Abstract
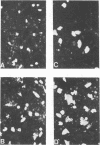
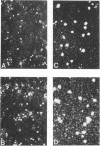
The effects of DEAE-dextran and cycloheximide on the infection of HeLa 229 cells with Chlamydia trachomatis serotype G were studied in terms of the number of cells infected and the yield of infectious progeny per infected cell. Pretreatment of the host cells with DEAE-dextran resulted in an increase in the number of infected cels but had no significant effect on the yield of infectious progeny per infected cell (burst size). In contrast, the addition of cycloheximide to the medium of infected cells had no significant effect on the number of infected cells but greatly enhanced the burst size. The burst size was calculated to be close to 500. The enhanced burst size was also observed in cells treated with DEAE-dextran and cycloheximide. In addition, there was an increase in the number of cells infected and an augmentation of the infectious progeny yield. Under the conditions of combined treatment, the yield of C. trachomatis serotype G cultivated in HeLa 229 cells was found to be approximately threefold higher than the yield of the organisms cultivated in McCoy cells. The results suggest that HeLa 229 cells treated with DEAE-dextran and cycloheximide offer a most suitable system for the high-yield cultivation of C. trachomatis organisms and possibly also for the diagnosis of infection with these organisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan I., Pearce J. H. Differential amino acid utilization by Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and its regulatory effect on chlamydial growth. J Gen Microbiol. 1983 Jul;129(7):1991–2000. doi: 10.1099/00221287-129-7-1991. [DOI] [PubMed] [Google Scholar]
- Becker Y. The chlamydia: molecular biology of procaryotic obligate parasites of eucaryocytes. Microbiol Rev. 1978 Jun;42(2):274–306. doi: 10.1128/mr.42.2.274-306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes S., McCormack W. M. Comparison of methods for cultivation and isolation of Chlamydia trachomatis. J Clin Microbiol. 1982 Nov;16(5):847–850. doi: 10.1128/jcm.16.5.847-850.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H. Growth characteristics of inclusion blennorrhea virus in cell cultures. Ann N Y Acad Sci. 1962 Mar 5;98:42–49. doi: 10.1111/j.1749-6632.1962.tb30530.x. [DOI] [PubMed] [Google Scholar]
- Croy T. R., Kuo C. C., Wang S. P. Comparative susceptibility of eleven mammalian cell lines to infection with trachoma organisms. J Clin Microbiol. 1975 May;1(5):434–439. doi: 10.1128/jcm.1.5.434-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. CYCLOHEXIMIDE: ASPECTS OF INHIBITION OF PROTEIN SYNTHESIS IN MAMMALIAN CELLS. Science. 1964 Dec 11;146(3650):1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- FURNESS G., GRAHAM D. M., REEVE P. The titration of trachoma and inclusion blennorrhoea viruses in cell cultures. J Gen Microbiol. 1960 Dec;23:613–619. doi: 10.1099/00221287-23-3-613. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. ISOLATION OF THE TRACHOMA AGENT IN CELL CULTURE. Proc Soc Exp Biol Med. 1965 Feb;118:354–359. doi: 10.3181/00379727-118-29841. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis P., Hobson D., Lee N. Effect of cycloheximide on the infective yield of a genital strain of Chlamydia trachomatis in McCoy cells. Infect Immun. 1981 Jul;33(1):309–311. doi: 10.1128/iai.33.1.309-311.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaipoel R. J., van Duin A. M. Isoelectric focusing of Chlamydia trachomatis. Infect Immun. 1979 Nov;26(2):775–778. doi: 10.1128/iai.26.2.775-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Keddell J. E. Efficacy of various cell culture procedures for detection of Chlamydia trachomatis and applicability to diagnosis of pediatric infections. J Clin Microbiol. 1981 Apr;13(4):705–708. doi: 10.1128/jcm.13.4.705-708.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota T. R. Chlamydia trachomatis in cell culture. II. Susceptibility of seven established mammalian cell types in vitro. Adaptation of trachoma organisms to McCoy and BHK-21 cells. In Vitro. 1977 May;13(5):280–292. doi: 10.1007/BF02616172. [DOI] [PubMed] [Google Scholar]