Ca2+ release-activated Ca2+ (CRAC) channels generate sustained Ca2+ signals that are essential for a range of cell functions, including antigen-stimulated T lymphocyte activation and proliferation1, 2. Recent studies3 have revealed that the depletion of Ca2+ from the endoplasmic reticulum (ER) triggers the oligomerization of STIM1, the ER Ca2+ sensor, and its redistribution to ER-plasma membrane (ER-PM) junctions4–8 where the CRAC channel subunit Orai1 accumulates in the plasma membrane and CRAC channels open9–12. However, how the loss of ER Ca2+ sets into motion these coordinated molecular rearrangements remains unclear. Here we define the relationships among [Ca2+]ER, STIM1 redistribution, and CRAC channel activation and identify STIM1 oligomerization as the critical [Ca2+]ER–dependent event that drives store-operated Ca2+ entry (SOCE). In cells expressing an ER-targeted Ca2+ indicator, CRAC channel activation and STIM1 redistribution follow the same function of [Ca2+]ER, with a K1/2 of ~200 μM and a Hill coefficient of ~4. Because STIM1 binds only a single Ca2+ ion5, the high apparent cooperativity suggests that STIM1 must first oligomerize to enable its accumulation at ER-PM junctions. To assess directly the causal role of STIM1 oligomerization in SOCE, we replaced the luminal Ca2+-sensing domain of STIM1 with the rapamycin-binding proteins FRB or FKBP. A rapamycin analog oligomerizes the fusion proteins and causes them to accumulate at ER-PM junctions and activate CRAC channels without depleting Ca2+ from the ER. Thus, STIM1 oligomerization is the critical transduction event through which Ca2+ store depletion controls store-operated Ca2+ entry, acting as a switch that triggers the self-organization and activation of STIM1-Orai1 clusters at ER-PM junctions.
The defining feature of store-operated channels is their activation in response to ER Ca2+ ([Ca2+]ER) depletion. However, their sensitivity to [Ca2+]ER and the factors that determine this sensitivity have never been established, largely because of the technical difficulty of quantifying [Ca2+]ER. To address this issue we generated a Jurkat T cell line stably expressing the Ca2+-sensitive cameleon protein, YC4.2er. YC4.2er is selectively retained in the ER, as shown by its colocalization with the resident ER protein calnexin but not with mitochondrial or Golgi markers, and by its functional response to agents that deplete ER Ca2+ (Fig. 1a and Supp. Fig. 1). In situ calibration of the YC4.2er FRET signal indicates a responsivity to [Ca2+]ER in the range of ~1 μM to >1 mM (Supp. Fig. 2).
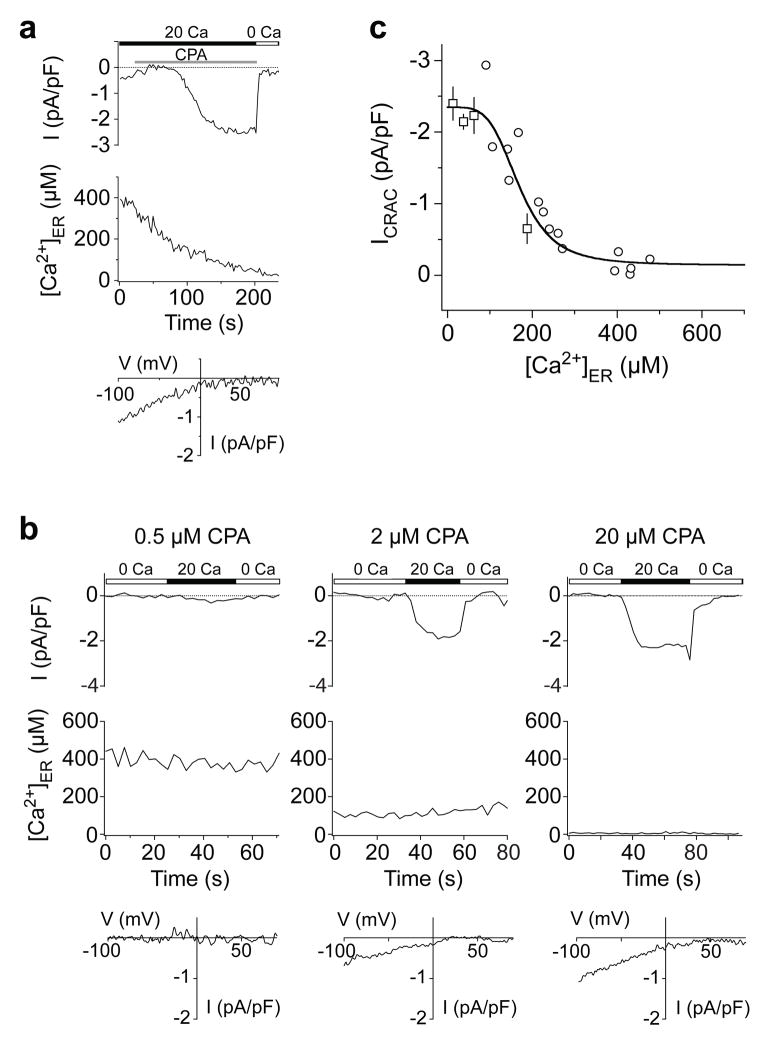
Figure 1. The [Ca2+]ER-response relation for the CRAC channel.
Simultaneous measurements of [Ca2+]ER and ICRAC in individual Jurkat T cells. a, Non-stationary measurements of ICRAC and [Ca2+]ER. Store depletion with 20 μM CPA induces an increase in ICRAC (top) that follows a decrease in [Ca2+]ER (middle) monitored with YC4.2er. The I-V relationship shows the inward rectification typical of ICRAC (bottom). In this cell, a small inward current through outwardly-rectifying Cl− channels is also present initially but disappears before ICRAC is induced. b, Recordings of ICRAC (top) and [Ca2+]ER (middle) under steady-state conditions. Each cell was treated with the indicated CPA concentration for 8–15 min prior to recording, and CPA was maintained throughout the experiment. I-V relations are typical for ICRAC (bottom). c, Steady-state ICRAC and [Ca2+]ER are plotted for 40 cells after treatment with 0.5–20 μM CPA. A fit of the Hill equation with a K1/2 of 169 μM and Hill coefficient of 4.2 is superimposed on the data. Squares: mean ± s.e.m. of 3–12 cells. Circles: single cells (see Supplementary Information).
To determine the dependence of CRAC channel activation on [Ca2+]ER, we measured ICRAC in perforated-patch recordings from Jurkat YC4.2er cells treated with cyclopiazonic acid (CPA), a reversible SERCA inhibitor. CPA evokes a time-dependent decline in [Ca2+]ER in parallel with the activation of ICRAC measured in the same cell (Fig. 1a). However, because ICRAC responds slowly to rapid changes of [Ca2+]ER, non-stationary measurements like these will distort estimates of the true [Ca2+]ER dependence of the CRAC channel. For this reason we determined instead the [Ca2+]ER-ICRAC relationship under steady-state conditions, by pretreating cells with 0.5–20 μM CPA for 8–15 min in the absence of extracellular Ca2+ to generate a range of constant [Ca2+]ER values. This passive depletion approach also minimizes spatial variations of [Ca2+]ER, allowing the [Ca2+]ER dependence of SOCE to be determined from whole-cell YC4.2er measurements. Following readdition of 20 mM Ca2+ to the bath, current was monitored during brief hyperpolarizations from the resting potential of +30–50 mV at constant [Ca2+]ER (Fig. 1b). The current was identified as ICRAC based on its delayed response to extracellular Ca2+ reflecting Ca2+-dependent potentiation (Fig. 1b), an inwardly rectifying I/V relation (Fig. 1b), and extremely low current noise17. Measurements from 40 cells show that ICRAC is a steep function of [Ca2+]ER with a K1/2 of 169 μM and a Hill coefficient (nH) of 4.2 (Fig. 1c). Interestingly, a decline of >100 μM from the resting [Ca2+]ER of ~400 μM is required to initiate CRAC channel opening in these cells, which may help explain the ability of IP3 to release small amounts of ER Ca2+ without activating ICRAC in some cells18.
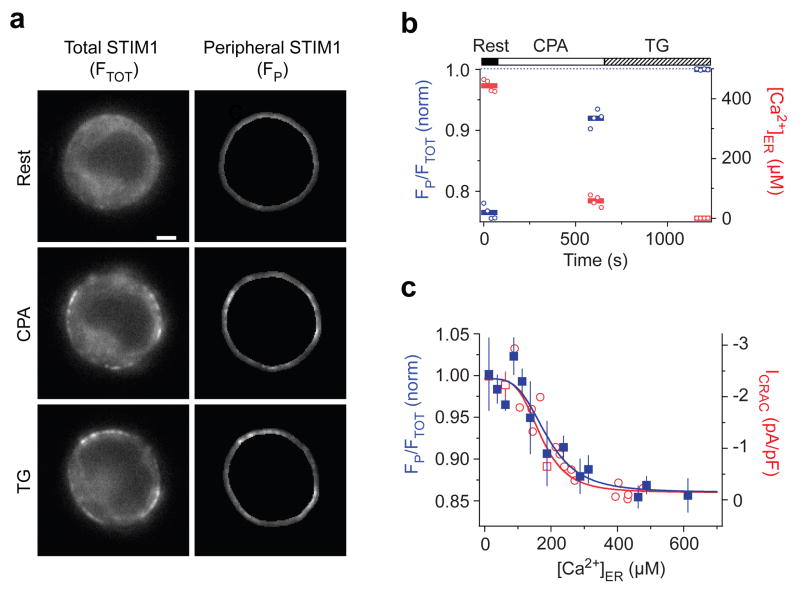
We next addressed the source of the CRAC channel’s steep dependence on [Ca2+]ER. Because STIM1 is known to be the Ca2+ sensor for SOCE6, 7 and its redistribution to ER-PM junctions is linked to ICRAC activation6, 8, 11, we measured the dependence of STIM1 redistribution on [Ca2+]ER. Exposure to 0.5–3 μM CPA for > 8 min causes a partial redistribution of Cherry-STIM1 to the cell periphery, which can be seen by widefield imaging at the cell equator (Fig. 2a). We quantified the redistribution of Cherry-STIM1 as the ratio of the mean peripheral fluorescence to the mean total fluorescence (Fig. 2b); this method gives results that agree quantitatively with TIRF measurements of STIM1 puncta (Supp. Fig. 3) while facilitating the separation of the cameleon and Cherry fluorescence signals (see Supplementary Information). Measurements from 41 cells show that STIM1 redistribution is a steep function of [Ca2+]ER that closely resembles that of ICRAC activation, with a K1/2 of 187 μM and a Hill coefficient of 3.8 (Fig. 2c). The value of K1/2 is close to the binding affinity of the recombinant EF-hand/SAM domain of STIM1 measured in vitro (KD = 200–600 μM; ref 5), consistent with its role as an ER Ca2+ sensor. Importantly, the close correspondence between the STIM1 and ICRAC curves indicates that CRAC channels open in direct proportion to the concentration of STIM1 at ER-PM junctions and that the CRAC channel derives its highly nonlinear dependence on [Ca2+]ER from the ER Ca2+ dependence of STIM1 redistribution. A recent study of HeLa cells found a similar dependence of STIM1 redistribution on [Ca2+]ER (ref 13). In that study, the homolog STIM2 redistributed to ER-PM junctions at higher [Ca2+]ER (K1/2 = 406 μM) than did STIM1 (K1/2 = 210 μM), and it was proposed that STIM2 functions as a homeostatic ER Ca2+ sensor by activating Orai1. Our findings that ICRAC and STIM1 redistribution follow the same function of [Ca2+]ER implies that in Jurkat cells STIM2 activates at most a minor fraction of endogenous CRAC channels, consistent with its low level of expression in T cells14.
Figure 2. The [Ca2+]ER dependence of STIM1 redistribution determines the [Ca2+]ER-response relation of the CRAC channel.
a,Widefield epifluorescence images of a cell expressing Cherry-STIM1 at rest (top) and following store depletion with 3 μM CPA (middle) and TG (bottom). The redistribution of Cherry-STIM1 in single cells was monitored as the ratio of the mean Cherry fluorescence in the most peripheral 0.5 μm of the cell (FP, right) to the mean fluorescence of the entire cell (FTOT, left). Scale bar = 2μm. b, In the same cell, STIM1 redistribution represented by FP/FTOT normalized to the maximum ratio with TG (blue). FP/FTOT increases as [Ca]ER (red) declines. Individual data points (open symbols) and the mean response (bars) are shown. c, STIM1 redistribution (FP/FTOT, blue) plotted against [Ca2+]ER after treatment with 0–3 μM CPA (means ± s.e.m. of 3–4 cells; 41 cells total). A fit of the Hill equation (blue line) indicates a K1/2 of 187 μM and Hill coefficient of 3.8. Steady-state ICRAC data fitted with the Hill equation are re-plotted from Fig. 1 (red).
The shape of the STIM1 redistribution curve has important implications for the mechanism underlying SOCE. The Hill coefficient of ~4 shows that puncta formation is a nonlinear process with respect to [Ca2+]ER without necessarily indicating a cooperative mechanism or that the active form of STIM1 is a tetramer. However, the high Hill coefficient implies that STIM1 puncta at ER-PM junctions do not form by the independent accretion of STIM1 monomers, which contain only a single luminal Ca2+ binding site5, but suggests instead that only oligomers of STIM1 can accumulate at these sites. There are two ways in which STIM1 is known to oligomerize. In resting cells STIM1 self-associates with an undetermined stoichiometry via its cytosolic coiled-coil domains15, 16; in addition, removal of Ca2+ from the EF-hand of STIM1 drives further oligomerization in vitro5 and in vivo4. Store-dependent oligomerization of STIM1 occurs within seconds, slightly in advance of puncta formation, and a causal role in SOCE has been hypothesized but never tested4, 5.
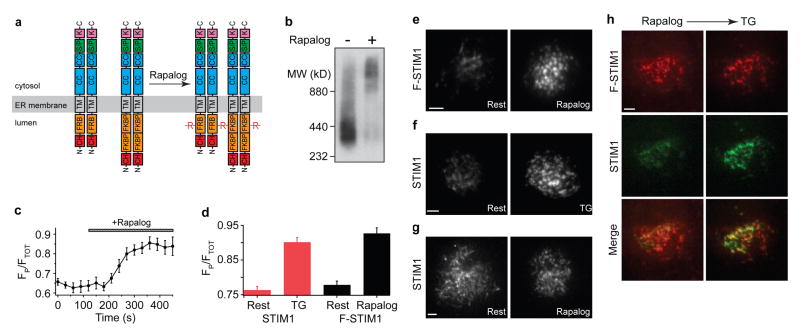
To address the possible role of STIM1 oligomerization in SOCE, we adopted an approach based on rapamycin-induced protein heterodimerization17, 18. We replaced the EF-SAM domain of Cherry-STIM1 with a tandem dimer of FK506-binding protein (FKBP12) or a variant of the rapamycin-binding domain of mTOR (FRB) to generate STIM1 chimeras that will heterodimerize when bound to a rapamycin analog (AP21967, or rapalog). Given that STIM1 is known to self-associate at rest15, 16, rapalog would thus be expected to link multimers containing FRB with those containing FKBP to form extended oligomers of STIM1 (Fig. 3a). We assayed oligomer formation in HEK293 cells expressing Cherry-FRB-STIM1 and Cherry-FKBP-STIM1 (abbreviated hereafter as F-STIM1) using blue native polyacrylamide gel electrophoresis (BN-PAGE)19. The >2-fold increase in apparent mass following rapalog treatment confirms its ability to oligomerize F-STIM1, and because crosslinking of monomers would be expected to at most double the mass, indicates that the resting state of FRB-STIM1 and FKBP-STIM1 is at least a dimer (Fig. 3b).
Figure 3. STIM1 oligomerization induces the accumulation of STIM1 at ER-plasma membrane junctions.
a, The cartoon depicts the oligomerization of F-STIM1 induced by rapalog (R). At rest, FKBP-STIM1 and FRB-STIM1 are expected to form homo- and heterodimers; only intermolecular crosslinks between homodimers are shown here. Abbreviations: EF (EF hand), SAM (sterile-α motif), CC (coiled-coil), S/P (serine-proline-rich), K (lysine-rich), CH (mCherry). b, BN-PAGE and Western blot of transiently expressed F-STIM1 harvested from HEK293 cells. Untreated (left) and rapalog-treated (right) F-STIM1 was detected using a monoclonal anti-STIM1 antibody. c, Rapalog induces a time-dependent peripheral redistribution of F-STIM1 (n=10 cells). d, Peripheral redistribution of Cherry-STIM1 by TG (red bars; n=31 for each) and redistribution of F-STIM1 by rapalog (black bars; n=39, rest; n=42, rapalog). Values expressed as mean ± s.e.m. (c, d). e–h, TIRF images of Jurkat cells, scale bar = 2 μm. e, F-STIM1 before (left) and after (right) incubation with rapalog. f, Cherry-STIM1 before (left) and after (right) store depletion with TG. g, Cherry-STIM1 before (left) and after (right) incubation with rapalog. h, F-STIM1 (top row), GFP-STIM1 (middle row) and merged images (bottom row) from a single cell after rapalog treatment (left column) and subsequent store depletion with TG (right column). Cherry and GFP intensities are scaled to the maximal intensity of each fluorophore after TG treatment.
We first examined the effects of rapalog on the localization of F-STIM1 in Jurkat cells. Rapalog evoked a redistribution of F-STIM1 to the cell periphery that was complete within several minutes (Fig. 3c). Quantitative analysis shows that rapamycin triggers the redistribution of F-STIM1 as effectively as Ca2+ store depletion induces the redistribution of wild-type STIM1 (Fig. 3d). When examined by TIRF microscopy, the rapalog-driven peripheral accumulations of F-STIM1 (Fig. 3e) resemble the puncta of wild-type STIM1 that form in response to store depletion (Fig. 3f). Similar results were obtained in HEK293 cells. Rapalog did not affect the localization of wild-type STIM1 (Fig. 3g), nor did it deplete Ca2+ stores (see below). Finally, rapalog-induced F-STIM1 puncta co-localize with store depletion-induced GFP-STIM1 puncta in the same cell, confirming that rapalog causes F-STIM1 to accumulate at the same ER-PM junctions where STIM1 and Orai1 are known to interact. Thus, we conclude that oligomerization of STIM1 is sufficient to drive the redistribution of STIM1 to ER-PM junctions.
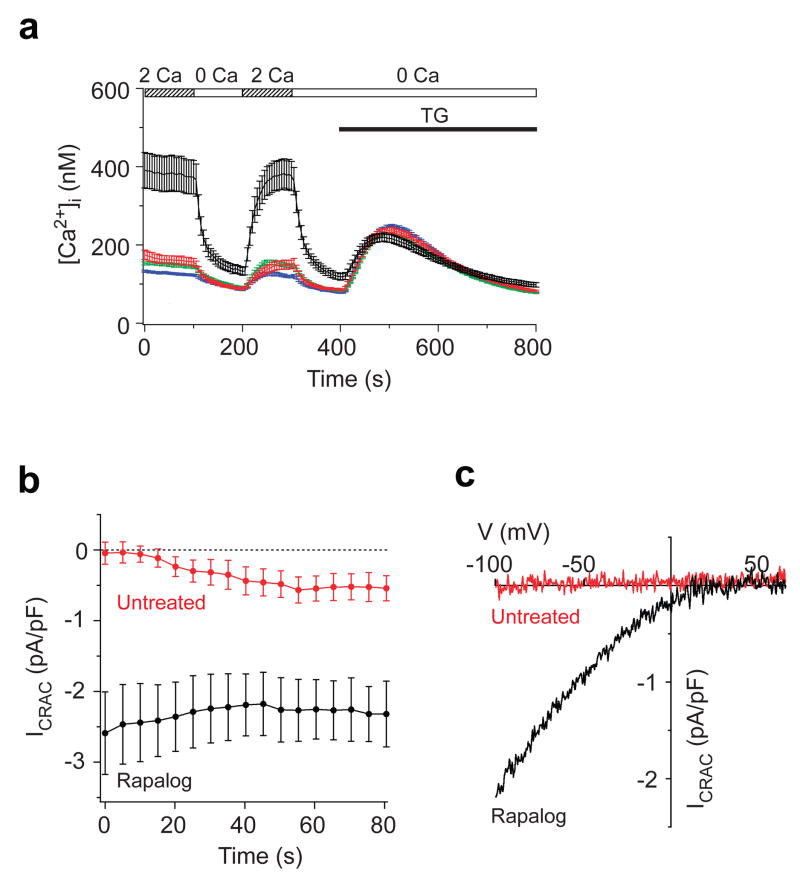
Heterodimerization of FRB-STIM1 and FKBP-STIM1 also activates endogenous CRAC channels. Rapalog elevated the mean resting [Ca2+]i in Jurkat cells expressing F-STIM1 (Cherry-positive cells) from 170 ± 11 nM (untreated; n = 61) to 388 ± 45 nM (Fig. 4a; n = 45), but did not affect [Ca2+]i in untransfected Jurkat cells. The elevated basal [Ca2+]i was dependent on extracellular Ca2+ (Fig. 4a) and was inhibited by 2-APB and low concentrations of La3+ (Supp. Fig. 4), consistent with constitutive Ca2+ entry through open CRAC channels. Importantly, TG released similar amounts of ER Ca2+ in rapalog-pretreated and resting cells, indicating that rapalog stimulates Ca2+ entry without depleting Ca2+ stores (Fig. 4a). Whole-cell recordings with a high-[Ca2+] pipette solution designed to minimize store depletion confirmed that heterodimerization of FRB-STIM1 and FKBP-STIM1 directly activates ICRAC. In untreated Jurkat cells expressing F-STIM1, ICRAC was negligible upon breaking in to the whole-cell configuration and developed slowly to a small amplitude, presumably in response to partial store depletion. In contrast, in rapamycin-pretreated cells with visible puncta, large inward currents were evident immediately upon breaking in (Fig. 4b) and displayed essential features of ICRAC, including a dependence on extracellular Ca2+, inwardly rectifying current-voltage relation (Fig. 4c), low current noise, rapid Ca2+-dependent inactivation, and inhibition by 2-APB and La3+ (Supp. Fig. 4). The mean current amplitude (2.6 ± 0.6 pA/pF, n= 9) was similar to that produced by Ca2+ store depletion in Jurkat cells overexpressing Cherry-STIM1 (ref 11), consistent with the comparable degrees of STIM1 and F-STIM1 redistribution in response to TG or rapalog, respectively (Fig. 3). Together, these results suggest that F-STIM1 oligomers at ER-PM junctions are fully active and provide direct evidence that the oligomerization of STIM1, independently of changes in [Ca2+]ER, is sufficient to evoke CRAC channel activation.
Figure 4. STIM1 oligomerization activates Ca2+ entry through CRAC channels.
a, In rapalog-treated cells expressing F-STIM1 (black, n=45), resting [Ca2+]i is elevated and sensitive to the removal of extracellular Ca2+, indicating constitutive Ca2+ entry. In contrast, resting Ca2+ influx was largely absent in untreated F-STIM1-expressing cells (red, n=61) and in wt Jurkat cells with (green, n=617) or without (blue, n=517) rapalog. TG-induced Ca2+ release in rapalog-treated cells was similar to that of untreated cells. b, ICRAC development during whole-cell recording from rapalog-treated (black, n=9) and untreated (red, n=9) cells expressing F-STIM1. ICRAC was measured beginning within 5 s of break-in. c, I–V relations upon break-in, showing the inward rectification typical of ICRAC in the rapalog-treated cell (black) and the absence of current in the untreated cell (red). Values expressed as mean ± s.e.m. (a,b).
We have shown that STIM1 redistribution and ICRAC share a steep dependence on [Ca2+]ER and that oligomerization of F-STIM1 is sufficient to drive puncta formation and CRAC channel activation. These results define the input-output relation of the CRAC channel and identify STIM1 oligomerization as the primary transduction event through which this relation is determined. The EF hand and SAM domains of STIM1 appear to serve primarily to control the extent of oligomerization, considering that removal of Ca2+ causes a recombinant EF-SAM peptide to oligomerize in vitro5, and that the FRB and FKBP modules in F-STIM1 can effectively substitute for the EF-SAM domain and activate ICRAC when crosslinked by rapalog. That the latter occurs without Ca2+ store depletion suggests that once STIM1 oligomerizes, all subsequent steps leading to SOCE occur independently of ER Ca2+. Thus, we propose that the oligomerization of STIM1 acts as a switch to trigger the self-organization of STIM1 and Orai1 complexes at ER-PM junctions and the consequent activation of CRAC channels.
How might this oligomerization “switch” operate? In its resting state, Ca2+-bound STIM1 moves freely throughout the ER membrane4 but following store depletion, STIM1 oligomers accumulate in ER subregions located 10–25 nm from the PM, close enough to allow trapping by binding to targets in the PM8. These targets have not yet been positively identified, but suggested candidates include Orai1 (refs 20, 21) or an associated protein22, and PM phospholipids4, 23. Once localized at ER-PM junctions STIM1 then promotes the accumulation of Orai1 at apposed sites, leading to channel activation11, 12, 24. Oligomerization may promote the binding of STIM1 to its targets in two ways: an affinity-based mechanism in which a conformational change exposes a previously masked cytosolic binding domain, and an avidity-based mechanism in which clustering of the binding domains increases their local concentration at ER-PM junctions. Both of these mechanisms are likely to contribute to the assembly and function of CRAC channel complexes that constitute the final stage of the SOC activation process.
METHODS SUMMARY
[Ca2+]ER measurements
[Ca]ER was measured in a Jurkat E6-1 cell line stably expressing a modified YC4er (V68L and Q69M; refs 25–27). Cells were pre-treated with CPA (0.5–20 μM) in Ca2+-free Ringer’s for 8–15 min, and emission intensities at 485 and 535 nm were averaged across the cell to yield a raw emission ratio. Ratios were calibrated in situ for every cell as described (Supplementary Information).
Heterodimerizer experiments
To generate F-STIM1, mutant FRB and tandem FKBP sequences were substituted for the EF-SAM domain (wtSTIM1 aa 35–207) in Cherry-STIM1 using plasmids provided by Ariad Pharmaceuticals (Cambridge, MA). F-STIM1 was crosslinked using 1 μM rapalog (AP21967, Ariad Pharmaceuticals). Unless indicated otherwise, cells were pre-incubated in full medium at 37°C for 30 min, with or without rapalog, and subsequent measurements were performed at 22–25°C in standard Ringer’s solutions. Timelapse imaging was performed at 37°C in full medium with or without rapalog. Only cells with ~3–10% of the fluorescence of the brightest cells in each experiment were analyzed. BN-PAGE was performed essentially as described19. A mAb against the STIM1 C-terminus (1:250; Abnova, Taipei City, Taiwan) and an alkaline phosphatase-conjugated 2° antibody (1:30,000; Sigma) were used for Western blotting.
Perforated-patch and whole-cell recording
ICRAC in YC4.2er cells (Fig. 1) was recorded in the perforated-patch configuration28 with 20 mM extracellular Ca2+, using a stimulus of a 50-ms step to −100 mV followed by a ramp from −100 to +100 mV, delivered from a holding potential of +30 or +50 mV. Whole-cell recording of ICRAC (ref 29; Fig. 4) was performed with 20 mM Ca2+ Ringer’s, with stimuli consisting of a 100-ms step to −112 mV followed by a 100-ms voltage ramp from −112 to +88 mV applied from the holding potential of +38 mV beginning within 5 s of break-in.
Supplementary Material
Acknowledgments
We thank Nirav Bhakta and Diana Bautista for assistance and advice during the initial phase of these studies, Roger Tsien for the gift of cameleon YC4er, Priti Bacchawat for advice on BN-PAGE, and Ricardo Dolmetsch for comments on the manuscript. This work was supported by a grant from the National Institutes of Health (NIH) and the Mathers Charitable Foundation.
References
- 1.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 3.Wu MM, Luik RM, Lewis RS. Some assembly required: constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–172. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 10.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu P, et al. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 13.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams RT, et al. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 17.Bayle JH, et al. Rapamycin analogs with differential binding specificity permit orthogonal control of protein activity. Chem Biol. 2006;13:99–107. doi: 10.1016/j.chembiol.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schägger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 20.Muik M, et al. Dynamic Coupling of the Putative Coiled-coil Domain of ORAI1 with STIM1 Mediates ORAI1 Channel Activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 21.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 23.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 25.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 27.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 28.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol. 2002;541:877–894. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 30.Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.