Abstract
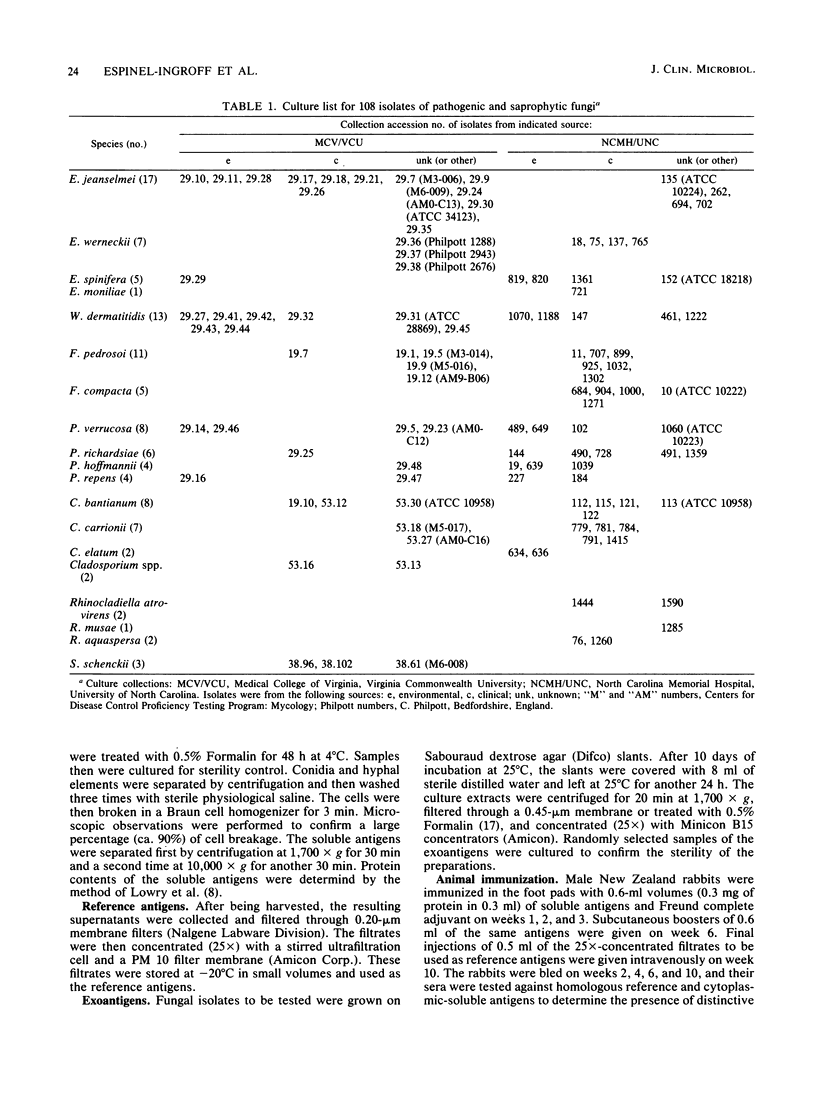
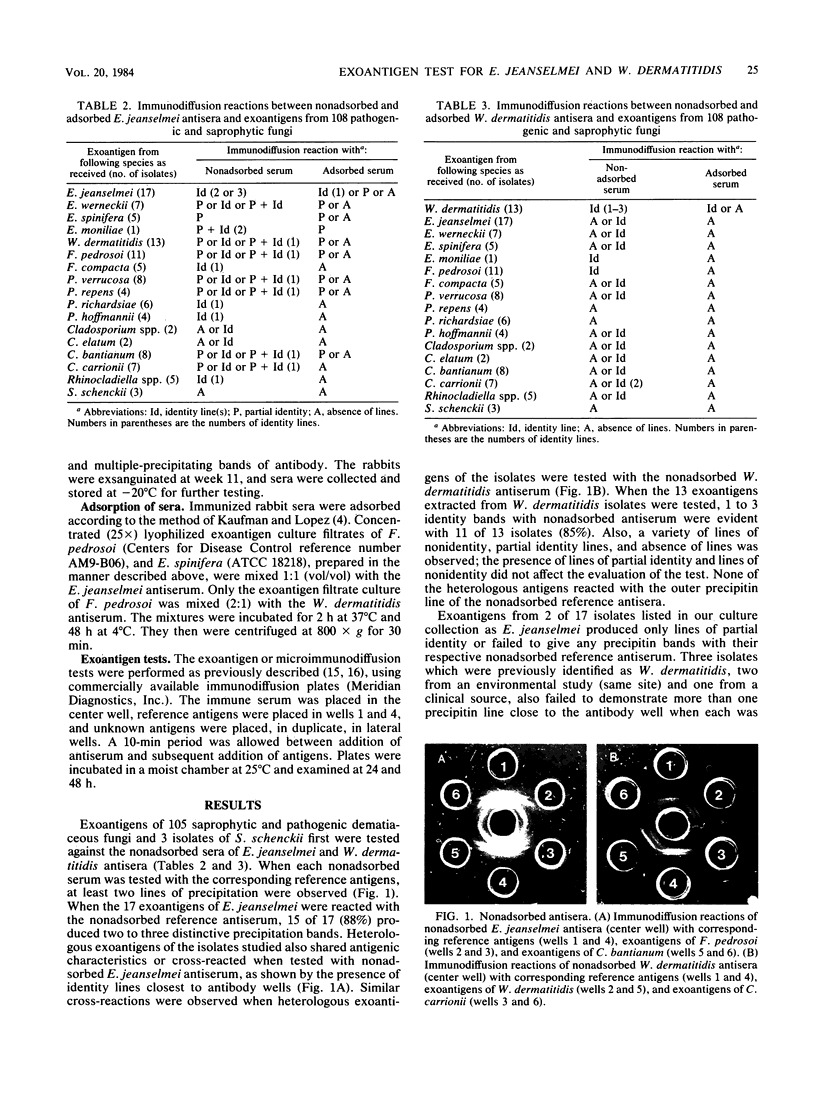
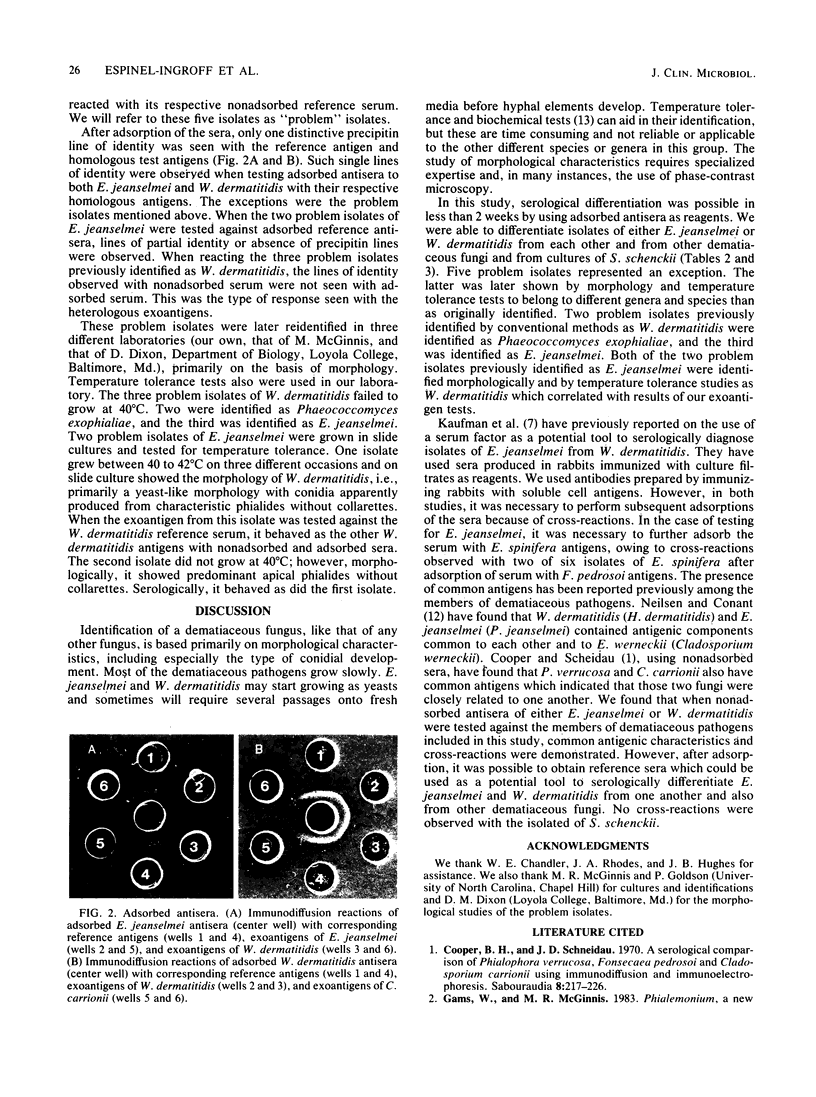
Concentrated (25X) exoantigens of 105 isolates of pathogenic and saprophytic dematiaceous fungi and 3 isolates of Sporothrix schenckii were analyzed by the microimmunodiffusion method. The reagents used were nonadsorbed and adsorbed sera produced in New Zealand rabbits. One set of rabbits was immunized with soluble antigens of a 1-month-old culture of Exophiala jeanselmei (ATCC 34123), and the other set was immunized with soluble antigens from a culture of Wangiella dermatitidis (ATCC 28869). The reference antigens were 25X-concentrated exoantigens of the above cultures. This exoantigen test permitted the differentiation of E. jeanselmei and W. dermatitidis from one another as well as from other Exophiala species, Fonsecaea species, Phialophora species, Cladosporium species, Rhinocladiella species, and Sporothrix schenckii by presence or absence of lines of identity or of partial identity, or lines of nonidentity. Using adsorbed serum eliminated the problems with cross-reactivity seen with nonadsorbed serum. Thus, with an adsorbed serum as the reagent, it was possible to presumptively differentiate E. jeanselmei and W. dermatitidis from one another and from other dematiaceous fungi.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper B. H., Schneidau J. D. A serological comparison of Phialophora verrucosa, Fonsecaea pedrosoi and Cladosporium carrionii using immunodiffusion and immunoelectrophoresis. Sabouraudia. 1970 Nov;8(3):217–226. [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Rice E. H. Specificity of exoantigens for identifying cultures of Coccidioides immitis. J Clin Microbiol. 1978 Sep;8(3):346–348. doi: 10.1128/jcm.8.3.346-348.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. Improved version of the exoantigen test for identification of Coccidioides immitis and Histoplasma capsulatum cultures. J Clin Microbiol. 1978 Jul;8(1):42–45. doi: 10.1128/jcm.8.1.42-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morace G., Polonelli L. Exoantigen test for identification of Petriellidium boydii cultures. J Clin Microbiol. 1981 Sep;14(3):237–240. doi: 10.1128/jcm.14.3.237-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. S., Jr, Conant N. F. Practical evaluation of antigenic relationships of yeastlike dematiaceous fungi. Sabouraudia. 1967 Jun;5(4):283–294. doi: 10.1080/00362176785190541. [DOI] [PubMed] [Google Scholar]
- Padhye A. A., McGinnis M. R., Ajello L. Thermotolerance of Wangiella dermatitidis. J Clin Microbiol. 1978 Oct;8(4):424–426. doi: 10.1128/jcm.8.4.424-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonelli L., Morace G. Exoantigen Studies of Sporothrix schenckii, Ceratocystis minor, and Graphium penicilliodes cultures. J Clin Microbiol. 1982 Mar;15(3):362–365. doi: 10.1128/jcm.15.3.362-365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Immunological procedure for the rapid and specific identification of Coccidioides immitis cultures. J Clin Microbiol. 1977 Feb;5(2):149–153. doi: 10.1128/jcm.5.2.149-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Safety considerations in handling exoantigen extracts from pathogenic fungi. J Clin Microbiol. 1982 Apr;15(4):663–667. doi: 10.1128/jcm.15.4.663-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Specific immunological test for the rapid identification of members of the genus Histoplasma. J Clin Microbiol. 1976 Feb;3(2):191–199. doi: 10.1128/jcm.3.2.191-199.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




