Abstract
A sensitive enzyme-linked immunosorbent capture assay with biotin and streptavidin (capture B/SA ELISA) was developed to detect herpes simplex virus (HSV) antigen. Rabbit anti-HSV antibody (immunoglobulin G fraction) was coated on flat-bottom, irradiated, 96-well polystyrene microtiter plates and served to capture HSV antigen. Clinical specimens from patients with genital herpes were added. Biotin-linked rabbit anti-HSV immunoglobulin G was used as the second antibody. The antigen-antibody complex was detected with alkaline phosphatase-conjugated streptavidin, which linked to the biotin. With clinical specimens, the test had a sensitivity of 95.6% and a specificity of 91.4% when compared with the tissue culture method. The presence of HSV antigen in specimens devoid of infectivity was confirmed by blocking the reaction with unlabeled rabbit and human antibody to HSV. The level of antigen detected by the capture B/SA ELISA did not necessarily correlate with the infectivity titer of the specimens. HSV antigens could be detected by the capture B/SA ELISA when the virus infectivity was destroyed at 37 degrees C, by UV irradiation, or by Triton X-100 treatment, but not when hypochlorite treatment was used. Greater sensitivity was obtained when HSV-1- and HSV-2-specific antibody reagents were used simultaneously in each test. The capture B/SA ELISA provides a relatively rapid method (4.5 h) which is quite sensitive and specific when compared with other non-tissue culture, direct assay methods.
Full text
PDF
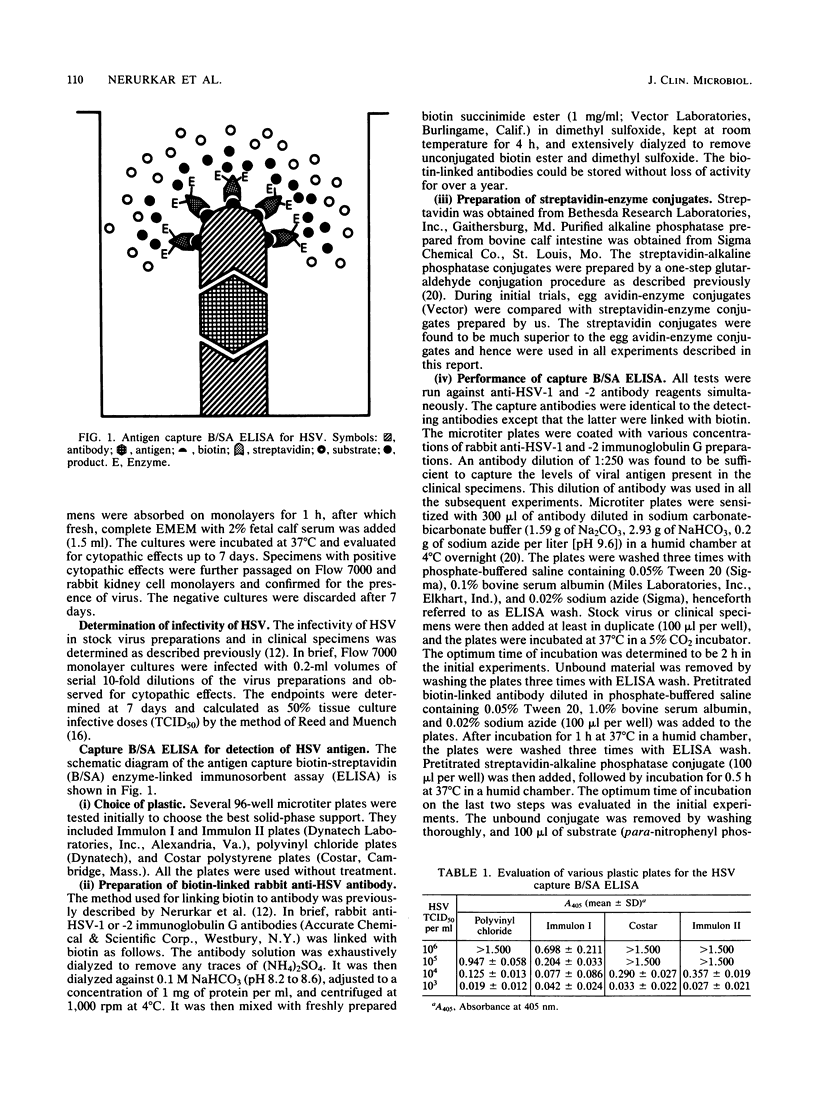
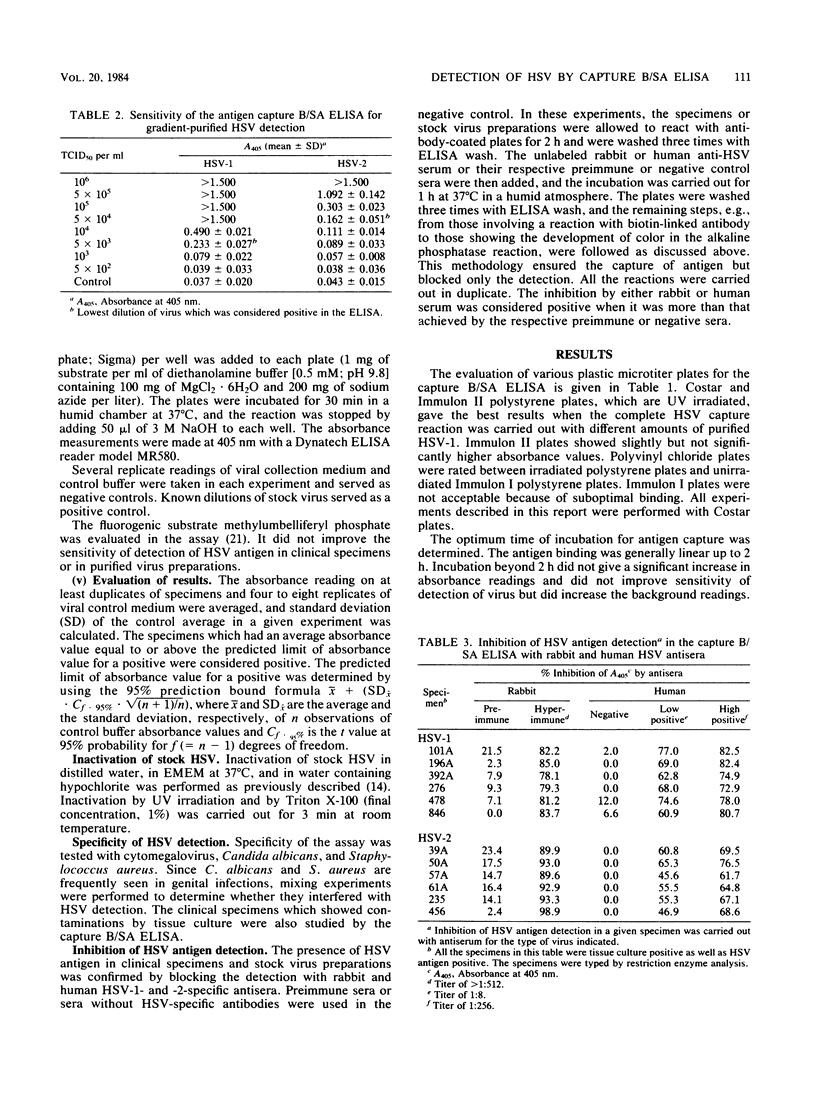
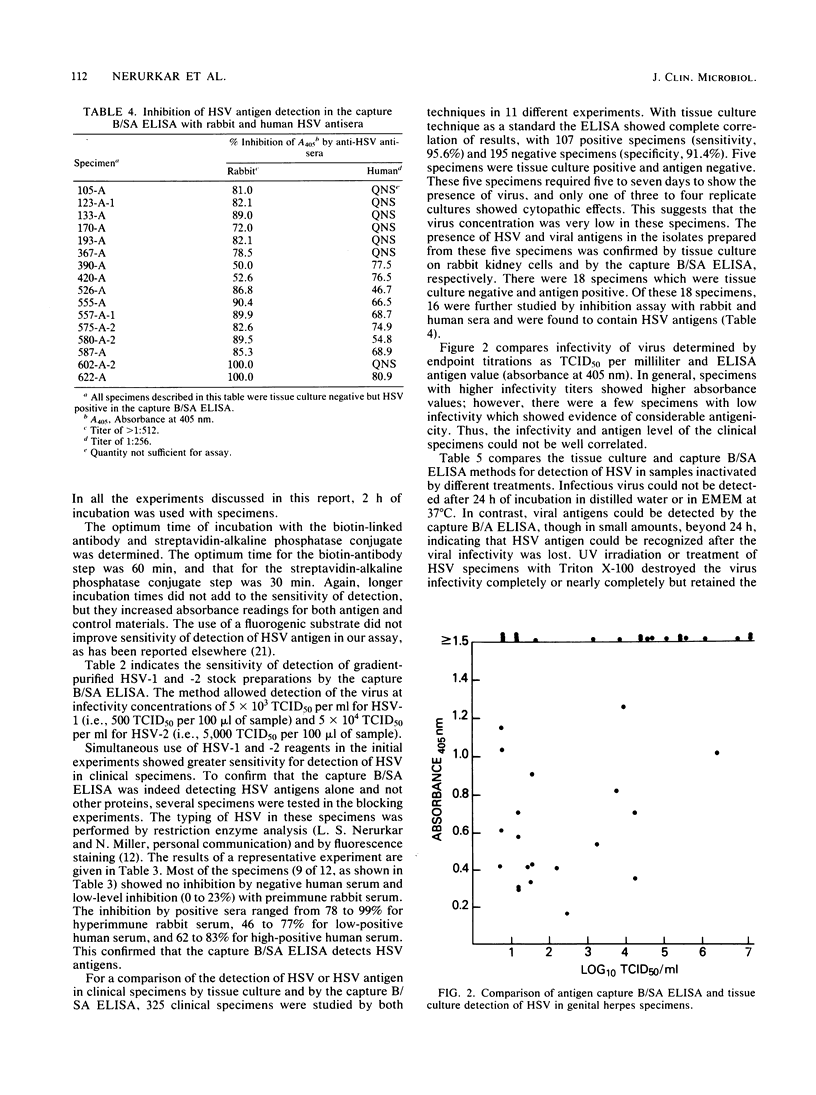
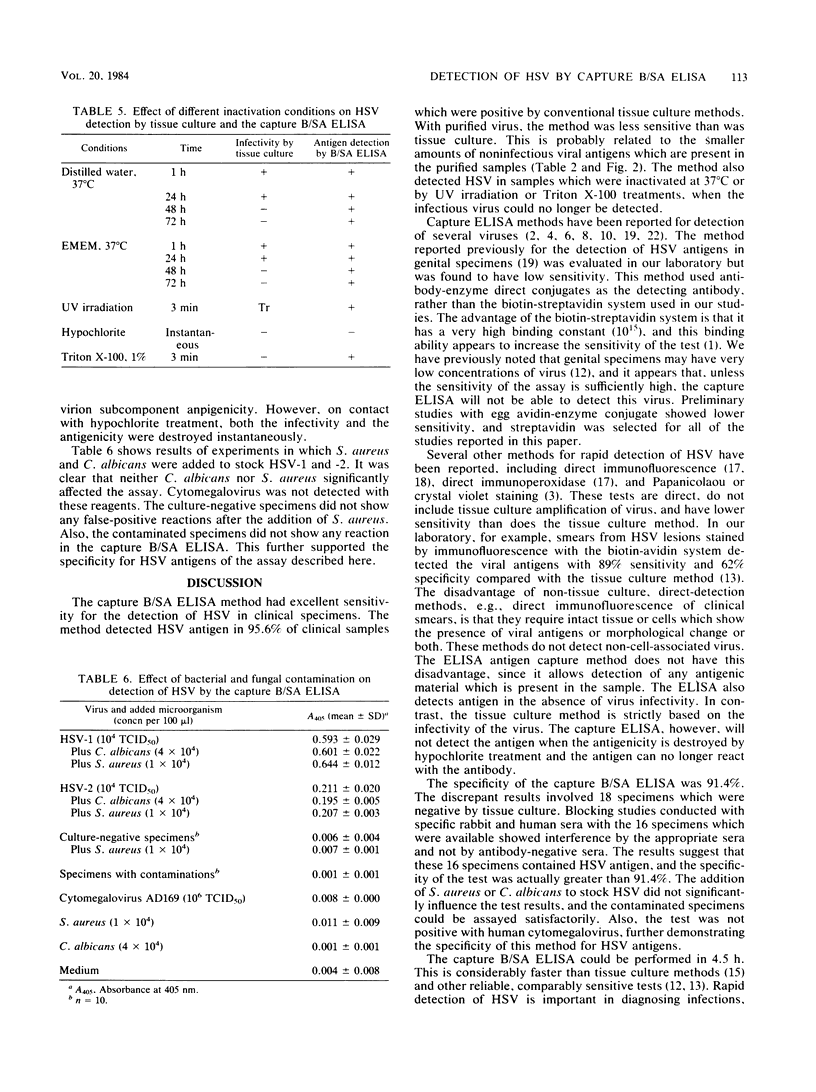

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Storthz K., Kendall C., Kennedy R. C., Henkel R. D., Dreesman G. R. Biotin-avidin-amplified enzyme immunoassay for detection of herpes simplex virus antigen in clinical specimens. J Clin Microbiol. 1983 Dec;18(6):1329–1334. doi: 10.1128/jcm.18.6.1329-1334.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Bryden A. S. Evaluation of a new enzyme-linked immunosorbent assay test for rotavirus antigen in faeces. J Clin Pathol. 1981 Dec;34(12):1388–1391. doi: 10.1136/jcp.34.12.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. T., Jaffe H. W., Zaidi A., Filker R., Herrmann K. L., Lylerla H. C., Jove D. F., Budell J. W. Sensitivity and specificity of diagnostic tests for genital infection with herpesvirus hominis. Sex Transm Dis. 1979 Jan-Mar;6(1):10–13. doi: 10.1097/00007435-197901000-00003. [DOI] [PubMed] [Google Scholar]
- Coleman R. M., Bailey P. D., Whitley R. J., Keyserling H., Nahmias A. J. ELISA for the detection of herpes simplex virus antigens in the cerebrospinal fluid of patients with encephalitis. J Virol Methods. 1983 Sep;7(3):117–125. doi: 10.1016/0166-0934(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Conradie J. D., Govender M., Visser L. ELISA solid phase: partial denaturation of coating antibody yields a more efficient solid phase. J Immunol Methods. 1983 May 13;59(3):289–299. doi: 10.1016/0022-1759(83)90190-4. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Hendry R. M., Collins M. F. Factors involved in enzyme-linked immunoassay of viruses and evaluation of the method for identification of enteroviruses. J Clin Microbiol. 1979 Aug;10(2):210–217. doi: 10.1128/jcm.10.2.210-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. E., Lang D. J. Rapid diagnosis of herpes simplex infection: amplification for electron-microscopy by short-term in vitro replication. J Infect. 1982 Jan;4(1):37–41. doi: 10.1016/s0163-4453(82)90959-8. [DOI] [PubMed] [Google Scholar]
- Miranda Q. R., Bailey G. D., Fraser A. S., Tenoso H. J. Solid-phase enzyme immunoassay for herpes simplex virus. J Infect Dis. 1977 Oct;136 (Suppl):S304–S310. doi: 10.1093/infdis/136.supplement_2.s304. [DOI] [PubMed] [Google Scholar]
- Nerurkar L. S., Jacob A. J., Madden D. L., Sever J. L. Detection of genital herpes simplex infections by a tissue culture-fluorescent-antibody technique with biotin-avidin. J Clin Microbiol. 1983 Jan;17(1):149–154. doi: 10.1128/jcm.17.1.149-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., Namba M., Sever J. L. Comparison of standard tissue culture, tissue culture plus staining, and direct staining for detection of genital herpes simplex virus infection. J Clin Microbiol. 1984 May;19(5):631–633. doi: 10.1128/jcm.19.5.631-633.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., West F., May M., Madden D. L., Sever J. L. Survival of herpes simplex virus in water specimens collected from hot tubs in spa facilities and on plastic surfaces. JAMA. 1983 Dec 9;250(22):3081–3083. [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Devlin V., Gallo D., Mills J. Comparison of direct immunofluorescence and direct immunoperoxidase procedures for detection of herpes simplex virus antigen in lesion specimens. J Clin Microbiol. 1983 Aug;18(2):445–448. doi: 10.1128/jcm.18.2.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Gallo D., Devlin V., Woodie J. D., Emmons R. W. Direct immunofluorescence staining for detection of herpes simplex and varicella-zoster virus antigens in vesicular lesions and certain tissue specimens. J Clin Microbiol. 1980 Nov;12(5):651–655. doi: 10.1128/jcm.12.5.651-655.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Comparison of fluorescent and colorigenic substrates for enzyme immunoassays. J Clin Microbiol. 1982 May;15(5):757–760. doi: 10.1128/jcm.15.5.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Analysis of nonspecific reactions in enzyme-linked immunosorbent assay testing for human rotavirus. J Clin Microbiol. 1979 Nov;10(5):703–707. doi: 10.1128/jcm.10.5.703-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


