense PAD. A dorsal root reflex would induce antidromic potentials with longer latencies (for review, see Willis 1999) and with significant jitter in their timing because of the variability in synaptic transmission (Shefner et al. 1992). Such fluctuations in latency were not seen in successive averages of the same ADVs (Figs. 5, 6, 8, and 10). Therefore the ADVs with stable longer latencies were unlikely to be caused by a dorsal root reflex but probably represent antidromic volleys of slowly conducting fibers, possibly unmyelinated nociceptive fibers. These long-latency ADVs were relatively rare (3% in the nociceptor range), possibly because the intraspinal site of ISMS were deeper than layers I–III, where small fibers terminate most densely (Willis and Coggeshall 1991).
Modulation of PAD during voluntary movement
We previously reported that the monosynaptic responses of spinal first-order interneurons evoked from SR are suppressed during active wrist movements and that descending motor commands probably contribute to the suppression, because it starts 400 ms before the onset of EMGs (Seki et al. 2003). We further showed preliminary evidence that ADVs evoked by ISMS can be facilitated during active movement in the same task, suggesting that presynaptic inhibition contributes to the suppression of monosynaptic responses. The results presented here document more fully the facilitation of ADVs found during active flexion and extension (Fig. 9), its onset before EMGs (Fig. 12), and its dependence on stimulus intensity (Figs. 5 and 6).
MODULATION AFTER EMG ONSET.
As shown in Fig. 12, the ADV size increased rapidly after the onset of muscle activity. This probably reflects increased PAD from self-induced afferent feedback after movement onset. Activity in SR occurred predominantly during active extension and the hold period of extension trials. We recently found that natural stimulation (brushing) applied to the receptive field of SR increases the PAD of SR (Seki and Takei 2006). (for effects on the PAD of muscle afferents, see Aimonetti et al. 2000). Therefore during active extension ADV facilitation is probably induced, at least in part, by reafference. However, ADV size also increased during flexion trials. We recently found that the intraspinal terminals of some SR afferents can be depolarized by brushing the palm, in the receptive field of the median nerve (Seki and Takei 2006). Active wrist flexion could mechanically stimulate the palmar skin, allowing reafference through the median nerve to facilitate PAD in SR terminals. It seems that reafference from both the same and different afferents (Eccles et al. 1963c) can contribute to PAD modulation of SR afferents after the onset of movements.
MODULATION BEFORE EMG ONSET.
As shown in Fig. 12, the size of ADVs started to increase before the onset of EMG in both flexion and extension trials. Such pre-EMG modulation cannot be explained by movement-induced reafference and consequently is attributed to descending motor commands. The results identify two interesting features of PAD modulation in awake behaving monkeys.
First, in extension trials, the size of ADVs increased 400 ms before EMG onset. The suppression of the monosynaptic responses of interneurons to SR stimulation during active movement (cf. Fig. 5 in Seki et al. 2003) follows the same time course. However, 100 ms before the onset of EMG, the size of the ADVs returned to control levels and then increased again after EMG onset (Fig. 12). It is possible that a transient suppression of PAD of cutaneous afferents may contribute to the changes of reflex output at movement initiation, as in the case of presynaptic inhibition on the muscle afferents of human subjects (Hultborn et al. 1987; Kagamihara and Tanaka 1985; Nielsen and Kagamihara 1993). Because most intraspinal sites that receive monosynaptic responses from SR have inhibitory projections to motoneuron pools, the facilitation of presynaptic inhibition may induce disinhibition of agonistic muscles (Seki et al. 2003). As shown previously (Seki et al. 2003), inhibitory projections from these intraspinal sites are widespread and suppress both agonist and antagonist muscles. Therefore reducing disinhibition to both agonistic and antagonistic muscles just before voluntary movements could reduce the stiffness of the wrist joint and facilitate movement initiation.
Second, in flexion trials, the facilitation of ADVs began 900 ms before the onset of EMG (Fig. 12) and increased gradually during the instructed delay and reaction time periods (Fig. 9A). This suggests that, during an instructed delay period, descending commands preferentially suppress the input from SR for flexion but not extension movements. Recently, it has been shown that spinal interneurons show instructed delay activity using the same behavioral paradigm as this study (Prut and Fetz 1999). Many interneurons exhibit increases in activity during the delay, and these changes are mostly unidirectional. Unidirectional facilitation of PAD may also contribute to “set-related” activity of spinal neurons.
It has been proposed that normal ADVs may serve to control sensory input during fictive (Beloozerova and Rossignol 1999) and treadmill (Beloozerova and Rossignol 2004) locomotion. If such ADVs occur during voluntary wrist movement and collide with orthodromic discharge (Gossard et al. 1999) in the SR afferent, the descending command for voluntary movement could also control afferent input by inducing ADVs. Further study is needed to confirm this possibility.
Target specificity of PAD modulation in SR afferents
Growing evidence suggests that presynaptic modulation of synaptic transmission is rather target specific (Rudomin and Schmidt 1999; Schmidt 1971). For example, the degree of presynaptic inhibition on the terminal of group II afferents are different between the dorsal horn and intermediate zone of the lumber spinal cord in the cat (Jankowska et al. 2002). We found two properties that might suggest the target specificity of presynaptic inhibition of cutaneous afferent during voluntary movement.
First ADVs showing task-related facilitation had smaller amplitudes at rest than those which were suppressed or unmodulated during the task (Fig. 9, G and H). This suggests that the terminals with large ADVs that could not be facilitated may have PAD at rest that is saturated. On the other hand, ADV+ and ADV− volleys were both evoked by relatively low stimulus currents (Fig. 9I). Therefore it seems likely that the amplitude of the applied current does not account entirely for the size of the evoked ADVs. Instead, there seems to be a difference in the intrinsic properties of the ADV+ and ADV− terminals.
Second, as shown in Fig. 11, task-related modulations of the ADVs were more frequently observed in faster-conducting SR afferents, suggesting that sensory feedback from tactile receptors is suppressed by presynaptic inhibition during movement. This result may contradict the hypothesis that feedback from tactile receptors coding limb position is an important signal for movement control (Edin and Abbs 1991). However, it has also been proposed (Collins et al. 1998) that without some attenuation, the magnitude of sensory inflow to the CNS during movement would be so large as to be unusable. It is possible that that PAD-mediated presynaptic inhibition may reduce sensory feedback from tactile receptors to keep its modulation in a functional range.
As mentioned above, we cannot reject the possibility that the size of some ADVs reflects the number of recruited axons with the same CVs, not just the firing probability of single terminals. In this case, the smaller amplitudes of the ADV+ than ADV− volleys suggests a smaller average PAD in the terminals of the axons making up the ADV+ than ADV− groups (resulting in recruitment of fewer axons for a given stimulus current). Furthermore, it seems likely that the average level of PAD of the terminals of axons with the faster CVs could be preferentially modulated during movement.
Comparison with behavior related modulation of PAD in other systems
In their ground-breaking study, Ghez and Pisa (1972) were the first to apply excitability testing in an awake behaving animal. They stimulated the cuneate nucleus with an implanted metal electrode and recorded antidromic responses in the superficial radial nerve in cats performing voluntary movements with the ipsilateral forelimb. Their results in feline dorsal column nuclei were quite similar to ours in primate cervical spinal cord. They showed that facilitation of the ADV occurred for both flexor and extensor movements and preceded the onset of EMG by >100 ms. The similarity of the results in these two studies indicates that inputs from cutaneous afferents are attenuated in similar fashion at both major first-order relay points. At both sites, attenuation of sensory input is mediated at least partially by presynaptic inhibition and induced by descending motor commands before movement. This phenomenon explains the increase in sensory detections thresholds observed during active movements (Chapman and Beauchamp 2006; Milne et al. 1988). In addition to modulating ascending conduction, presynaptic inhibition at the spinal level also serves to protect the descending motor commands to interneurons from interference from variable reafferent input (Seki et al. 2003). To the extent that supraspinal motor centers are also subject to such interference, the same protective function could result from presynaptic inhibition of ascending conduction at both relay sites.
In addition to the somatic cutaneous pathways, presynaptic inhibition is also known to operate in various relays of the visual (Pecci-Saavedra et al. 1966), olfactory (Ennis et al. 2001), and trigeminal (Baldissera et al. 1967) systems. Comparison of the functional roles of presynaptic inhibition at other sensory relays could show general principles of sensorimotor integration during voluntary behavior.
GRANTS
This work was supported by National Institutes of Health Grants NS-12542, NS-040867, and RR-00166 and Human Frontiers Science Program Grant LT0070/1999-B.
Acknowledgments
We thank J. Garlid, S. Gilbert, L. Shupe, and S. Votaw for technical assistance.
REFERENCES
- Aimonetti JM, Vedel JP, Schmied A, Pagni S. Mechanical cutaneous stimulation alters Ia presynaptic inhibition in human wrist extensor muscles: a single motor unit study. J Physiol 522: 137–145, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol 406: 225–246, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Sears TA. Cortically evoked depolarization of primary afferent fibers in the spinal cord. J Neurophysiol 27: 63–77, 1964. [DOI] [PubMed] [Google Scholar]
- Baev KV, Panchin Iu V, Skryma RN. Depolarization of primary afferents during fictitious scratching of thalamic cats. Neirofiziologiia 10: 173–176, 1978. [PubMed] [Google Scholar]
- Baldissera F, Broggi G, Mancia M. Primary afferent depolarization of trigeminal fibres induced by stimulation of brain stem and peripheral nerves. Experientia 23: 398–400, 1967. [DOI] [PubMed] [Google Scholar]
- Beloozerova I, Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. I. Studies at rest and during fictive locomotion. Brain Res 846: 87–105, 1999. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. II. Studies during treadmill locomotion. Brain Res 996: 227–236, 2004. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Lundberg A, Norrsell U. Effects from the pyramidal tract on primary afferents and on spinal reflex actions to primary afferents. Experientia 18: 337–338, 1962. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Beauchamp E. Differential controls over tactile detection in humans by motor commands and peripheral reafference. J Neurophysiol 96: 1664–1675, 2006. [DOI] [PubMed] [Google Scholar]
- Collins DF, Cameron T, Gillard DM, Prochazka A. Muscular sense is attenuated when humans move. J Physiol 508: 635–643, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Task-dependent presynaptic inhibition. J Neurosci 23: 1886–1893, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Gynther BD, Beattie DT, Lacey G. An in vivo electrophysiological investigation of group Ia afferent fibres and ventral horn terminations in the cat spinal cord. Exp Brain Res 106: 403–417, 1995. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lodge D. The depolarization of feline ventral horn group Ia spinal afferent terminations by GABA. Exp Brain Res 46: 215–233, 1982. [DOI] [PubMed] [Google Scholar]
- Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res 70: 15–25, 1988. [DOI] [PubMed] [Google Scholar]
- Eccles J, Schmidt RF, Willis WD. Depolarization of central terminals of group Ib afferent fibers of muscle. J Neurophysiol 26: 1–27, 1963b. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol 161: 237–257, 1962a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Krnjevic K. Potential changes recorded inside primary afferent fibers within the spinal cord. J Physiol 149: 250–273, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol 160: 62–93, 1962b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J Physiol 168: 500–530, 1963a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Depolarization of the central terminals of cutaneous afferent fibers. J Neurophysiol 26: 646–661, 1963c. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991. [DOI] [PubMed] [Google Scholar]
- Eguibar JR, Quevedo J, Jimenez I, Rudomin P. Selective cortical control of information flow through different intraspinal collaterals of the same muscle afferent fiber. Brain Res 643: 328–333, 1994. [DOI] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86: 2986–2997, 2001. [DOI] [PubMed] [Google Scholar]
- Enriquez-Denton M, Manjarrez E, Rudomin P. Persistence of PAD and presynaptic inhibition of muscle spindle afferents after peripheral nerve crush. Brain Res 1027: 179–187, 2004. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol 275: 263–282, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Pisa M. Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Res 40: 145–155, 1972. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Bouyer L, Rossignol S. The effects of antidromic discharges on orthodromic firing of primary afferents in the cat. Brain Res 825: 132–145, 1999. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 62: 1177–1188, 1989. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. Phase-dependent modulation of primary afferent depolarization in single cutaneous primary afferents evoked by peripheral stimulation during fictive locomotion in the cat. Brain Res 537: 14–23, 1990. [DOI] [PubMed] [Google Scholar]
- Graham B, Redman S. A simulation of action potentials in synaptic boutons during presynaptic inhibition. J Neurophysiol 71: 538–549, 1994. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol 258: 33–61, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Primary afferent depolarization of central terminals of group II muscle afferents in the cat spinal cord. J Physiol 411: 71–83, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland M A flexible method for fabrication of nerve cuff electrodes. In: 18th annual international conference of the IEEE Engineering in Medicine and Biology Society. Amsterdam: the Conference organizing commitee and IFESS, 1996.
- Hoffer J Techniques to record spinal cord, peripheral nerve and muscle activity in freely moving animals. In: Neurophysiological Techniques: Applications to Neural Systems, edited by Boulton AA, Baker GB, Vanderwolf CH. Humana: Clifton, NJ, p. 65–145, 1990.
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389: 757–772, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol 542: 287–299, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez I, Rudomin P, Solodkin M. PAD patterns of physiologically identified afferent fibres from the medial gastrocnemius muscle. Exp Brain Res 71: 643–657, 1988. [DOI] [PubMed] [Google Scholar]
- Kagamihara Y, Tanaka R. Reciprocal inhibition upon initiation of voluntary movement. Neurosci Lett 55: 23–27, 1985. [DOI] [PubMed] [Google Scholar]
- Martin RF, Haber LH, Willis WD. Primary afferent depolarization of identified cutaneous fibers following stimulation in medial brain stem. J Neurophysiol 42: 779–790, 1979. [DOI] [PubMed] [Google Scholar]
- Miller RJ Presynaptic receptors. Annu Rev Pharmacol Toxicol 38: 201–227, 1998. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE, Gandevia SC. Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res 70: 569–576, 1988. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464: 575–593, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecci-Saavedra J, Wilson PD, Doty RW. Presynaptic inhibition in primate lateral geniculate nucleus. Nature 210: 740–742, 1966. [DOI] [PubMed] [Google Scholar]
- Perl ER Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J Physiol 197: 593–615, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol 80: 2475–2494, 1998. [DOI] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594, 1999. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Jimenez I, Rudomin P. Raphe magnus and reticulospinal actions on primary afferent depolarization of group I muscle afferents in the cat. J Physiol 482: 623–640, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. [DOI] [PubMed] [Google Scholar]
- Schmidt RF Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol Biol Chem Exp Pharmakol 63: 20–101, 1971. [DOI] [PubMed] [Google Scholar]
- Seki K, Perlmutter S, Fetz EE. Task-dependent modulation of primary afferent depolarization in the spinal cord of behaving monkey. Soc Neurosci Abstr 656.4, 2004. [DOI] [PMC free article] [PubMed]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003. [DOI] [PubMed] [Google Scholar]
- Seki K, Takei T. Primary afferent depolarization evoked by natural stimulation of cutaneous afferent in monkey. Soc Neurosci Abstr 54.58, 2006.
- Shefner JM, Buchthal F, Krarup C. Recurrent potentials in human peripheral sensory nerve: possible evidence of primary afferent depolarization of the spinal cord. Muscle Nerve 15: 1354–1363, 1992. [DOI] [PubMed] [Google Scholar]
- Stoney SD Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol 31: 659–669, 1968. [DOI] [PubMed] [Google Scholar]
- Wall PD Excitability changes in afferent fibre terminations and their relation to slow potential. J Physiol 142: 1–21, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Graham B, Nicol MJ. Serial E-M and simulation study of presynaptic inhibition along a group Ia collateral in the spinal cord. J Neurophysiol 74: 616–623, 1995. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanism of the Spinal Cord. New York: Plenum Press, 1991.
- Willis WD Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124: 395–421, 1999. [DOI] [PubMed] [Google Scholar]