Abstract
A case of de novo acute hepatitis B that showed symptoms of general malaise and anorexia during rituximab therapy with the CHOP regimen for diffuse large B cell lymphoma is reported. Lamivudine was strikingly effective, showing a rapid recovery from liver damage with jaundice. Hepatitis B virus (HBV) DNA in serum became and stayed undetectable even after the withdrawal of lamivudine, although HBsAg remained positive over 42 months from the onset. Liver biopsy showed a picture suggestive of acute viral hepatitis with multinucleated giant hepatocytes and CD38-positive plasma cell infiltration into liver parenchyma. Immunohistochemically, CD3-positive T-cells were predominant cells that infiltrated in liver parenchyma, whereas CD20-positive B cells were essentially null. Hence, it is suggested from these findings that B lymphocytes might be crucial for the continuous latency in HBV infection and may give rise to de novo acute hepatitis B if totally deleted. Moreover, the CHOP regimen might have some additive effects with the repeated on–off use of corticosteroids to the onset of the disease. In addition, significance of plasma cell infiltration in this setting is discussed.
Keywords: De novo acute hepatitis B, Rituximab, Lamivudine
Introduction
Chronic hepatitis B virus (HBV) infection has become a matter of worldwide healthcare issue, because more than one-third of the world’s population is now infected with HBV and there are 350 million people with chronic infection, most of whom live in southwest Asia and the western Pacific region [1–3]. Therefore, the reactivation of HBV, which is caused by anticancer chemotherapy, organ transplantation, and/or immunosuppressive therapies for autoimmune disorders, is more often encountered in such regions including Japan.
Anti-CD20 chimeric monoclonal antibodies, such as rituximab, have been developed and are widely used for the treatment of B cell malignancies as one of the molecular-targeted therapies. It is well known that it eventually causes de novo acute hepatitis by HBV reactivation even in HBsAg-negative patients, some of which are fatal [4–9]. Simultaneously, however, it may give a clue to elucidate the essential mechanisms for the maintenance of latent HBV infection in such individuals [10–12].
Here we report a case of de novo acute hepatitis B that was induced by the administration of rituximab in combination with the CHOP regimen to a patient with B cell lymphoma who is initially negative for HBsAg. Lamivudine, one of the nucleoside analogues that block RNA-dependent DNA polymerase in the replication cycle of HBV, was immediately administered and was strikingly effective as a treatment modality, showing a rapid recovery from acute hepatitis. The liver histology in the convalescent stage revealed a typical histologic picture of suggestive acute viral hepatitis with multinucleated giant hepatocytes. Immunohistochemically, CD20-positive cells were null, whereas CD3-positive cells and CD38-positive plasma cells were numerously infiltrated in the liver. The patient no longer showed a flare-up of transaminases even after the withdrawal of lamivudine and stayed positive for HBsAg for more than 42 months from the onset.
Case report
A 57-year-old woman visited an otolaryngo-pharyngologist for swelling of her right tonsil and submandibular lymph nodes. Her history of illness was not contributory. She was suspected to have malignant lymphoma and referred to the Division of Oto-Laryngo-Pharyngology of our hospital in late September 2003, where she was diagnosed with the diffuse large B cell lymphoma on tonsil biopsy. Contrast-enhanced abdominal computer tomography revealed multiple swelling of mesenteric lymph nodes of <1 cm in diameter. HBsAg and anti-HCV in her serum at the time of visit were both negative. She was admitted to our hospital for chemotherapy in June 2004, and she was prescribed the biweekly CHOP regimen with rituximab (R-CHOP). She was given 488 mg of anti-CD20 monoclonal antibodies (rituximab) 12 times every prior day of the CHOP regimen. She complained of general malaise on early November 2004. HBsAg turned out to be positive and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated to 1,491 and 1,113 IU/l, respectively. She was diagnosed with acute hepatitis B, and she was referred to our division for the treatment of hepatitis.
Laboratory data on admission are shown in Table 1. Bicytopenia (red blood cells and white blood cells, WBC), which was caused by the R-CHOP, was apparent. The transaminases were moderately elevated, but no jaundice was obvious. HBsAg was positive, whereas anti-HBc IgM was negative, indicating that this acute hepatitis B was caused by the HBV reactivation. The titer of HBV DNA was high at 7.5 log copies/ml. The HBV genotype was Bj in Sugauchi et al.’s classification [13], which was determined by the PCR invader method [14]. The precore mutant HBV was 60%, whereas the wild-type HBV was 40% on the basis of the PCR enzyme-linked minisequence assay. According to DNA sequencing, the core promoter mutation was of wild type.
Table 1.
Laboratory data on admission
| RBC | 265 × 104/mm3 | ALP | 423 IU/l | Anti-HBs (−) 0.1 mlU/ml | |
| Hb | 8.5 g/dl | LDH | 825 IU/l | ||
| Hct | 25.8% | γ-GTP | 92 IU/l | HBeAg (−) 0.1-fold | |
| WBC | 1,500/mm3 | Total bilirubin | 0.5 mg/dl | Anti-HBe (+) 100% | |
| Band | 17% | BUN | 12.2 mg/dl | ||
| Seg | 35% | Cr | 0.69 mg/dl | ||
| Eo | 0% | UA | 3.7 mg/dl | Anti-HBc (2+) 80.8% | |
| Baso | 2% | T.P | 6.1 g/dl | ||
| Mono | 25% | Alb | 63.4% | Anti-HBc lgM (−) C·O.I 0.11 | |
| Lympho | 18% | α1-gl | 4.8% | ||
| At.lym | 3% | α2-gl | 10.9% | HBV DNA 7.5 log copies/ml | |
| Plt | 14.8 × 104/mm3 | β−gl | 8.5% | HBV genotype Bj | |
| γ-gl | 12.4% | HBV precore mutation wild:mutant = 40:60 | |||
| CRP | 2.26 mg/dl | TC | 161 mg/dl | HBV core promoter wild type | |
| TG | 91 mg/dl | ||||
| AST | 1,491 IU/l | PT | 69% | Anti-HCV (−) | |
| ALT | 1,113 IU/l | HBsAg | (+) 2,000 U/ml | Anti-HAlgM (−) | |
RBC; red blood cell counts; Hb, hemoglobin; Hct, hematocrit; WBC, white blood cell counts; Band, Band neutrophils; Seg, segmented neutrophils; Eo, eosinophils; Baso, basophils; Mono, monocytes; Lympho, lymphocytes; At.lym, atypical lymphocytes; Plt, platelet counts; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; γ-GTP, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; T.P., total protein; Alb, albumin; α1-gl, α1-globulin; α2-gl, α2-globulin; β-gl, β-globulin; γ-gl, γ-globulin; TC, total cholesterol; TG, triglyceride; PT, prothrombin time
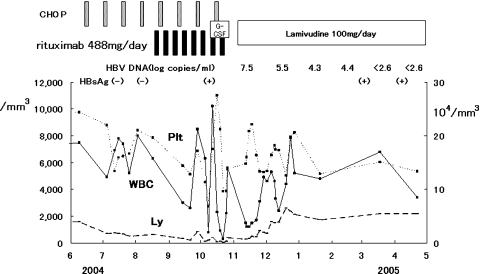
The time-elapsed course in three blood cell types (WBC, Plt, platelet count; and Ly, lymphocyte count) is shown in Fig. 1. Note the rapidly and largely waving (shaking) pattern of these three blood cell counts just around the usage of granulocyte-colony-stimulating factors (G-CSF) for four consecutive days and the subsequent appearance of HBsAg.
Fig. 1.
Serial white blood cells (WBC), lymphocyte count (Ly), and platelet count (Plt) counts during the R-CHOP regimen and after the onset of de novo acute hepatitis B. G-CSF, granulocyte-colony-stimulating factor
Based on the diagnosis of de novo acute hepatitis B that was caused by the reactivation of HBV, an oral daily dose (100 mg) of lamivudine was immediately started on the day when elevated transaminases were pointed out for the first time.
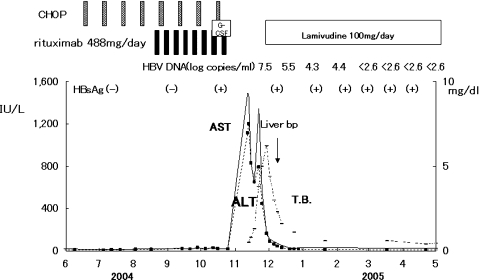
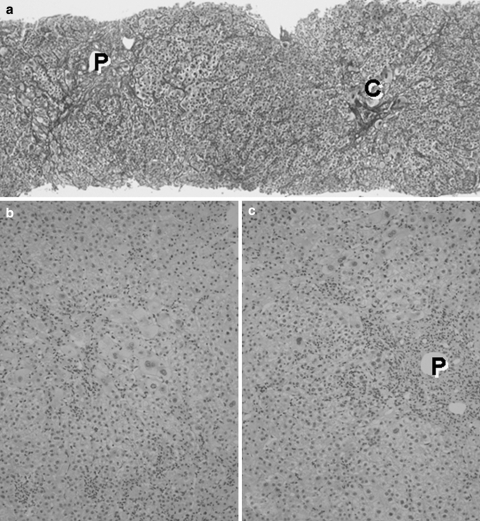
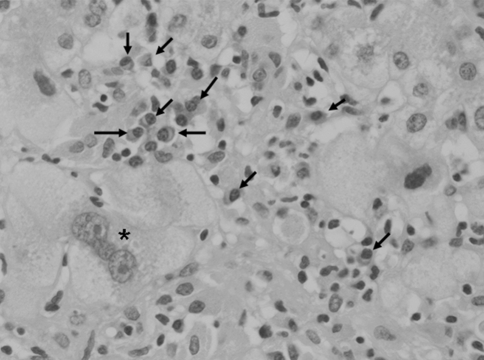
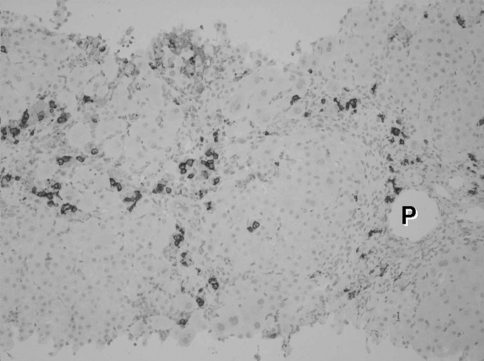
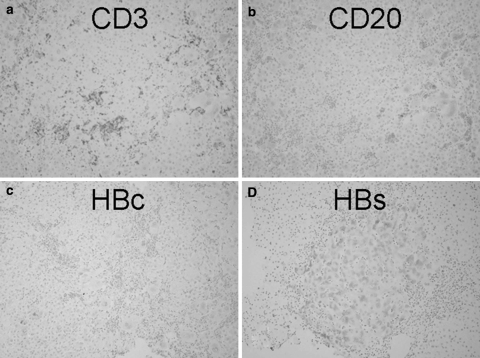
The clinical course of de novo acute hepatitis B is shown in Fig. 2. AST and ALT levels rapidly elevated above 1,200 IU/l soon after the emergence of HBsAg. The titer of HBV DNA reached to 7.5 log copies/ml just before the peak of transaminases and gradually fell down to 5.5 log copies/ml following lamivudine administration on the tenth hospital day. The level of HBV DNA in serum finally fell down under the lower limit of detection on the 116th day. Jaundice appeared only temporarily and rapidly disappeared. Echo-guided liver biopsy was performed in the convalescent phase under full informed consent of the patient. The liver biopsy specimen revealed a typical histologic picture of suggestive acute viral hepatitis (Fig. 3), namely, the fibrous thickening of central venous wall, edematous enlargement of portal tracts with the moderate degree of inflammatory cell infiltration, many focal necroses in liver parenchyma, the appearance of giant multinucleated hepatocytes in the central zone of hepatic lobule (Fig. 4), and the accumulation of diastase-resistant-periodic acid Schiff-positive materials in the migrated macrophages in hepatic lobules. Unlike the usual acute hepatitis B, the marked plasma cell infiltration in focal necroses in hepatic parenchyma was a unique finding. This finding was immunohistochemically reconfirmed by the CD38 staining that specifically labeled plasma cells (Fig. 5) by using avidin–biotin–peroxidase complex method. CD3-positive T lymphocytes infiltrated in the liver parenchyma (Fig. 6a), whereas CD20-positive B lymphocytes were essentially null throughout the whole liver tissue (Fig. 6b), indicating that the total depletion of B cells was accomplished by rituximab. Among CD3-positive T lymphocytes, CD8-positive cytotoxic/suppressor T lymphocytes were the main effecter cells that infiltrated in liver parenchyma (data not shown). As to the HBV-related antigens, HBcAg was not detected throughout liver parenchyma (Fig. 6c), whereas HBsAg was positive in liver cell cytoplasm forming positive cell clusters (Fig. 6d).
Fig. 2.
Serial serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels in de novo acute hepatitis B
Fig. 3.
Histologic picture of the liver biopsy specimen in de novo acute hepatitis B. (a) Hepatic lobular architecture is preserved and fibrous thickening of the walls of terminal hepatic venules (C) is seen. Portal triads (P) are not fibrously enlarged. The regular trabecular pattern of hepatocytes is slightly disrupted. Silver impregnation, 10×. (b) In hepatic lobules, anisocytosis of hepatocytes is conspicuous and many areas of focal necroses are seen. Inflammatory mononuclear cells are markedly infiltrated into hepatic sinusoids. Hematoxylin-eosin stain, 25×. (c). Portal triads are not fibrously enlarged, indicating an acute nature of this disease condition. Focal necroses are also conspicuous around portal triads. Hematoxylin-eosin stain, 25×
Fig. 4.
Multinucleated giant hepatocytes (asterisk) is found in hepatic lobules. Plasma cells (arrows) are infiltrated in hepatic lobules and gathered into the area of focal necroses. Hematoxylin-eosin stain, 100×
Fig. 5.
Immunohistochemistry of CD38 antigen. Avidin–biotin–peroxidase complex stain, 25×. CD38-positive cells (plasma cells) are abundant in periportal (P) and in focal necroses
Fig. 6.
Immunohistochemistry of CD3 (a), CD20 (b), HBc (c), and HBs antigen (d). Avidin–biotin–peroxidase complex stain, 25×. (a) CD3-positive T lymphocytes are scattered throughout hepatic lobules and preferentially aggregated in focal necroses. (b) CD20-positive B lymphocytes are null. Only diastase-resistant PAS positive, ceroid materials show false-positive reactions. (c) HB core antigen (HBc) is negative throughout hepatic lobules. (d) HB surface antigen (HBs) is positive in the cytoplasm of hepatocytes. These HBsAg-positive hepatocytes are gathered in a form of a nodular mass
After the lamivudine administration, the serum HBV DNA level gradually fell down and became undetectable at 4 months from the onset. The HBV DNA stayed undetectable until now (42 months). Lamivudine was finally withdrawn at 16 months after the disappearance of HBV DNA. Any serious biochemical or virological reactivation has not occurred following the lamivudine withdrawal. However, HBsAg continuously remained positive even after 42 months from the onset of the disease.
Discussion
The reactivation of HBV eventually occurs in antitumor chemotherapy for hematologic malignancies not only in HBV carriers, but also in individuals negative for serum HBsAg irrespective of the presence of anti-HBs [4–9, 15–19]. The previous episode of HBV infection or occult HBV infection [12] is thought to underlie the latter situation. This HBV reactivation often results in fulminant hepatitis that is apt to be fatal. Until the mid-1990s, the immunosuppressive effect of antitumor agents and/or repeated on–off use of corticosteroids in fixed chemotherapeutic regimens have been discriminated as a main cause of this reactivation [15]. Subsequently, the development and worldwide clinical use of rituximab for the treatment of B cell malignancies have made this HBV reactivation more common [4–9].
Occult HBV infection is defined as the detection of HBV DNA without HBsAg with or without the presence of HBV antibodies outside the acute-phase window period [12]. The molecular mechanism of occult HBV infection is closely related to the presence of covalently closed circular or supercoiled DNA that corresponds to a replicative intermediate of HBV DNA in the liver [11].
The exact mechanism by which the immunosuppressive condition can induce HBV reactivation is not fully understood as yet, although only some speculations exist. In addition to various conditions that may evoke HBV reactivation, the total depletion of CD20-positive lymphocytes by rituximab can offer a detailed model of HBV reactivation as it would be done in experimental animal models.
Wands et al. [15] observed that the titer of anti-HBs gradually decreased and finally disappeared in serum as WBC counts fell by the administration of anticancer agents. HBsAg then became positive in serum followed by the HBV reactivation in such patients. Recently, Hui et al. [19] revealed that HBV DNA appears in patients’ sera about 18.5 weeks prior to the onset of the disease. They also revealed that HBV DNA appears in serum at 10 weeks in average prior to HBsAg. Thus, the most efficient way to predict HBV reactivation earlier is to periodically monitor (e.g., once in a month) this abrupt emergence of HBV DNA in serum during chemotherapy in high-risk patients for de novo acute hepatitis B.
In our case, changes in WBC counts during R-CHOP were extremely wavy, because a short-term administration of G-CSF caused this wavy pattern in neutrophil counts within WBC counts in addition to the total depletion of B lymphocytes by rituximab. A possibility exists that this extreme up and down in WBC counts may provoke the HBV reactivation by shaking the immunologic status that is responsible for the suppression of HBV reactivation [20].
The reason why plasma cells infiltrate in liver parenchyma as shown in our case is unclear other than some speculations. It is well known that B lymphocytes lose CD20 antigens from their cell surface at the final differentiation stage of antibody-producing cells (plasma cells). Thus, one explanation is that mature plasma cells survive even under the presence of rituximab and those that produce anti-HBs are recruited to liver parenchyma putatively as one of the protective mechanisms that is activated by the increased HBsAg production in the liver during the HBV reactivation. Another possibility is that some autoimmune process may develop in coordination with the HBV reactivation, although any clinical manifestation of autoimmunity is not found in the laboratory data on admission as shown in Table 1. Anyway, our report is the first that demonstrated the abundance of plasma cells in liver parenchyma of de novo acute hepatitis B.
Male gender, younger age, a higher ALT level before chemotherapy, and the use of steroids are reported [18] to be significant risk factors for HBV reactivation, although omitting steroids in fixed regimens to reduce the risk of HBV reactivation also reduced the effect of chemotherapy for hematologic malignancies [21]. Since the HBV genome possesses glucocorticoid-responsive elements in its own DNA sequence [22], there is an opinion that nucleoside analogues should always be administered preemptively whenever steroids are used in such patients.
Lamivudine has been used in the treatment of de novo acute hepatitis B, and it has resulted in the favorable outcome in many reports [23–25] only if it is used very early. However, it is still controversial whether lamivudine should be used for all patients who are scheduled to have the rituximab therapy for the prophylaxis of de novo acute hepatitis B since the incidence of HBV reactivation in this setting is estimated to be as little as 3.3% even in endemic areas [19]. Moreover, it is well known that 38% of treated patients show lamivudine-resistant viruses within 2 years [26] and these mutant viruses may cause the breakthrough hepatitis that can be once again fatal. Therefore, the first thing to do is to screen the serum markers, including HBsAg, anti-HBs, and anti-HBc, and HBV DNA, for HBV infection in some instances prior to the rituximab therapy. Lamivudine should be used only in those patients in whom the persistent and previous HBV infection is demonstrated. However, there is still a problem of occult HBV infection where any screening serum marker of HBV other than HBV DNA is undetectable by routine detection methods. Thus, more convenient and reliable guideline is necessary to prevent the HBV reactivation.
Lamivudine was strikingly effective in our case, providing the rapid normalization of transaminases. However, it is still controversial as to when lamivudine should be withdrawn [27]. In our case, lamivudine was withdrawn at the time when HBV DNA stayed negative for 16 months. After the withdrawal of lamivudine, liver function remained under upper limit of normal for 22 months, although HBsAg remained positive in serum. Thus, our recommendation that is deduced from our experience is that it should be withdrawn at least 12 months after the disappearance of HBV DNA and the normalization of transaminases.
Entecavir, a new nucleoside analogue, is reported to induce few drug-resistant mutant viruses unlike lamivudine, and therefore may replace it in the near future [28], although evidences are still few that entecavir is more favorable than lamivudine in the treatment of de novo acute hepatitis B.
Interferon may be another choice [29] or additively used since it effects more rapidly than nucleoside analogues, although there is always a risk that interferon may aggravate or accelerate the recurrence of lymphoma.
A consensus seems to be already reached as to the preemptive use of nucleoside analogues in the rituximab treatment of B-cell malignancies in HBV carriers. It is recommended that it should be used 2 weeks prior to the start of the chemotherapies including rituximab (e.g., R-CHOP), and that it should be continued until at least 6 months from the last use of rituximab. However, as we have mentioned, there are a lot of controversies concerning the necessity of preemptive use of nucleoside analogues in patients with previous episodes of HBV infection.
In addition, more efficient method to prevent the de novo acute hepatitis B is necessary to improve the mortality as a result of this disease and of B-cell malignancies that need rituximab.
References
- 1.Millman I, London WT, Blumberg BS. Immunofluorescent identification of Australian antigen. N Engl J Med. 1973;288:108–109. doi: 10.1056/NEJM197301112880222. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Lee W. Hepatitis B infection. N Engl J Med. 1997;337:1735–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 4.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68–69. doi: 10.1056/NEJM200101043440120. [DOI] [PubMed] [Google Scholar]
- 5.Ng HJ, Lim LC. Fulminant hepatitis B virus reactivation with concomitant listeriosis after fludarabine and rituximab therapy: case report. Ann Hematol. 2001;80:549–552. doi: 10.1007/s002770100346. [DOI] [PubMed] [Google Scholar]
- 6.Skrabs C, Müller C, Agis H, Mannhalter C, Jäger U. Treatment of HBV-carrying lymphoma patients with rituximab and CHOP: a diagnostic and therapeutic challenge. Leukemia. 2002;16:1884–1885. doi: 10.1038/sj.leu.2402567. [DOI] [PubMed] [Google Scholar]
- 7.Westhoff M, Jochimsen TH, Schmittel F, Stöfer-Meilicke A, Schäfer JH, Zidek W, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood. 2003;102:1030. doi: 10.1182/blood-2003-05-1403. [DOI] [PubMed] [Google Scholar]
- 8.Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother. 2005;11:189–191. doi: 10.1007/s10156-005-0385-z. [DOI] [PubMed] [Google Scholar]
- 9.Umemura T, Kiyosawa K. Fatal HBV reactivation in a subject with anti-HBs and anti-HBc. Intern Med. 2006;45:747–748. doi: 10.2169/internalmedicine.45.0158. [DOI] [PubMed] [Google Scholar]
- 10.Rehermann B, Ferrari C, Pasquinelli C, Chizari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of cytotoxic T lymphocyte response. Nat Med. 1996;10:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 11.Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998;27:1736–1742. doi: 10.1002/hep.510270638. [DOI] [PubMed] [Google Scholar]
- 12.Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004;11:18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu D, et al. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology. 2003;125:1916–1917. doi: 10.1053/j.gastro.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Tadokoro K, Kobayashi M, Yamaguchi T, Suzuki F, Miyauchi S, Egashira T, et al. Classification of hepatitis B virus genotypes by the PCR-invader method with genotype-specific probes. J Virol Methods. 2006;138:30–39. doi: 10.1016/j.jviromet.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105–112. [PubMed] [Google Scholar]
- 16.Kawatani T, Suou T, Tajima F, Ishiga K, Omura H, Endo A, et al. Incidence of hepatitis virus infection and severe liver dysfunction in patients receiving chemotherapy for hematologic malignancies. Eur J Haematol. 2001;67:45–50. doi: 10.1034/j.1600-0609.2001.067001045.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi Y, Kawamura T, Saitoh S, Yamada M, Obara S, Miura T, et al. Hepatitis B virus reactivation in a case of non-Hodgkin’s lymphoma treated with chemotherapy and rituximab: necessity of prophylaxis for hepatitis B virus reactivation in rituximab therapy. Leuk Lymphoma. 2004;45:627–629. doi: 10.1080/1042819031000151923. [DOI] [PubMed] [Google Scholar]
- 18.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–220. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 19.Hui CK, Cheung WWW, Zhang HY, Au WY, Yueng YH, Leung AYH, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Lau GK, Yiu HHY, Fong DYT, Cheng HC, Au WY, Lai LSY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Lymphoma Committee of Taiwan Cooperative Oncology Group. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–1328. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 22.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83:1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persico M, Marino FD, Russo GDG, Severino A, Parmentieri B, Picardi M, et al. Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus-infected patients treated for non-Hodgkin lymphoma. Blood. 2002;15:724–725. doi: 10.1182/blood.V99.2.724. [DOI] [PubMed] [Google Scholar]
- 24.Shibolet O, Ilan Y, Gillis S, Hurbert A, Shouval D, Safadi R. Lamivudine therapy for prevention of immunosuppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood. 2002;100:391–396. doi: 10.1182/blood.V100.2.391. [DOI] [PubMed] [Google Scholar]
- 25.Rossi G. Prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HBsAg carriers with hemato-oncological neoplasias treated with chemotherapy. Leuk Lymphoma. 2003;44:759–766. doi: 10.1080/104281903100006351. [DOI] [PubMed] [Google Scholar]
- 26.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 27.Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769–774. doi: 10.1007/s00277-004-0899-y. [DOI] [PubMed] [Google Scholar]
- 28.Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis. 2007;4:965–991. doi: 10.1016/j.cld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Leaw SJ, Yen CJ, Huang WT, Chen TY, Su WC, Tsao CJ. Preemptive use of interferon or lamivudine for hepatitis B reactivation in patients with aggressive lymphoma receiving chemotherapy. Ann Hematol. 2004;83:270–275. doi: 10.1007/s00277-003-0825-8. [DOI] [PubMed] [Google Scholar]