Abstract
It has been reported that the IgE response to allergens is influenced by γδ T cells. Intrigued by a study showing that airway challenge of mice with OVA induces the development of γδ T cells in the spleen that suppress the primary IgE response to i.p. injected OVA/alum, we investigated the γδ T cells involved. We found that the induced IgE-suppessors are contained within the Vγ4+ subset of γδ T cells of the spleen, that they express Vδ5 and CD8, and that they depend on IFN-γ for their function. However, we also found that normal non-challenged mice harbor IgE-enhancing γδ T cells, which are contained within the larger Vγ1+ subset of the spleen. In cell transfer-experiments, airway-challenge of the donors was required to induce the IgE-suppressors among the Vγ4+ cells. Moreover, this challenge simultaneously turned off the IgE-enhancers among the Vγ1+ cells. Thus, airway allergen challenge differentially affects two distinct subsets of γδ T cells with non-overlapping functional potentials, and the outcome is IgE-suppression.
Keywords: T cells, Allergy, Lung, Spleen and Lymph Nodes, Transgenic/Knockout Mice, IgE
Introduction
Antibodies of the IgE class are prominent in the host response to parasitic infections and in allergic responses to many non-pathogenic antigens (1). The interest in IgE has heightened as ever-increasing proportions of the world’s population suffer from allergies (2). In healthy mammals, IgE antibodies acquired via the gastrointestinal tract by the newborn may serve as a first line of defense (3). IgE is synthesized and functions in the normal adult largely in the mucosal tissues where the IgE concentrations are high, whereas concentrations of IgE in the circulation remain low by comparison with other Igs (1). Mechanisms responsible for this biased anatomical distribution include the distribution and longevity of cells that express the receptors for IgE, as most of the IgE in the tissues is cell-bound and thus protected from degradation, and local IgE synthesis, which is favored by the Th2-environment of the mucosal tissues that maintains local IgE levels (1).
IgE antibodies are also induced during vaccination. Aluminum adjuvants (alum), currently the most widely used adjuvants in human and animal vaccines, stimulate the innate system and can favor Th2-biased reactivity (4). Immunization of previously untreated laboratory animals with soluble inert protein antigens using alum typically elicits Th2-type responses, accompanied by the development of IgE antibodies. However, the outcome of this type of immunization also depends on prior exposure. Mucosal exposure to the same antigen may result in non-responsiveness and the failure of the immunization to elicit Th2 reactivity (5). For example, repeated airway challenge of rodents with ovalbumin (OVA) without adjuvant leads to non-responsiveness to a subsequent intra-peritoneal injection of OVA/alum, which otherwise would induce Th2-reactivity and a strong OVA-specific IgE response (6). How airway exposure alters the outcome of OVA/alum immunization is not yet fully understood (7).
Several groups have provided evidence that γδ T cells can modulate the OVA/alum-induced IgE response. Investigating the development of tolerance to inhaled OVA, McMenamin et al. found that γδ T cells from tolerized mice efficiently and selectively suppressed primary OVA/alum-induced IgE responses (8). In apparent contrast, others reported that γδ T cells are required for the development of IgE responses to OVA and other antigens (9, 10). The underlying mechanisms responsible for these apparently opposing observations remained unresolved.
That γδ T cells can exert both Th1-like and Th2-like effects on the immune responses to pathogens has been recognized (11), and later studies revealed the surprising circumstance that these different and sometimes opposed functional effects on the host responses segregate with TCR-Vγ-definable subsets of γδ T cells, such as Vγ4+ and Vγ1+ γδ T cells (12, 13). Specific functional contributions of these and other TCR-defined subsets suggested that the γδ TCR not only determines ligand-specificity of γδ T cells but also their functional potential (14). Consistent with this concept, a recent study showed that the ability of γδ T cells with specificity for the T22-molecule to express IL-17 and IFN-γ depends on TCR-ligand interactions during their development (15).
Given the divergent observations regarding the role of γδ T cells in the IgE response, we were interested in determining if different TCR-Vγ-definable subsets of γδ T cells also exert opposed effects on IgE-production. In the current study, we took advantage of the observation that normal mice immunized with a single i.p injection of OVA/alum make a primary IgE-response to OVA. The results of these experiments indicate that Vγ1+ γδ T cells are able to enhance the primary IgE response induced by OVA/alum, whereas Vγ4+ cells in contrast are able to suppress it. Moreover, we found that in addition to their different functional potentials, the overall effect of the IgE-modulating γδ T cells critically depends on the exposure history of the animal. Whereas the IgE-enhancing γδ T cells lose this ability upon airway allergen-exposure, the IgE-suppressive γδ T cells gain theirs under the same circumstance.
Materials and Methods
Animals
Female C57BL/6 mice and several mutant strains of the same genetic background (B6.TCR-β−/−, B6.TCR-δ−/−, B6.TCR-β−/−/δ−/−) were obtained from The Jackson Laboratory (Bar Harbor, Maine). TCR-Vγ4−/−/6−/− mice deficient in Vγ4+ and Vγ6+ T cells were a gift from Dr. K. Ikuta (Kyoto University, Japan). They were backcrossed to the C57BL/6 genetic background and used after 11 backcross generations. B6.TCR-β−/−/IFN-γ−/− mice were generated by crossing the single mutants and breeding double mutants identified in the F2 generation. B6.TCR-Vγ1 transgenic mice were a gift from Dr. Pablo Pereira (Institut Pasteur, Paris, France). All mice were 8–12 wk old at the time of the experiments. Mice were maintained on an OVA-free diet, and were cared for at National Jewish Health (Denver, Colorado), following guidelines for immune deficient animals. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Antigen exposure and immunization
The animals were exposed to 1% OVA (wt/vol) (5x crystalline; EMD Biosciences, La Jolla, CA) in saline aerosol inhalation for 30 minutes daily, 5 d per week for up to 2 wk, and subsequently once per week (designated 10N in this paper), following a method described by others (8). To induce OVA-specific IgE, mice were immunized by i.p. injection of 10 µg OVA in aluminum hydroxide (AlumImject; Pierce, Rockford, IL) (8).
Treatment with Abs against the TCR
Hamster pan anti-TCR-Cδ mAbs (clone GL3), anti-Vγ4 mAb (clone UC3), and anti-Vγ1 mAb (clone 2.11) were purified from hybridoma culture supernatants using a protein G-Sepharose affinity column (Amersham Pharmacia Biotech, Uppsala, Sweden). T cells were targeted by injection of 200 µg of hamster anti-TCR-δ, anti-Vγ4 or anti-Vγ1 mAbs into the tail veins of mice 4 days before the i.p. immunization with OVA. The effect of these treatments on the targeted T cells was monitored as previously described (13). This approach, which is based on staining with non-cross-blocking anti TCR mAbs, allows an assessment of the treatment-effect on TCR-expression but it does not assess the fate of the targeted T cells. The antibody-treatments transiently reduce TCR-expression by > 90%. Sham Ab treatments were performed with nonspecific hamster IgG (The Jackson Laboratory). Throughout this article, we use the nomenclature for murine TCR-Vγ genes introduced by Heilig and Tonegawa (16).
T cell purification from spleen and lung
Total spleens and lungs were harvested from naïve or 10N mice at the time of the experiments. Lungs were dissected into small pieces and exposed to an enzymatic digestion cocktail containing 0.125% dispase II (Roche, Indianapolis, IN), 0.2% collagenase II (Sigma-Aldrich), and 0.2% collagenase IV (Sigma-Aldrich) for 90 min at 37° C. After enzymatic digestion, a single-cell suspension was prepared by pushing the lung tissue fragments through a 70 µm diameter nylon mesh (BD Falcon). A suspension of splenocytes was prepared by mechanical dispersion. Cell suspensions were treated with Gey’s red cell lysis solution and passed through nylon wool columns to obtain T lymphocyte-enriched cell preparations containing >75% T cells as previously described (13). Total cell counts were determined using a Coulter counter.
Adoptive transfer of γδ T cells
Splenic nylon wool-nonadherent (NAD) cells from naïve and 10N mice (B6.TCR-β−/−, B6.TCR-β−/−/IFN-γ−/−) were incubated with biotinylated anti-Vγ4 mAb (clone UC3) or anti-Vγ1 mAb (clone 2.11) for 15 min at 4°C, washed and incubated with streptavidin-conjugated magnetic beads (Streptavidin Microbeads, Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min at 4°C, and passed through magnetic columns to purify Vγ4+ or Vγ1+ cells as previously described in detail (17). This produced a cell population containing >90% Vγ4+ or Vγ1+ viable cells as determined by two-color staining with anti-TCR-δ and anti-Vγ4 or anti-Vγ1 mAbs. These splenic Vγ4+ or Vγ1+ cells were washed in PBS and re-suspended to a concentration of 1.5 × 105 cells/ml PBS, and 3 × 104 cells/mouse were injected in 200 µl PBS via the tail vein into B6.TCR-δ−/− mice directly before the OVA immunization.
In some experiments, subpopulations of Vγ4+ or Vγ1+ cells were purified using the MoFlo cell sorter. NAD cells were incubated with FITC-conjugated anti-Vγ4 mAb (clone UC3) or FITC-conjugated anti-Vγ1 mAb (clone 2.11) and PE-conjugated anti-CD8α (clone 53–6.7; BD Pharmingen) or biotinylated anti-Vδ5 (clone F45-152) followed by PE-conjugated streptavidin (20 min at 4°C), and then washed. Cells were next sorted based on their expression of Vγ chain and Vδ5 or CD8α using a MoFlo cell sorter (Dako Cytomation, Inc.). Purified cells were washed in PBS and re-suspended to a concentration of 1.5× 105 cells/ml PBS, and 3 × 104 cells/mouse injected in 200 µl of PBS via the tail vein into B6.TCR-δ−/− mice directly before the OVA immunization. In general, the cell-sorter purified cells were less effective than those prepared by magnetic bead-selection and therefore have only been compared to each other.
Flow cytometric analysis
For flow cytometric analyses, NAD cells (2 × 105/well) in 96-well plates (Falcon; BD Biosciences, Franklin Lakes, NJ) were stained for TCR-expression using a PE-labeled pan Cδ Ab (clone GL3) and FITC -conjugated anti-Vγ1 (clone 2.11), or anti-Vγ4 (clone UC3) Ab followed by biotinylated anti-Vδ mAbs [anti-Vδ4 (clone GL-2), anti-Vδ5 (clone F45-152), anti Vδ 6.3 (clone 17-C), anti Vδ6λ12 (clone F4.22) and anti Vδ8 (clone B20.1)] plus streptavidin-allophycocyanin. All samples were analyzed on a FACScan flow cytometer (BD Biosciences) counting a minimum of 25,000 events per gated region, and the data were processed using FlowJo 6.4.1 software (Tree Star).
Determination of serum IgE levels
Sera were harvested on day 14 after the i.p. immunization with OVA/alum. For OVA-specific serum IgE determinations, plates were coated overnight at 4°C with 2 µg/ml of rat anti-mouse IgE antibody (clone R35-72, BD Biosciences). The serum samples were then added, and biotin-labeled OVA subsequently added to the wells. Prior to biotinylation, OVA was first dialyzed at 4°C overnight against 0.1 M borate buffer (pH 8.4). Biotinylated OVA was then prepared by reacting 1 ml OVA in PBS (1 mg/ml) with 150 µl of N-hydroxy-succinimido-biotin in dimethyl sulfoxide (DMSO) (1 mg/ml) for 4 h at room temperature, followed by overnight dialysis against PBS at 4°C. The bound OVA-biotin was detected with streptavidin-conjugated HRP (BD Biosciences) followed by 100 µl/well of TMB substrate solution. OVA-specific IgE levels in the samples were compared to an internal standard obtained from pooled sera of hyper-immunized BALB/c mice, which was arbitrarily assigned to equal 1,000 ELISA Units. Total IgE levels were measured by sandwich ELISA using rat anti-mouse IgE at 2 µg/ml (clone R35-72, BD Biosciences) as a capture antibody followed by biotinylated rat anti-mouse IgE heavy chain mAb (clone R35-118, BD Biosciences) at 2 µg/ml, and detected as described above. All samples were read using a VERSAmax™ tunable microplate reader and processed using a SoftMax Pro 4.7.1 software.
Statistical analysis
Data are presented as means ± SEM. The unpaired t test was used for two group comparisons and ANOVA was used for analysis of differences in three or more groups. Statistically significant levels are indicated as follows: one star (*) = p < 0.05; two stars (**) = p < 0.01; three stars (***) = p < 0.001. NS, not significant.
Results
Altered IgE response in γδ T cell genetically modified mice, and in normal mice treated with antibodies against the γδ TCR
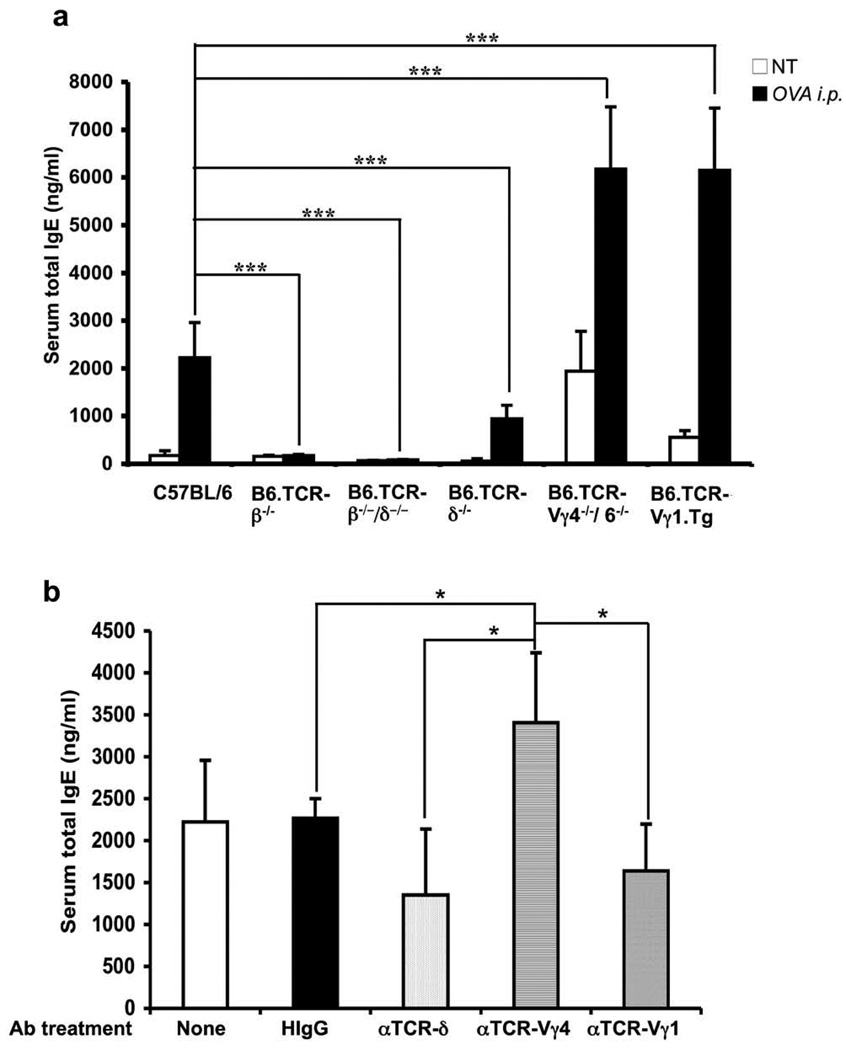
To test the concept that γδ T cells are capable of modulating the IgE response, we initially examined genetically modified mice, including mice lacking all T cells (B6.TCR-β−/−/δ−/−), all γδ T cells (B6.TCR-δ−/−), Vγ4+ and Vγ6+ γδ T cells only (B6.TCR-Vγ4−/−/6−/−), and mice expressing a rearranged Vγ1Jγ4Cγ4-transgene (B6.TCR-Vγ1 Tg), plus wild-type controls (C57BL/6). We also examined mice lacking all αβ T cells (B6.TCR-β−/−). Total serum IgE levels were measured by ELISA, in adult age/sex-matched untreated mice, and in mice injected i.p. with OVA/alum, 14 days after the injection (Fig.1a). Without treatment, basal serum IgE levels were low by comparison with wild-type controls in mice lacking all γδ T cells (B6.TCR-δ−/− vs C57BL/6, p <0.01), but high in mice deficient in Vγ4+ and Vγ6+ γδ T cells (B6.TCR-Vγ4−/−/6−/− vs C57BL/6, p <0.0001) and in mice expressing the Vγ1Jγ4Cγ4 transgene (B6.TCR-Vγ1 tg vs C57BL/6, p <0.001). Mice lacking αβ T cells did not express IgE at significant levels. At day 14 after OVA/alum injection, all mice except those lacking αβ T cells exhibited substantially increased serum IgE levels, and the relative increases in IgE resembled the pattern of the untreated mice.
Figure 1.
Altered IgE response in γδ T cell-modified mice. (a) Background serum IgE levels and primary OVA/alum-induced IgE responses in genetically altered mouse strains. Total serum IgE concentrations in C57BL/6, B6.TCR-β−/−, B6.TCR-β−/−/δ−/−, B6.TCR-δ−/−, B6.TCR-Vγ4−/−/6−/−, and B6.TCR-Vγ1 Tg mice was measured using sandwich ELISA 14 days after i.p. injection of OVA/alum, and in non-treated controls. Results for each group are expressed as the mean +/− SEM (n=18, 4, 4, 20, 15, 6, in the order shown). NT, Non-treated; ***, Significant difference (p < 0.001) compared to OVA-challenged C57BL/6 mice. Note: Significant differences in basal IgE levels between the mouse strains shown in Fig. 1a are listed in the text.
(b) Effect of treatment with anti TCR-mAbs on the primary OVA/alum-induced IgE response in C57BL/6 mice. Normal C57BL/6 mice were injected i.v. with non-specific hamster IgG (HIgG), anti-TCR-δ, anti-TCR-Vγ4, and anti-TCR-Vγ1 mAbs on day -4, and total serum IgE concentrations were determined on day 14 after i.p. injection of OVA/alum. Mice that received the OVA/alum injection but no antibody treatment were included as controls. Results for each group are expressed as the mean +/− SEM (n=4 in each group). *, Significant difference compared to untreated or HIgG-treated controls (p < 0.05).
In a follow-up experiment with wild-type C57BL/6 mice only, we again examined serum IgE-levels on day 14 after the i.p. OVA/alum injection, comparing groups that had been treated with antibodies against TCR-δ, TCR-Vγ4 and TCR-Vγ1 or with non-specific Ig, 4 days prior to the OVA/alum-sensitization (Fig. 1b). We have shown previously that the i.v. injected anti TCR mAbs inactivate the targeted T cells (13). Although the treatments with anti TCR-δ and TCR-Vγ1 mAbs did not significantly decrease serum IgE levels by comparison with the controls, the treatment with anti TCR-Vγ4 mAb significantly increased IgE levels, and there were significant differences between the different antibody-treated groups (Fig. 1b), consistent with the findings in the genetically modified mice (Fig. 1a). Taken together, the data shown in Fig.1 suggested that the overall-effect of γδ T cells in untreated mice, and in mice sensitized with OVA/alum, is to increase the primary IgE response, but also suggested that different Vγ-defined subsets are capable of modulating the IgE response in opposite directions: whereas Vγ1+ cells might enhance it, Vγ4+ cells might suppress it.
Adoptively transferred Vγ4+ cells from OVA-challenged but not untreated mice, inhibit the primary anti-OVA IgE response in TCR-δ−/− mice
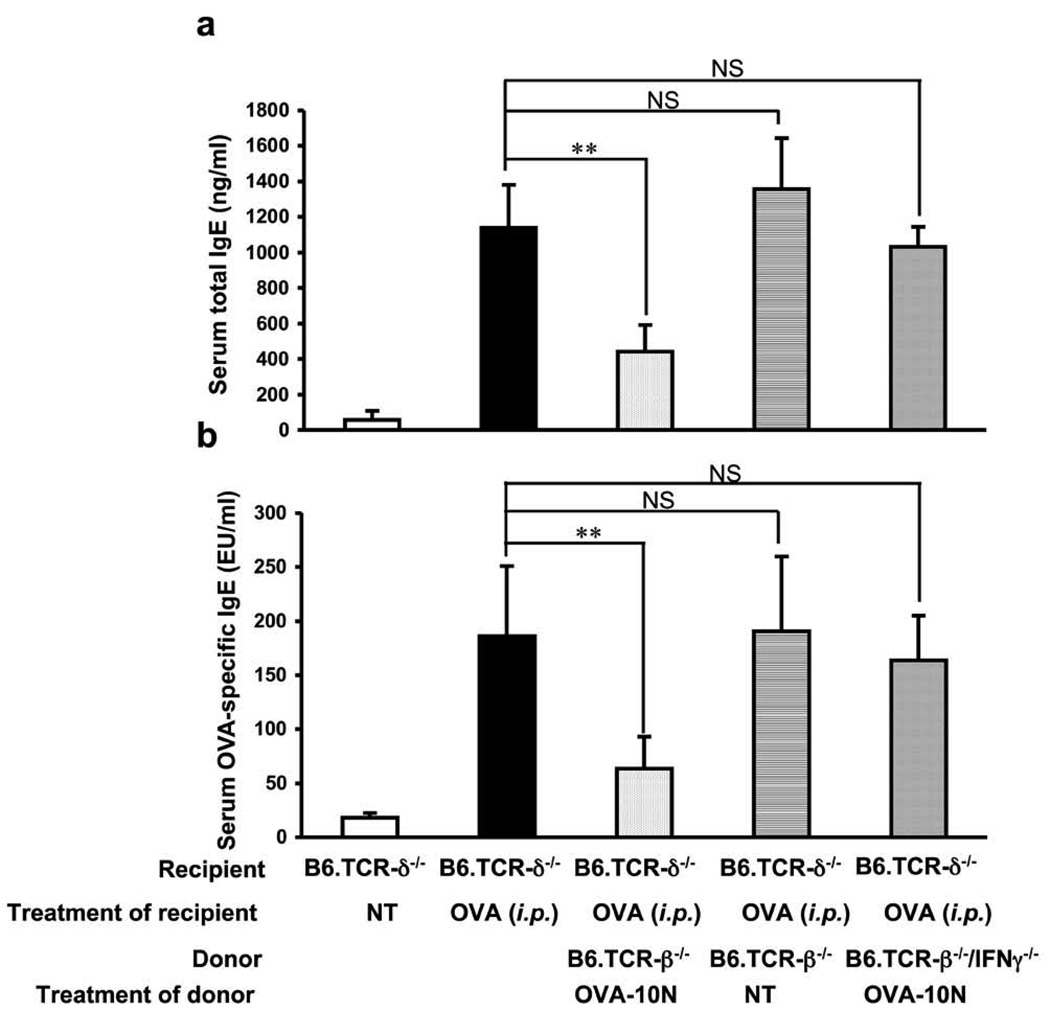
To further investigate the effect of Vγ4+ γδ T cells on the IgE response, we positively selected Vγ4+ cells from the spleen of untreated B6.TCR-β−/− mice (using magnetic beads), and transferred 3×104 of the purified cells to B6.TCR-δ−/− mice by i.v. injection, directly before the i.p. OVA/alum-sensitization. We examined both serum total IgE (Fig. 2a) and OVA-specific IgE in the cell transfer-recipients (Fig. 2b). Vγ4+ cells derived from the untreated donors did not significantly alter serum IgE levels in the OVA/alum-sensitized recipients. However, others have reported that mice exposed to aerosolized OVA by inhalation on at least 10 consecutive days (“10N” treated mice) lose their ability to mount an IgE response upon i.p. OVA/alum injection, and give rise to suppressive γδ T cells (8). We therefore prepared Vγ4+ cells from the spleen of 10N-treated B6.TCR-β−/− mice. Unlike those from the untreated donors, these cells markedly suppressed both total IgE and OVA-specific IgE levels in the serum of the B6.TCR-δ−/− cell-transfer-recipients (Fig. 2a, b). These data confirmed that IgE-suppressive γδ T cells are induced by airway allergen challenge (8) and showed that this occurs independently of αβ T cells. In an earlier study, we made essentially the same observations with AHR-suppressive γδ T cells and found that Vγ4+ γδ T cells derived from wild-type C57BL/6 mice and B6.TCR-β−/− mice are functionally equivalent (17). Finally, based on data of others suggesting that IgE-suppressive γδ T cells depend on IFN-γ (8), we tested whether Vγ4+ cells from the spleens of 10N-treated B6.TCR-β−/−/IFN-γ−/− mice had suppressive activity. In clear contrast to their wild-type counterparts, these cells failed to suppress the IgE response (Fig. 2a, b). These data confirmed that IFN-γ-dependent γδ T cells can become potent inhibitors of the IgE response (8), and identified Vγ4+ cells as the source population of the suppressors.
Figure 2.
Primary OVA/alum-induced IgE-response in B6.TCR-δ−/− mice is inhibited following adoptive transfer of Vγ4+ cells from airway-OVA-challenged B6.TCR-β−/−mice. Purified Vγ4+ cells (3×104/inoculum) from naïve or 10N-treated B6.TCR-β−/− mice, or from 10N-treated B6.TCR-β−/−/IFN-γ−/− - mice were adoptively transferred i.v. to B6.TCR-δ−/− recipients, just prior to i.p. OVA/alum injection. Serum total IgE (a) and OVA-specific IgE (b) levels were measured 14 days after the OVA/alum treatment.Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. **, p < 0.01; ***, p < 0.001. NS, not significant.
IgE-suppressive Vγ4+ cells express Vγ4Vδ5-TCRs and CD8
To further investigate the properties of IgE-suppressive Vγ4+ γδ T cells, we examined the Vδ usage of Vγ4+ and Vγ1+ cells in spleen and lung of untreated and 10N-treated B6.TCR-β−/− mice (Table I). Following the 10N treatment, the overall numbers and relative frequency of Vγ4+ cells remained stable or slightly decreased in spleen and increased in lung whereas Vγ1+ cells more generally decreased in spleen and less prominently increased in lung. Interestingly, certain Vγ4+ subpopulations (Vγ4/Vδ5+ cells and Vγ4/Vδ8+ cells) showed relative and absolute or at least relative increases in lung and spleen suggesting that these cells are selected in the 10N-treated mice and hence, that they might be enriched in IgE suppressors.
Table 1.
Sizes of γδ T cell subpopulations and their relative percentage in spleen and lung of naïve and 10N treated B6.TCR-β−/− mice
| Group | Vγ4δ4 | Vγ4δ5 | Vγ4δ6.3 | Vγ4δ6λ12 | Vγ4δ8 | Vγ1δ4 | Vγ1δ5 | Vγ1δ6.3 | Vγ1δ6λ12 | Vγ1δ8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Naïve spleen | 143±23 (11.5±0.6) |
296±69 (24±1.5) |
20±8 (1.6±0.5) |
21±5.6 (1.7±0.4) |
15±4.5 (1.2±0.4) |
163±45 (7.4±0.1) |
568±141 (25.9±0.5) |
114±50 (5.2±1.7) |
199±15 (9.4±1.7) |
23±7.5 (1.1±0.4) |
| 10N spleen | 128±33 (14±0.6) |
285±81 (30.7±3.7)* |
14±4 (1.6±0.6) |
14±5.3 (1.6±0.8) |
21±13 (1.4±0.6) |
115±67 (8.7±2.1) |
319±89 (25.8±3.0) |
84±11 (7.0±1.4) |
101±22 (8.3±1.0) |
14±5.8 (1.1±0.1) |
| Naïve lung | 14 (11.4) |
34 (26.9) |
1.8 (1.4) |
2.7 (2.1) |
5.2 (4.1) |
3.3 (1.9) |
43 (24.1) |
10 (5.7) |
8.5 (4.9) |
2.5 (0.9) |
| 10N lung | 21 (11.5) |
68 (37.1) |
4.8 (2.7) |
1.4 (0.8) |
9.7 (5.4) |
5.7 (3.2) |
53 (35.6) |
11.4 (8.6) |
7.8 (5.0) |
2.9 (1.1) |
Nylon wool non-adherend (NAD) cells derived from spleen and lung were counted. The percentages of individual γδ T cell subpopulations in NAD cells (%) were determined cytofluorimetrically. Absolute cell numbers (×1,000) were calculated as P (percentage) × NAD cell numbers. The percentages of γδ T cell subpopulations in total γδ T cells (%) are shown in parentheses. Four mice were used in each group and data for spleen cells were expressed as mean ± SEM. Lung cells were pooled and the average numbers are shown. Significant differences between naïve and challenged mice are indicated.
p < 0.05.
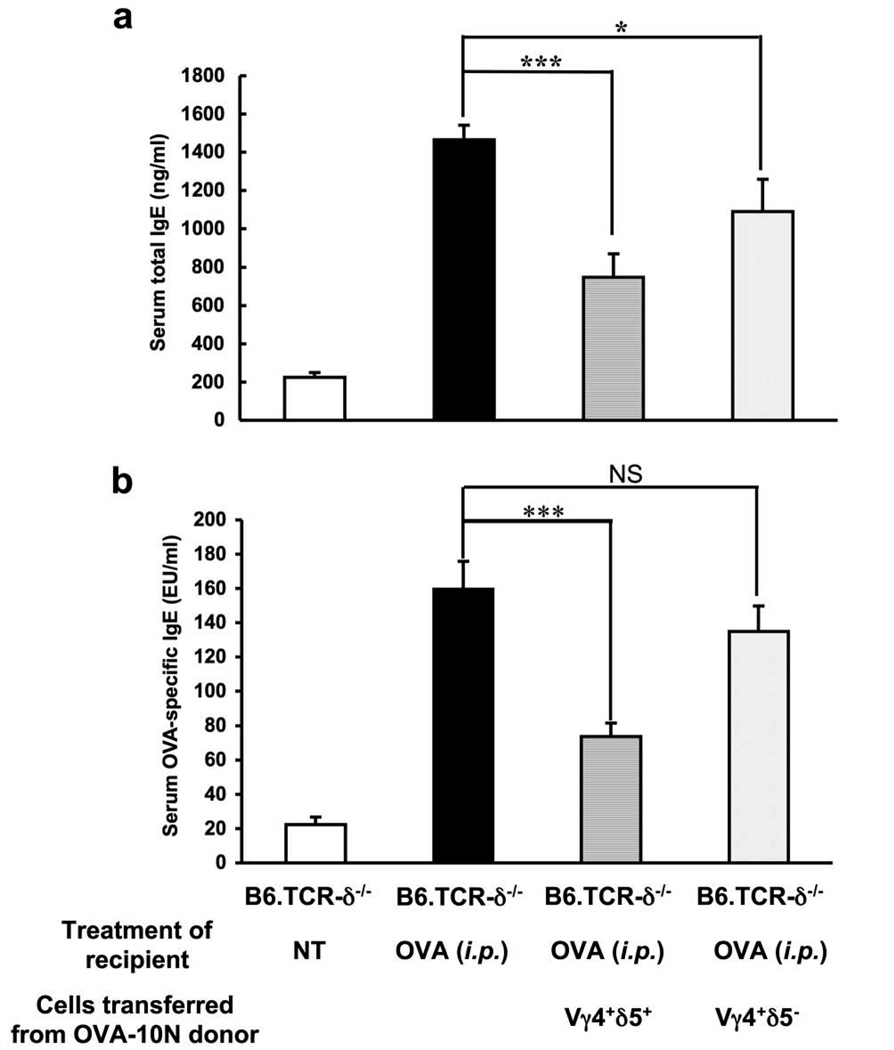
Of the two subpopulations of Vγ4+ cells, Vγ4/Vδ5+ cells are more accessible because they are present in larger numbers in both organs. Therefore, to test the idea that IgE suppressors among Vγ4+ cells are enriched during the 10N treatment, we purified Vγ4+/Vδ5+ and Vγ4+/Vδ5−cells by FACS sorting of splenocytes from 10N-treated B6.TCR-β−/− mice. Cells of either type (3×104 /inoculum) were then adoptively transferred to B6.TCR-δ−/− recipients by i.v. injection directly before i.p. OVA/alum-sensitization (Fig. 3). Overall, the FACS-sorted cells were less effective than the cells enriched by magnetic bead selection. However, the FACS-selected Vγ4+/Vδ5+ cells significantly decreased both serum total IgE (Fig. 3a) and OVA-specific IgE (Fig. 3b) whereas Vγ4+/Vδ5− cells had a smaller effect, and only serum total IgE was significantly reduced. These data confirm that Vγ4+/Vδ5+ γδ T cells can suppress IgE. Our experiment leaves open the possibility that other Vγ4+ cells, e.g. Vγ4+/Vδ8+ γδ T cells, also might function as IgE suppressors.
Figure 3.
IgE-suppressive Vγ4+ γδ T cells co-express Vδ5. Vδ5+ and Vδ5− Vγ4 cells were purified from the spleen of 10N-treated B6.TCR-β−/− mice by FACS-sorting. Purified Vγ4+/Vδ5+ or Vγ4+/Vδ5− cells (3×104/inoculum) were adoptively transferred i.v. to B6.TCR-δ−/− recipients just prior to i.p. injection of OVA/alum. Serum total IgE (a) and OVA-specific IgE (b) levels were measured 14 days after the OVA/alum injection. Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. ***, p < 0.001. NS, not significant.
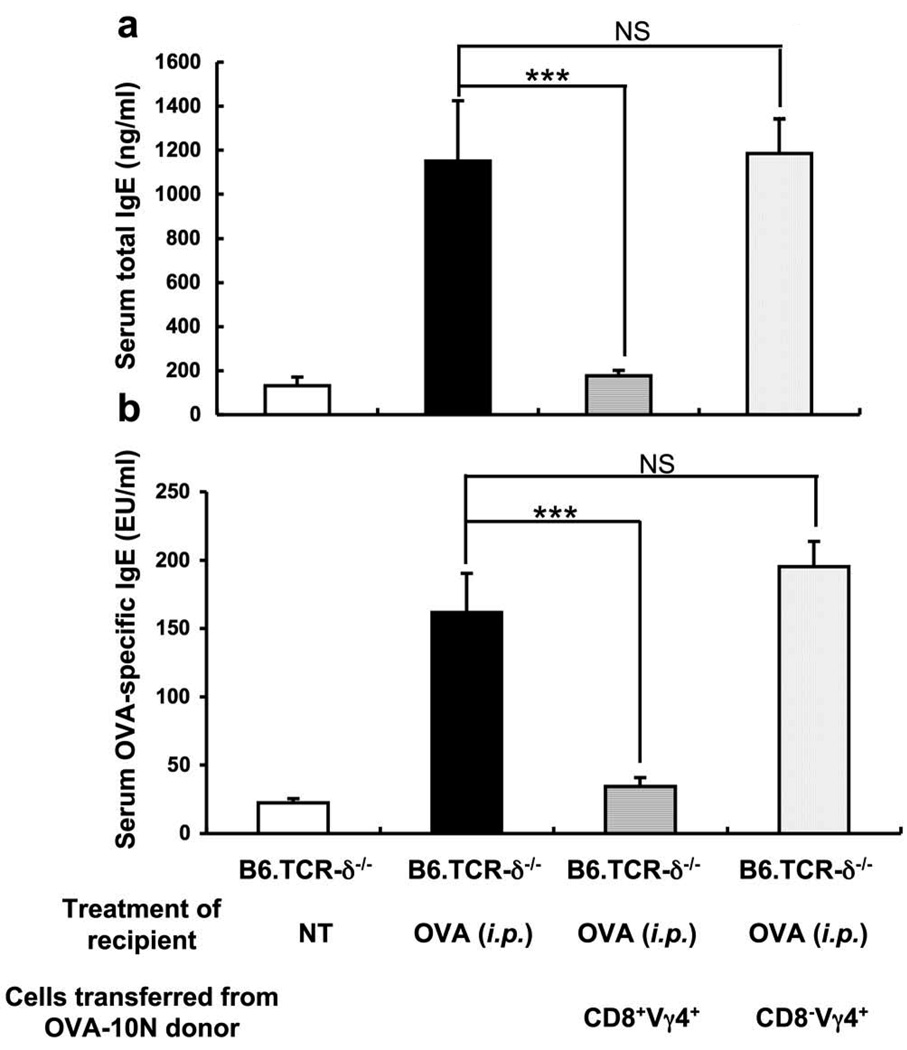
It has been proposed that IgE-suppressive γδ T cells express CD8 (8); in addition, Vγ4+ γδ T cells express CD8 more frequently than other γδ T cells (18). To examine the possibility that the Vγ4+ IgE suppressors co-express CD8, both Vγ4+/CD8α+ and Vγ4+/CD8α− cells were purified by FACS sorting of splenocytes from 10N-treated B6.TCR-β−/− mice, and subsequently tested in the cell transfer assay (Fig. 4). Whereas the Vγ4+/CD8α+ cells completely suppressed the OVA-specific IgE response, Vγ4+/CD8α−cells had no effect whatsoever. Taken together, these data suggest that a subset of γδ T cells expressing Vγ4/Vδ5-TCRs and CD8 contains most if not all IgE suppressors in our model.
Figure 4.
IgE-suppressive Vγ4+ γδ T cells express CD8. CD8α+ and CD8α− Vγ4 cells were purified from the spleen of 10N-treated B6.TCR-β−/− mice by FACS-sorting. 3×104 cells of each type were adoptively transferred i.v. to B6.TCR-δ−/− recipients just prior to i.p. injection of OVA/alum. Serum total IgE levels were measured 14 days after the OVA/alum injection. Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. ***, p < 0.001. NS, not significant.
Adoptively transferred Vγ1+ cells from untreated but not OVA-challenged mice enhance the primary anti-OVA IgE response in TCR-δ−/− mice
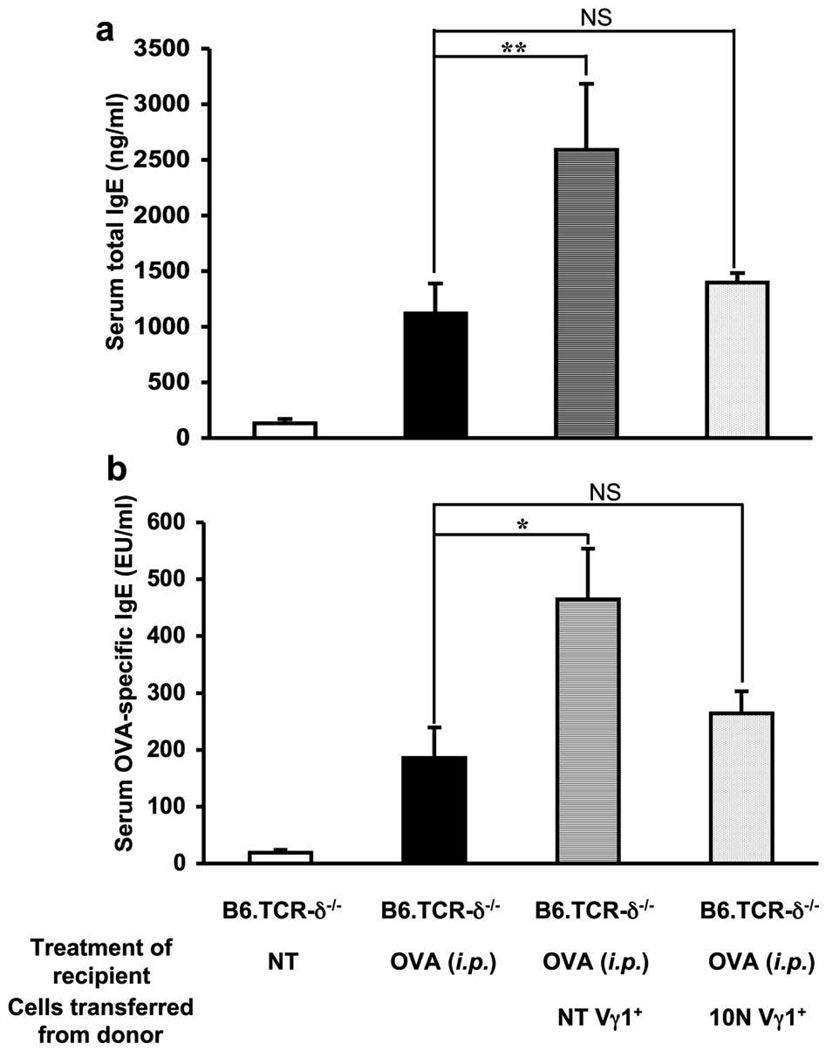
To further investigate the effect of Vγ1+ γδ T cells on the IgE response, we positively selected Vγ1+ cells from the spleens of untreated B6.TCR-β−/− mice, and transferred 3×104 of the purified cells to B6.TCR-δ−/− mice, shortly before the i.p. OVA/alum-sensitization. Vγ1+ cells derived from untreated donors significantly increased serum total IgE (Fig. 5a) and OVA-specific IgE levels (Fig. 5b) in the OVA/alum-sensitized recipients, in marked contrast to the Vγ4+ cells, which required OVA challenge of the donor mice to become functional IgE-suppressors (Fig. 2). However, Vγ1+ cells from 10N-treated B6.TCR-β−/−mice no longer had any significant effect on the IgE level (Fig. 5a, b). This is reminiscent of our earlier observation that Vγ1+ cells from untreated mice enhance AHR (19), and suggests that Vγ1+ γδ T cells in untreated mice already exert a Th2-like influence which enables them to promote both AHR and the IgE-responses.
Figure 5.
Adoptively transferred Vγ1+ γδ T cells from non-treated but not airway-OVA-challenged B6.TCR-β−/− mice enhance the primary OVA/alum-induced IgE response in B6.TCR-δ−/− mice. Purified Vγ1+ cells (3×104/inoculum) from non-treated or 10N-treated B6.TCR-β−/− mice were adoptively transferred i.v. to B6.TCR-δ−/− recipients just prior to i.p. OVA/alum injection. Serum total IgE (a) and OVA-specific IgE (b) levels were measured 14 days after the OVA/alum injection. Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. *, p < 0.05; **, p < 0.01. NS, not significant.
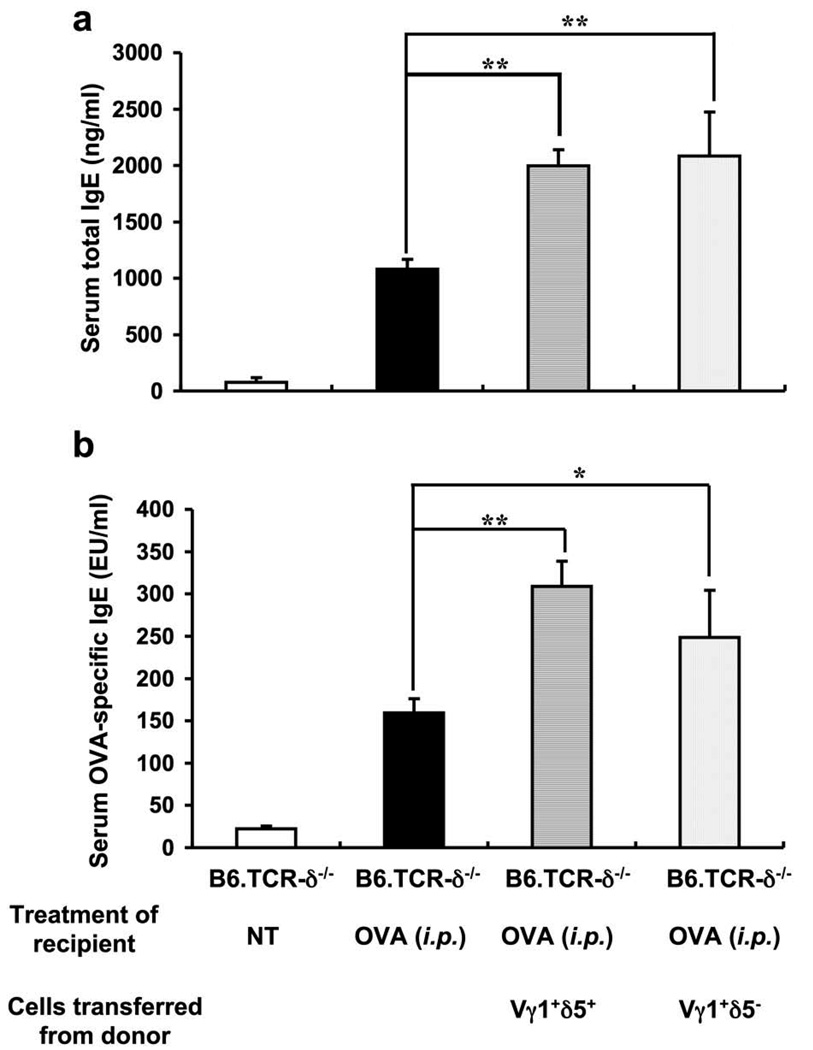
We had also found that AHR-enhancing Vγ1+ γδ T cells express Vδ5 (19). To test whether the IgE-enhancers also are Vγ1/Vδ5+ cells, we purified both Vγ1+/Vδ5+ and Vγ1+/Vδ5− cells by FACS sorting of splenocytes from non-treated B6.TCR-β−/− mice, and adoptively transferred either type to B6.TCR-δ−/− recipients by i.v. injection shortly before i.p. OVA/alum-sensitization (Fig. 6). However, both Vγ1+/Vδ5+ and Vγ1+/Vδ5− cells significantly increased serum total IgE and OVA-specific IgE in the B6.TCR-δ−/− cell-transfer recipients, indicating that the IgE-enhancers among Vγ1+ γδ T cells, unlike the AHR-enhancers, do not have to co-express Vδ5.
Figure 6.
Co-expression of Vδ5 is not required for Vγ1+ γδ T cells from non-treated mice to enhance the primary OVA/alum-induced IgE response. Purified Vγ1+/Vδ5+ or Vγ1+/Vδ5− cells (3×104/inoculum) from non-treated B6.TCR-β−/− mice were adoptively transferred i.v. to B6.TCR-δ−/− recipients directly before antigen challenge. Serum total IgE (a) and OVA-specific IgE (b) levels were measured 14 days after the OVA/alum injection. Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. *, p < 0.05; **, p < 0.01.
Net-effect of non-separated γδ T cells on the primary anti-OVA IgE response in TCR-δ−/− mice
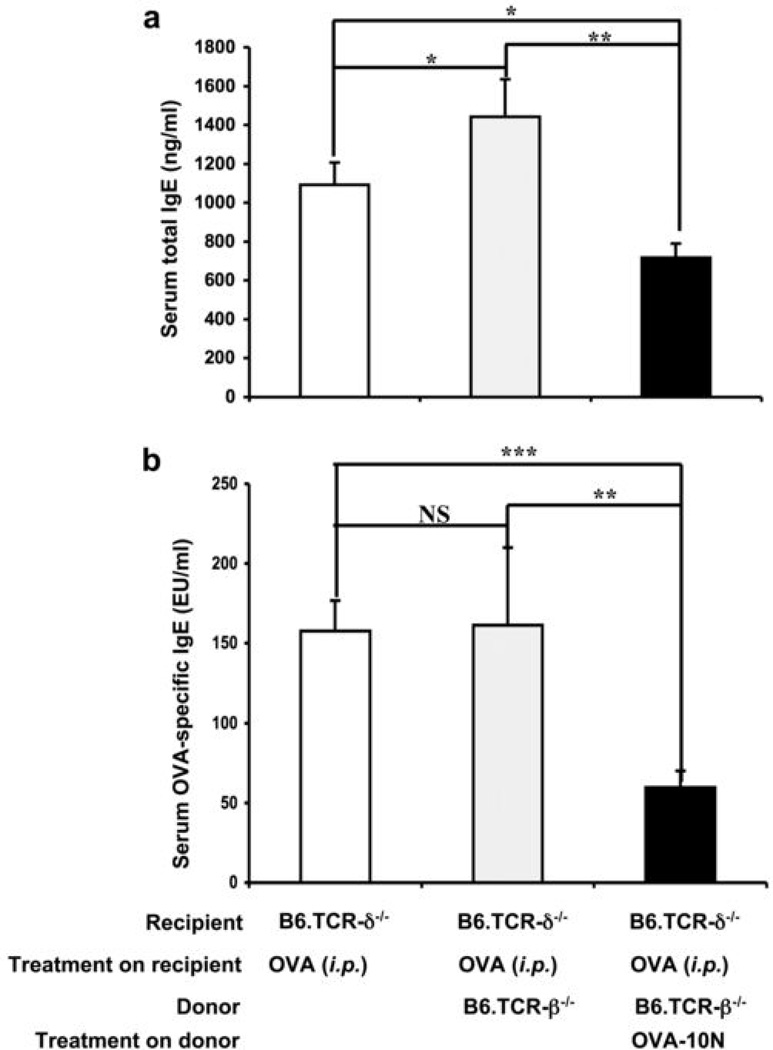
Since our findings with transferred purified cells representing the γδ T cell subsets suggested that the net-effect of all γδ T cells might be a switch from IgE-enhancement to IgE-suppression, we tested this idea by transferring total non-separated γδ T cells derived from donors that either remained untreated or were OVA-challenged (10N) (Fig.7). Since the total number of transferred cells (3×104 i.v.) remained the same, in this experiment fewer cells from either γδ T cell subset were transferred by comparison with the purified cells representing the individual subsets. As predicted, the non-separated γδ T cells from challenged donors suppressed the primary IgE response. However, non-separated gd T cells from untreated donors did not significantly enhance the IgE response (see discussion).
Figure 7.
Net-effect of total γδ T cells on the primary OVA/alum-induced IgE response. Purified γδ T cells (3×104/inoculum) from non-treated or OVA-challenged (10N) B6.TCR-β−/− mice were adoptively transferred i.v. to B6.TCR-δ−/− recipients directly before antigen challenge. Serum total IgE (a) and OVA-specific IgE (b) levels were measured 14 days after the OVA/alum injection. Results for each group are expressed as the mean +/− SEM (n=4 in each group). Significant differences between mice that received cells and those that received no cells are indicated. *, p < 0.05; **, p < 0.01, ***, p < 0.001.
Discussion
In this study we confirmed the earlier observation of others that γδ T cells exert a regulatory influence on the IgE response (8–10, 20). We extended these findings to show that there are IgE-suppressors and IgE-enhancers among IgE-regulatory γδ T cells, which belong to different subsets of γδ T cells and are distinguished by their TCR-expression. Furthermore, we demonstrate that while the suppressors require induction by airway exposure to allergen, the enhancers do not, and finally, that airway allergen challenge, previously reported to induce γδ T cell-dependent suppression of the IgE response (8, 20), actually has a dual effect: It induces the IgE-suppressors and inactivates the IgE-enhancers, thereby mediating γδ T cell-dependent IgE suppression in the challenged mice.
These observations raise several new questions. If γδ T cells indeed regulate the IgE response, when are they likely to play a role and how far-reaching might their influence be? In the current study, we have investigated the effect of γδ T cells on serum IgE levels following a single i.p. injection of OVA/alum. After transfer of non-separated γδ T cells derived from untreated or challenged donors, the change in the primary IgE-response was roughly two-fold, and after transfer of purified γδ T cells representing the regulatory subsets, the range of changes in total and OVA-specific IgE levels increased to approximately ten-fold. Considering that IgE-levels in circulation tend to be small (1), and that only 3×104 γδ T cells were transferred, the regulatory potential of γδ T cells may be substantial. We chose to investigate serum IgE and to transfer small numbers of splenic γδ T cells mainly for experimental convenience. Conceivably, the regulatory effect of γδ T cells on IgE in the mucosal tissues, where γδ T cells are more concentrated (21) and where IgE antibodies are present at much higher levels (2), might be greater. Our data also reveal differences in background serum IgE levels of γδ T cell genetically altered mice, and here the range of differences reaches approximately 100-fold. It seems clear, therefore, that γδ T cells profoundly influence the IgE response, and that their influence is not limited to the special case of immunization with OVA/alum.
What might be the significance of the correlation between TCR-expression and IgE-regulatory function? We found that the effect of Vγ4+ cells on the IgE response ranged from none to suppression, whereas that of Vγ1+ cells ranged from enhancement to none. These non-overlapping functional effects are reminiscent of other studies where the two types of γδ T cells also had non-overlapping and opposed functional effects (12). In particular, Vγ1+ cells also enhanced AHR whereas Vγ4+ cells suppressed it (13, 17, 19, 22). Our recent studies have shown that the Vγ1+ γδ T cell subset in normal untreated mice contains AHR-enhancing cells (19, 23) while the Vγ4+ subset does not. However, the AHR and IgE-regulatory γδ T cell populations do not appear to be identical. Whereas the AHR-enhancers seem to be limited to those cells that express Vγ1Vδ5 (19), IgE-enhancers were also found among Vγ1+Vδ5− cells. On the other hand, most of the IgE-suppressors appear to express Vγ4Vδ5 whereas the AHR-suppressors which also express Vγ4 might be less biased with regard to Vδ expression (17). Moreover, we found that the IgE-suppressors tend to express CD8, unlike the AHR-suppressors which may be CD8+ or CD8− (22). At least for those functional populations that depend on defined VγVδ pairs (the AHR-enhancers and the IgE-suppressors), it seems likely that the TCR is somehow involved with their function, either because TCR-ligand interactions help determine cellular differentiation or because they define function itself through ligand-specificity (14, 24). Consistent with the former possibility, a recent study showed that TCR-ligand interactions in the thymus determine the ability of peripheral γδ T cells to express certain cytokines (15). However, possible ligands for the IgE-regulatory γδ T cells remain unknown.
How can airway challenge mediate IgE-suppression? Having examined both IgE-suppressors and enhancers, it is clear that airway challenge does more than simply change the ratio of these cells in favor of the suppressors but rather affects the two regulatory populations separately. The experiments shown in this paper do not formally rule out that γδ T cells migrate from the challenged lung to the spleen. However, it would be difficult for pulmonary γδ T cells to change in the composition of splenic γδ T cells substantially because the splenic population of γδ T cells is much larger (25, 26). More likely perhaps, γδ T cells in the spleen are influenced by other signal carriers from the lung, e.g. pulmonary DC. Our preliminary studies with γδ T cells exposed to transferred non-T cells from challenged mice are consistent with such a mechanism (Yafei Huang, unpublished). Moreover, we found both Vγ1+ and Vγ4+ γδ T cells in the spleen in close proximity to CD8+ DC in the splenic PALS (22), a known destination of the peripheral signal carriers or “shuttles”. Furthermore, the splenic γδ T cells required the presence of CD8+ splenic dendritic cells (DC) for their functional development in another model (22). Therefore, we envision that the splenic γδ T cells might be compelled to change their function under the influence of CD8+ splenic DC, which must have received signals from the challenged lung (27). Indeed, such a mechanism has already been described, where CD8+ dendritic cells (DC), which are known to remain in the lymphoid tissues (28), depend on “shuttle” cells to receive stimulatory signals and antigen from the peripheral tissues including the lung (29–32).
Despite the clear IgE-enhancing effect of purified Vγ1+ cells derived from non-challenged donors, total γδ T cells derived from such donors enhanced IgE only weakly (detected in the assay for total serum IgE, but not for OVA-specific IgE). This simply might be due to the smaller number of transferred Vγ1+ cells in this experiment (only approximately ½ of total splenic γδ T cells are Vγ1+) or it might be caused by interactions among the IgE-regulatory γδ T cell subsets. In any case, the net effect of γδ T cells in untreated mice therefore might be only slightly supportive of the primary IgE response whereas their effect in challenged mice is clearly suppressive.
In sum, we found that airway challenge brings about coordinated and opposite functional changes of the IgE-enhancing and suppressive γδ T cells. This coordinated change of two antagonistic cell-types appears to represent an efficient mechanism in the regulation of the primary IgE response.
Acknowledgments
We would like to acknowledge the advice and support of Dr. Katja Aviskus, and the expert help of Shirley Sobus and William Townend with cell analysis and sorting.
Footnotes
This work was supported by National Institutes of Health Grants AI40611 and HL65410 to W.K.B., and AI44920 and AI063400 to R.L.O.
References
- 1.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IgE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 2.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature Reviews Immunology. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 3.Thornton CA, Holloway JA, Popplewell EJ, Shute JK, Boughton J, Warner JO. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin. Exp. Allergy. 2003;33:306–311. doi: 10.1046/j.1365-2222.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisenbarth SC, Colegio OR, O'Connor W, Jr, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1127. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowat AM. The regulation of immune responses to dietary protein antigens. Immunol. Today. 1987;8:93–98. doi: 10.1016/0167-5699(87)90853-X. [DOI] [PubMed] [Google Scholar]
- 6.Sedgwick JD, Holt PG. Down-regulation of immune responses to inhaled antigen: studies on the mechanism of induced suppression. Immunology. 1985;56:635–642. [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour BWP, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-γ/δ+ T cells or interferon (IFN)-γ in a murine model of allergen sensitization. J. Exp. Med. 1998;187:721–731. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 9.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 10.Svenson L, Lilliehook B, Larsson R, Bucht A. γδ T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1-and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 12.Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunology. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 13.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J.Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. γδ T cell receptors: functional correlations. Immunol. Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen KD, Su CX, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien Y-H. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 17.Jin N, Taube C, Sharp L, Hahn Y-S, Yin X, Wands JM, Roark CL, O'Brien RL, Gelfand EW, Born WK. Mismatched antigen prepares gd T cells for suppression of airway hyperresponsiveness. J. Immunol. 2005;174:2671–2679. doi: 10.4049/jimmunol.174.5.2671. [DOI] [PubMed] [Google Scholar]
- 18.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn Y-S, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl. Acad. Sci (USA) 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, J.L M, Gapin L, O'Brien RL, Gelfand EW, Born WK. Airway hyperresponsiveness through synergy of γδ T cells and NKT cells. J.Immunol. 2007;179:2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMenamin C, McKersey M, Kühnlein P, Hünig T, Holt PG. γδ T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J. Immunol. 1995;154:4390–4394. [PubMed] [Google Scholar]
- 21.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nature Reviews Immunology. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 22.Cook L, Miyahara N, Jin N, Wands JM, Taube C, Roark CL, Potter TA, Gelfand EW, O'Brien RL, Born WK. Evidence that CD8+ dendritic cells enable the development of gammadelta T cells that modulate airway hperresponsiveness. J. Immunol. 2008;181:309–319. doi: 10.4049/jimmunol.181.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin N, Roark CL, Miyahara N, Taube C, Aydintug MK, Wands JM, Huang Y, Hahn YS, Gelfand EW, O'Brien RL, Born WK. Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J. Immunol. 2009;182:2002–2010. doi: 10.4049/jimmunol.0803280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien RL, Lahn M, Born W, Huber SA. T cell receptor and function cosegregate in gamma-delta T cell subsets. Chemical Immunology. 2001;79:1–28. doi: 10.1159/000058829. [DOI] [PubMed] [Google Scholar]
- 25.Hahn Y-S, Taube C, Jin N, Takeda K, Park J-W, Wands JM, Aydintug MK, Roark CL, Lahn M, O'Brien RL, Gelfand EW, Born WK. Vγ4+ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J.Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 26.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn Y-S, Cook L, Yin X, Dalporto J, Lahn M, Hyde DM, Gelfand EW, Mason DY, O'Brien RL, Born WK. Distribution and leukocyte contacts of gd T cells in the lung. J. Leukocyte Biology. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 27.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nature Reviews Immunology. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 28.Shortman K, Naik SH. Steady-state and inflammatory dendritic cell development. Nature Reviews Immunology. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 29.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends in Immunology. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8a+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 31.Mount AM, Smith CM, Kupresanin F, Stoermer K, Heath WR, Belz GT. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS ONE. 2008;3:1–10. doi: 10.1371/journal.pone.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci (USA) 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]