Abstract
Sex differences in pharmacokinetics and pharmacodynamics characterize many drugs and contribute to individual differences in drug efficacy and toxicity. Sex-based differences in drug metabolism are the primary cause of sex-dependent pharmacokinetics and reflect underlying sex differences in the expression of hepatic enzymes active in the metabolism of drugs, steroids, fatty acids and environmental chemicals, including cytochromes P450 (P450s), sulfotransferases, glutathione transferases, and UDP-glucuronosyltransferases. Studies in the rat and mouse liver models have identified more than 1000 genes whose expression is sex-dependent; together, these genes impart substantial sexual dimorphism to liver metabolic function and pathophysiology. Sex differences in drug metabolism and pharmacokinetics also occur in humans and are due in part to the female-predominant expression of CYP3A4, the most important P450 catalyst of drug metabolism in human liver. The sexually dimorphic expression of P450s and other liver-expressed genes is regulated by the temporal pattern of plasma growth hormone (GH) release by the pituitary gland, which shows significant sex differences. These differences are most pronounced in rats and mice, where plasma GH profiles are highly pulsatile (intermittent) in male animals versus more frequent (nearly continuous) in female animals. This review discusses key features of the cell signaling and molecular regulatory mechanisms by which these sex-dependent plasma GH patterns impart sex specificity to the liver. Moreover, the essential role proposed for the GH-activated transcription factor signal transducer and activator of transcription (STAT) 5b, and for hepatic nuclear factor (HNF) 4α, as mediators of the sex-dependent effects of GH on the liver, is evaluated. Together, these studies of the cellular, molecular, and gene regulatory mechanisms that underlie sex-based differences in liver gene expression have provided novel insights into the physiological regulation of both xenobiotic and endobiotic metabolism.
Individual differences in drug metabolism and pharmacokinetics contribute to the individual-to-individual variation that characterizes the responses to many drugs and other foreign chemicals and presents a major challenge in clinical pharmacology. Individual variation in the expression of major drug-metabolizing enzymes (DMEs), including cytochromes P450 (P450s), sulfotransferases, glutathione transferases, and UDP-glucuronosyltransferase, is associated with substantial individual differences in the bioavailability and clearance of drugs and other xenobiotics. Given the crucial role of hepatic DMEs in regulating the pharmacological and biological activity of drugs as well as steroid and other endobiotics, it is important to understand the regulatory features that lead to individual differences in the expression of DMEs. Factors that contribute to interindividual differences in DME expression and drug metabolism include genetic polymorphisms (Hines et al., 2008), prior or concomitant exposure to drugs and environmental chemicals (Urquhart et al., 2007), dietary factors (Moon et al., 2006; Murray, 2007), pregnancy (Anderson, 2005a), diseased states (Mann, 2006), epigenetic factors (Szyf, 2007), and endogenous hormonal factors, which change with age and differ between male and female subjects (Cotreau et al., 2005; Kennedy, 2008).
Genetic polymorphisms of DMEs influence the clinical outcome of an estimated 20 to 25% of all drug therapies (Ingelman-Sundberg, 2004; Eichelbaum et al., 2006). Several key drug-metabolizing human P450 genes are highly polymorphic, with more than 350 distinct alleles identified for the 57 known human P450 genes. A well studied example is CYP2D6, where genetic polymorphisms contribute to large interindividual variability in the metabolism of many antidepressants, antipsychotics, analgesics, and anticancer drugs (Ingelman-Sundberg et al., 2007). Interindividual differences also arise from the induction of P450s and other DMEs after exposure to lipophilic drugs and environmental chemicals. For example, phenobarbital and other drugs can increase the rates of metabolism of many drugs and chemicals by increasing the expression of individual P450 genes (Waxman and Azaroff, 1992). This P450 induction response is mediated by ligand-activated transcription factors (nuclear receptors) that function as sensors of foreign compounds and can activate a large number of DME genes in liver and other tissues (Waxman, 1999; Handschin and Meyer, 2003; Timsit and Negishi, 2007), with the associated potential for drug interactions (Jana and Paliwal, 2007; Sinz et al., 2008). The xenobiotic-activated nuclear receptors constitutive androstane receptor, pregnane X-receptor, and peroxisome proliferator-activated receptor-α respond to a wide range of xenochemicals and induce the expression of CYP2B, CYP3A, and CYP4A genes, respectively. Aryl hydrocarbon receptor is a receptor/transcription factor that induces CYP1 family members and certain other DME genes upon binding polycyclic aromatic hydrocarbons and other drugs and environmental chemicals (Ramadoss et al., 2005). Hepatic expression of DMEs can also be altered by pathophysiological conditions such as diabetes, inflammation, alcohol consumption, and protein-calorie malnutrition (Kim and Novak, 2007; Morgan et al., 2008). In addition, circadian factors regulate the expression of certain DMEs (Gachon et al., 2006) and contribute to the dosing time-dependence of drug activity and toxicity (Levi and Schibler, 2007).
Sex-based differences1 in pharmacokinetics and pharmacodynamics are well recognized (Fletcher et al., 1994; Gandhi et al., 2004; Franconi et al., 2007) and can be an important source of individual differences in drug responses. Sex-based differences in pharmacokinetics reflect differences in bioavailability, distribution, metabolism, and/or excretion. Sex hormones influence bioavailability through effects on gastrointestinal motility; estrogen inhibits gastric emptying. Sex differences in pharmacokinetics can result from sex differences in distribution, which can be caused by differences in body weight (lower in women), body fat (higher in women), plasma volume (lower in women, but varies through the menstrual cycle and during pregnancy), and organ blood flow (higher in women). In addition, body-weight effects on glomerular filtration rate can contribute to lower renal clearance in women in the case of drugs that are actively eliminated via the kidney (Gandhi et al., 2004). However, sex differences in metabolism are thought to be the primary determinant of sex differences in pharmacokinetics. Male-female differences in the metabolism of barbiturates were documented in rats as early as 1932 (Nicholas and Barron, 1932) and were later found to characterize many drugs and steroids, as shown in both rat and mouse models (Kato, 1974; Skett, 1988). These sex differences in metabolism are now known to reflect sex differences in the expression of specific, individual hepatic P450 enzymes and other DMEs (Shapiro et al., 1995). Sex-linked differences in P450-dependent drug and steroid metabolism are also found in hamster (Teixeira and Gil, 1991; Sakuma et al., 1994), dog (Lin et al., 1996; Hay Kraus et al., 2000), chicken (Pampori and Shapiro, 1993), and fish (Gray et al., 1991; Lee et al., 1998). Sex differences in human hepatic P450-catalyzed drug metabolism are well documented, but are less dramatic than in the rat (Tanaka, 1999; Parkinson et al., 2004; Scandlyn et al., 2008). This review discusses some of the pharmacological consequences of the observed sex differences in drug metabolism. The role of hormones, in particular GH, in establishing and maintaining these sex differences is discussed, and recent mouse and rat model studies that elucidate the underlying cellular signaling and molecular regulatory mechanisms by which pituitary GH secretory profiles dictate sex-based differences in DME expression in the liver are evaluated, focusing on factors that regulate the transcription of genes that code for P450 enzymes.
Sex Differences in Hepatic Drug Metabolism
Sex-based differences characterize the metabolism of many drugs commonly used in humans (Gandhi et al., 2004; Anderson, 2005b; Schwartz, 2007); 6 to 7% of new drug applications that include a sex analysis show at least 40% difference in pharmacokinetics between men and women (Anderson, 2005b). For example, acetaminophen clearance rates are 22% higher in men than in women because of a higher rate of glucuronidation (Miners et al., 1983). The higher bioavailability of aspirin in women than in men has been linked to decreased conjugation with glycine and glucuronic acid (Franconi et al., 2007). Men show a higher rate of clearance of the benzodiazepines diazepam, chlordiazepoxide, and olanzapine (MacLeod et al., 1979; Roberts et al., 1979; Bigos et al., 2008). Likewise, women display greater sensitivity to diazepam, which can lead to impairment of psychomotor skills (Palva, 1985). CYP3A4, the predominant cytochrome P450 catalyst of oxidative metabolism in human liver, is expressed at a higher protein and mRNA level in women than in men (Hunt et al., 1992; Wolbold et al., 2003; Diczfalusy et al., 2008). Accordingly, certain CYP3A4 drug substrates, including cyclosporine (Kahan et al., 1986), erythromycin (Austin et al., 1980), and nifedipine (Krecic-Shepard et al., 2000), show higher clearance rates in women. CYP3A4-catalyzed hepatic microsomal N-dechloroethylation of the anticancer drug ifosfamide is also more rapid in women, suggesting that women could be more susceptible to the neurotoxic side affects associated with this metabolic pathway (Schmidt et al., 2001). CYP3A4 also metabolizes steroids, such as cortisol, whose conversion to 6β-hydroxycortisol is more rapid in women than in men and serves as a biomarker for CYP3A4 metabolic activity (Inagaki et al., 2002).
Higher CYP2B6 activity has been observed in women than in men of Hispanic origin (Lamba et al., 2003). There is also evidence for higher CYP2A6 activity in women (Sinues et al., 2008), although that could, in part, reflect the use of estrogen-containing contraceptives, given the estrogen responsiveness of the human CYP2A6 gene (Higashi et al., 2007). CYP1A2 substrates, including caffeine and the antipsychotic agents olanzapine and clozapine, show higher clearance rates in men (Schwartz, 2007). As a result, women show greater improvement in psychotic symptoms but suffer a greater number of adverse side effects after treatment with these drugs. Men also show more rapid clearance of CYP2E1 substrates (e.g., chlorzoxazone) and certain CYP2D6 substrates, including propanolol, metoprolol, dextromethorphan, desipramine, and mirtazapine (Franconi et al., 2007; Schwartz, 2007). Increased rates of glucuronidation (UGT2B15 activity with oxazepam as substrate) also characterize male compared with female livers (Court et al., 2004). Overall, women are more likely to experience adverse drug reactions compared with men, due in part to sex differences in metabolic activity (Rademaker, 2001), with notable effects for cancer chemotherapeutic drugs (Mader, 2006; Wang and Huang, 2007) and antiretroviral agents (Ofotokun, 2005).
Studies carried out in rats and mice have established that sex-based differences in liver function also characterize many phase II DMEs, including sulfotransferases (Klaassen et al., 1998; Clodfelter et al., 2006; Kocarek et al., 2008), glutathione transferases (Srivastava and Waxman, 1993; Clodfelter et al., 2006; Knight et al., 2007), and UDP-glucuronosyltransferases (Takeuchi et al., 2004; Clodfelter et al., 2006; Buckley and Klaassen, 2007). In addition to drugs and other xenobiotics, P450s and other DMEs metabolize endogenous sex steroids (Waxman, 1988; Song and Melner, 2000; You, 2004; Chouinard et al., 2008), suggesting that the sex differentiation of drug metabolism reflects a need for sex-specific steroid metabolism. The sexual dimorphism of liver gene expression is not confined to DMEs; it is quite extensive and affects more than 1000 individual genes in both rats and mice (Clodfelter et al., 2006, 2007; Yang et al., 2006; Wauthier and Waxman, 2008). These include plasma lipoproteins (Oscarsson et al., 1991; Rudling et al., 1992), pheromone binding proteins (Johnson et al., 1995), regulators of fatty acid homeostasis (Amador-Noguez et al., 2005; Cheung et al., 2007), nuclear receptors (such as peroxisome proliferator-activated receptor-α and prolactin receptor) (Robertson et al., 1990; Jalouli et al., 2003), and other transcription factors, including sterol regulatory element binding protein (Lahuna et al., 1997; Améen et al., 2004; Laz et al., 2007). The extent to which sex differences occur in human liver is largely unknown; however, sex differences in genes such as these are likely to contribute to the observed sex differences in hepatic pathophysiology (Yokoyama et al., 2005), including differences in susceptibility to hepatocellular carcinoma seen both in animal models and in the clinic (Rogers et al., 2007; Shimizu et al., 2007).
Gonadal and Pituitary Hormonal Determinants of Hepatic Sex Differences
Early studies carried out in rats demonstrated that sex-based differences in P450 activities were abolished by castration, were re-established by testosterone replacement (Kato and Onoda, 1970), and were reversed by treatment with estradiol (Kramer et al., 1978). It was initially presumed that the gonadal hormones exerted their effects on steroid and drug metabolism via direct actions on the liver. However, an intact pituitary gland was later found to be required for testosterone and estradiol to regulate hepatic corticosterone metabolism, a sexually dimorphic metabolic activity (Colby et al., 1973). Several other groups later confirmed the requirement of an intact pituitary gland for gonadal hormones to regulate sex-dependent drug metabolism (Denef, 1974; Gustafsson and Stenberg, 1974; Lax et al., 1974). Further investigations implicated GH, a 191-amino acid protein hormone secreted by the anterior pituitary gland, in mediating the effects of gonadal hormones on liver drug and steroid metabolism. Wilson first observed that GH treatment of male but not female rats suppressed total P450 protein and drug metabolic activity (Wilson, 1973; Wilson and Bass, 1973). These effects of GH were later shown by Kramer et al. (1978) and Rumbaugh and Colby (1980) to mimic those of estradiol and were manifested in testosterone-treated female rats, establishing a clear link between sex steroids and the responsiveness of hepatic drug metabolism to GH treatment.
A key finding was the subsequent discovery by Edén (1979) that GH was secreted by the pituitary gland in a sexually dimorphic manner: in adult male rats, GH release into the bloodstream occurs in discrete pulses every ∼3.5 h, with little or no GH detectable between pulses. In adult female rats, however, GH secretion is more frequent, resulting in a more continuous presence of GH in circulation, albeit at a much lower level than peak male levels (Tannenbaum and Martin, 1976; Edén, 1979). Mice also display sex differences in the ultradian pattern of GH secretion, male mice having less frequent GH pulses and longer baseline intervals compared with female mice (MacLeod et al., 1991). These sex-dependent patterns of pituitary GH release are set (“imprinted”) by the action of gonadal hormones on the hypothalamus during the neonatal period, are manifested at puberty, and continue into adulthood (Jansson et al., 1985). The sexually dimorphic expression of liver steroid metabolic activity was later shown to be determined by the temporal pattern of GH stimulation: continuous but not pulsatile GH treatment restored female liver enzyme profiles in hypophysectomized female rats, whereas estrogen treatment of intact male rats feminized liver enzyme profiles in association with a feminization of the plasma GH profile (Mode et al., 1982). Subsequent studies by many laboratories demonstrated that 1) the differential effect of pulsatile versus continuous GH administration on hepatic drug and steroid metabolism (mimicking male- and female-like plasma GH patterns, respectively) is due to the action of GH on individual P450s and other sex-specific liver genes, and 2) these regulatory responses are largely determined at the level of gene transcription (Mode and Gustafsson, 2006; Waxman and O'Connor, 2006).
Humans also display plasma GH patterns that are sex-dependent and gonadal hormone-regulated (Veldhuis, 1998; Veldhuis and Bowers, 2003), with sex-dependent effects on certain human DMEs, albeit much smaller than those seen in rats and mice. CYP3A4 drug metabolic activity is increased in acromegalic patients and in men treated with GH-releasing hormone every 2 h (Watkins et al., 1993). In other studies, GH treatment of elderly men using a once-daily treatment schedule led to induction of CYP1A2 and, to a lesser extent, inhibition of CYP2C19 metabolic activity, but no effects were seen on CYP2D6 and CYP3A4 activity (Jürgens et al., 2002). However, in a separate study that investigated the pattern-dependence of GH responses in GH-deficient male and female patients, pulsatile GH was found to be more effective than continuous GH treatment with respect to stimulation of osteocalcin (a GH-responsive marker of bone formation) and down-regulation of CYP1A2 metabolic activity, whereas several other GH targets (serum IGF-1, serum IGF binding protein-3, and hepatic CYP3A4 activity) were increased more effectively using a continuous GH treatment schedule (Jaffe et al., 2002). Thus, pulsatile and continuous GH treatment can induce distinct responses of individual GH target genes in humans, in a manner similar to that in the rat and mouse models.
GH regulation of CYP3A4 can be recapitulated in primary human hepatocyte cultures, where CYP3A4 protein and mRNA can be induced by continuous GH treatment and suppressed by intermittent (pulsatile) GH (Liddle et al., 1998; Dhir et al., 2006). Moreover, as discussed elsewhere (Waxman and O'Connor, 2006), in transgenic mouse lines that contain the complete CYP3A4 gene, including 5′ and 3′ flanking DNA and the associated cis regulatory sequences, hepatic CYP3A4 expression shows a pattern of postnatal developmental regulation and adult sex specificity indistinguishable from that of several endogenous female-specific mouse Cyp3a gene products. Thus, hepatic CYP3A4 protein and RNA are expressed in immature male transgenic mice but are strongly down-regulated and become undetectable by 6 weeks of age, whereas in female mice, the CYP3A4 transgene is expressed in both immature and adult livers. Furthermore, continuous GH treatment of the transgenic male mice substantially increased liver CYP3A4 protein and RNA (Cheung et al., 2006). Thus, the human CYP3A4 gene contains all of the DNA sequence elements required to respond to the endogenous mouse hormonal environment, leading to a pattern of postnatal developmental regulation, adult sexual dimorphism, and plasma GH-responsiveness very similar to that of the endogenous female-specific, GH-regulated mouse Cyp3a genes. Sex-specificity and GH regulation have also been observed for CYP2C18 and CYP2C19 in a transgenic mouse liver model, where introduction of these tandem human genes together with flanking regulatory sequences results in hepatic expression that is male-specific and suppressed by continuous GH treatment (Löfgren et al., 2008). Further investigation will be required to ascertain the relevance of these findings in transgenic mice to the expression of these P450 genes in humans, which differ from mice in terms of their plasma GH profiles and other physiological factors.
Sex differences in DME expression have been observed in the kidney, where testosterone rather than GH is the proximal stimulus of male-specific gene expression. For example, the male-specific expression of rat CYP4A2 is stimulated by GH pulses in the liver but by testosterone in the kidney (Sundseth and Waxman, 1992). This, together with similar findings in studies of other DMEs in a mouse model (Henderson and Wolf, 1991), indicates that, in the kidney, male-specific DMEs are regulated by androgen receptor-dependent mechanisms, rather than by GH-dependent mechanisms. It is conceivable that a direct androgen-stimulatory mechanism might be ineffective in hepatocytes, where androgen receptor levels are generally low and high steroid metabolic activity may lead to rapid and extensive inactivation of androgens.
Class I and Class II Sex-Specific Genes
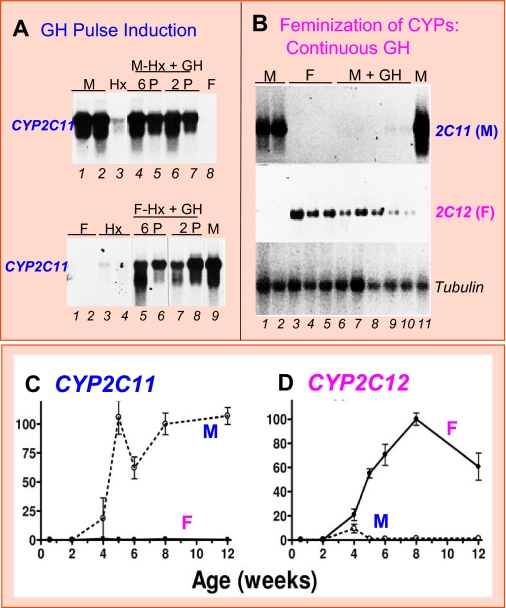
Male-specific genes can be classified based on their response to pituitary hormone ablation. Class I male-specific genes require pituitary hormones (principally GH) for full expression and are therefore decreased in expression after hypophysectomy, whereas class II male-specific genes are primarily regulated by the repressive actions of the female GH pattern in both rats (Table 1) (Wauthier and Waxman, 2008) and mice (Table 2; V. Wauthier and D. J. Waxman, unpublished observations). Thus, plasma GH pulses positively regulate class I male-specific P450 genes, whereas class II male-specific P450 genes do not require GH; indeed, they are expressed in liver at their highest levels when GH is ablated by hypophysectomy (Table 1). Rat CYP2C11 is a prototypic class I male-specific gene: it is induced after 4 weeks of age by the male, pulsatile GH secretion pattern, whereas a more continuous, female-like plasma GH profile abolishes CYP2C11 expression (Fig. 1 and Table 1). A GH-free interpulse interval of at least 100 to 140 min is required for full expression of CYP2C11 in male rat liver (Waxman et al., 1991; Agrawal and Shapiro, 2001), whereas GH ablation in male rats decreases CYP2C11 expression to 25 to 30% of intact male levels (Morgan et al., 1985; Janeczko et al., 1990). Class II male-specific genes include rat genes CYP2A2, CYP2C13, and CYP3A2 (Yamazoe et al., 1986; Waxman et al., 1988; McClellan-Green et al., 1989). These liver-expressed genes are not down-regulated by hypophysectomy; i.e., they do not require male GH pulse stimulation. Most strikingly, class II male-specific genes are up-regulated to near normal adult male liver levels in hypophysectomized female rats (Yamazoe et al., 1986; Waxman et al., 1988; McClellan-Green et al., 1989), evidencing the strong suppressive action of the female pituitary hormone profile (Pampori and Shapiro, 1999). Male GH pulses also repress the expression of class II male P450 genes (Agrawal and Shapiro, 2000) in a process that is rapid (detected within 30 min), as revealed by analysis of the effects of a single short-term GH pulse on hepatic levels of the primary, unspliced transcripts (hnRNAs) of CYP2A2 and CYP2C13 in hypophysectomized male rat liver (Wauthier and Waxman, 2008). These latter findings suggest that the transcription of class II male-specific genes is restricted to the GH-free interpulse interval in adult male rat liver.
TABLE 1.
Classification of sex-specific rat liver P450 genes and other genes
Genes are classified based on their response to hypophysectomy (Hx), pulsatile GH treatment of Hx rats, and continuous GH infusion in intact male rats. Male class II genes are expressed at high levels in both male and female Hx rats; they are subject to partial repression by male GH pulses (Wauthier and Waxman, 2008) and nearly complete repression by continuous GH treatment. Male class I genes, such as CYP2C11, are positively regulated by plasma GH pulses but are also repressed by continuous GH treatment, hence their increase in expression in Hx female liver to a level similar to that found in Hx male liver.
|
Gene Class
|
Prototypic Rat Genes
|
Loss of GH
|
GH Treatment
|
||
|---|---|---|---|---|---|
| Male Hx | Female Hx | GH Pulses | GH Continuous | ||
| Male | |||||
| Class I | CYP2C11 | ↓ | ↑ | ↑ | ↓ |
| Class II | CYP2A2, CYP2C13, CYP3A2 | - | ↑ | ↓ | ↓ |
| Female | |||||
| Class I | CYP2C12, HNF6, A1BG | ↓ | ↓ | ↓ | ↑ |
| Class II | ADH4, IGFBP1 | ↑ | ↑ | ↓ | ↑ |
↑, increase in gene expression compared with untreated controls; ↓, decrease in gene expression compared with untreated controls; -, no major changes in expression.
TABLE 2.
Regulation of sex-specific genes in mouse liver by GH, STAT5b, and HNF4α
Data are based on Sakuma et al. (2002) and Holloway et al. (2006, 2007, 2008). Sex-specific mouse liver genes are classified based on the response to hypophysectomy (Hx), as described for rat liver (Wauthier and Waxman, 2008), with the male class I genes further divided into subclasses A, B, and C based on the impact of Hx in female liver, as shown in this table. The responses to Hx in male liver reflect ablation of the stimulatory effects (male class IA, IB, and IC genes) as well as the inhibitory effects (female class II genes) of both STAT5b and male GH pulses, which activate STAT5b. In male mice, STAT5b deficiency and HNF4α deficiency have essentially the same impact on sex-specific gene expression, suggesting that these two factors coregulate gene expression, as discussed in the text. “Mups” refers to several closely related Mup family genes. The Cyp3a genes (last column) do not respond to GH pulse treatment or STAT5b deficiency; they also respond unusually slowly (≥7 days) to continuous GH treatment in intact male mice, indicating a unique regulatory mechanism. The response of Cyp2d9 to STAT5b deficiency in female mice is based on analysis of hepatocyte-specific Stat5a/Stat5b-deficient mice (Holloway et al., 2007).
|
Male
|
Female
|
||||
|---|---|---|---|---|---|
| Class IA | Class IB | Class IC | Class II | Other | |
| Prototypic mouse genes | Cyp2d9 | Cyp7b1, Mups | Cyp4a12, Gstp | Cyp2a4, Cyp2b9 | Cyp3a16, Cyp3a41,Cyp3a44 |
| GH deficiency | |||||
| Hx Male | ↓ | ↓ | ↓ | ↑ | - |
| Hx Female | - | ↓ | ↑ | - | ↓ |
| GH treatment | |||||
| Hx Male + GH pulses | ↑ | ↑ | ↑ | ↓ | - |
| Male + continuous GH | ↓ | ↓ | ↓ | ↑ | ↑ |
| STAT5b deficiency | |||||
| Male | ↓ | ↓ | ↓ | ↑ | - |
| Female | ↑ | ↓ | - | - | - |
| HNF4α deficiency | |||||
| Male | ↓ | ↓ | ↓ | ↑ | - |
| Female | ↓ | ↓ | - | - | ↓ |
↑, increase in gene expression compared with untreated controls; ↓, decrease in gene expression compared with untreated controls; -, no major changes in expression.
Fig. 1.
Regulation of rat liver CYP2C11 (male-specific) and CYP2C12 (female-specific) by plasma GH profile (A and B) and through postnatal development (C and D). A and B, Northern blots probed for the indicated liver mRNAs isolated from intact male (M) and female (F) rats, or from hypophysectomized (Hx) rats given physiological GH pulses (P) by intravenous injection six or two times per day for 7 days (A; data from Waxman et al., 1991). GH pulse treatment induces CYP2C11 in both male (top) and female (bottom) rats. Intact male rats were infused with GH continuously for 7 days using an osmotic mini-pump (B; data from Sundseth and Waxman, 1992), which suppresses CYP2C11 and induces CYP2C12. Tubulin RNA serves as a loading control. Each lane represents an individual rat liver. C and D show rat hepatic mRNA levels for CYP2C11 and CYP2C12 at various postnatal ages, as determined by quantitative polymerase chain reaction (data from Laz et al., 2007).
Female-specific genes can also be classified into sets of class I and II genes based on their response to hypophysectomy and GH replacement (Wauthier and Waxman, 2008). The steroid sulfate 15β-hydroxylase CYP2C12 is a class I female-specific gene; it requires continuous exposure to GH to restore its expression in hypophysectomized rat liver (MacGeoch et al., 1985; Pampori and Shapiro, 1996). It can also be induced to near-female levels by continuous GH infusion in intact male rats (Fig. 1B and Table 1). CYP2C12 is induced in intact female but not male rat liver at puberty, at the time when strong pituitary GH secretion commences, reflecting the requirement for continuous GH stimulation for high level expression (Fig. 1D). ADH4 and IGFBP1 are examples of class II female-specific genes: they are strongly derepressed (i.e., up-regulated) in both male and female liver after hypophysectomy, with a loss of sex specificity, and they are rapidly suppressed by physiological GH pulse replacement (Wauthier and Waxman, 2008). Continuous GH infusion in hypophysectomized female rats at doses that yield only ∼3% of the mean female plasma GH concentration (∼40 ng/ml) induces female P450 genes and suppresses both class I and II male P450 genes (Pampori and Shapiro, 1996). The effectiveness of such low GH concentrations is consistent with the low Kd for GH binding exhibited by GH receptor, ∼2 ng/ml (Fuh et al., 1992), which corresponds to only ∼1% of the peak plasma GH concentration in male rats (∼ 200 ng/ml).
Sex differences in the intrinsic responsiveness of the liver to continuous GH are apparent, with the effects of continuous GH on liver P450 expression being less complete and/or requiring a higher GH dose in a male hypophysectomized rat model compared with a female hypophysectomized rat model (Pampori and Shapiro, 1999). These intrinsic sex differences in continuous GH responsiveness can be recapitulated in primary hepatocyte cultures (Thangavel et al., 2004). Likewise, male hepatocytes are more responsive than female hepatocytes to GH pulses applied in culture (Thangavel et al., 2006), which could reflect elevated levels of the JAK2-STAT5 signaling inhibitors CIS and SOCS2 (Ram and Waxman, 1999; Ram and Waxman, 2000) in the female-derived liver cells (Thangavel and Shapiro, 2007). Epigenetic mechanisms, such as DNA methylation and histone methylation, could underlie these sex differences, reflecting a memory of the sexual differentiation of intact liver in vivo. Alternatively, these sex differences could be genetically determined [e.g., by Y-chromosome-encoded genes, several of which show strong expression in liver (Clodfelter et al., 2006)] and could potentially modulate responsiveness to GH stimulation.
Requirement of STAT5b for the Sex-Dependent Actions of GH
GH activates multiple intracellular signaling pathways (Herrington and Carter-Su, 2001), one of which involves STAT5b, a GH-responsive transcription factor that has been implicated in many of the sex-dependent actions of GH on the liver (Waxman and O'Connor, 2006). GH signaling is initiated at the cell surface, where GH binds to its receptor and induces a conformational change that activates Janus kinase 2 (JAK2), a GH receptor-associated tyrosine kinase. The activated JAK2 phosphorylates GH receptor on several intracellular tyrosine residues, thereby forming docking sites for STAT5b and other downstream signaling molecules that have SH2 (phosphotyrosine-binding) domains. JAK2 subsequently phosphorylates the GH receptor-bound STAT5b on tyrosine 699, which leads to STAT5b dimerization and translocation to the nucleus, where the active STAT5b dimer binds DNA response elements containing the consensus sequence TTC-NNN-GAA and activates gene transcription (Darnell, 1997; Herrington and Carter-Su, 2001). STAT5b is one of seven mammalian STAT transcription factors that share a conserved SH2-domain and a carboxyl-terminal tyrosine phosphorylation site (Grimley et al., 1999). STAT5a is closely related to STAT5b, with >90% sequence similarity, but is apparently unable to compensate for the loss of STAT5b in a gene knockout mouse model (Udy et al., 1997) and in humans with deleterious mutations in the STAT5b gene (Rosenfeld et al., 2007). STAT5a is essential for normal mammary gland development (Liu et al., 1997), whereas STAT5b is required for GH-stimulated pubertal and adult growth and for the sex-dependent effects of GH on liver gene expression (Udy et al., 1997; Teglund et al., 1998), as discussed below.
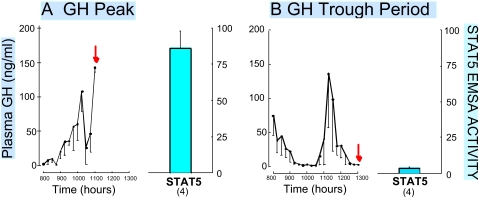
STAT5b is rapidly activated by each incoming plasma GH pulse in adult male rat liver (Fig. 2), whereas in adult female rats, liver STAT5b activity is maintained at a low but persistent level by the more continuous plasma GH pattern (Waxman et al., 1995; Choi and Waxman, 1999; Choi and Waxman, 2000). Hepatic STAT5b activity seems to have a similar sex dependence in mouse liver (Sueyoshi et al., 1999), although differences can be expected based on the unique features of the sexual dimorphic plasma GH profiles reported for mice (MacLeod et al., 1991). The sexual dimorphism of hepatic nuclear STAT5b activity, and its direct dependence on male plasma GH pulse stimulation, suggested that STAT5b might mediate the sex-dependent effects of GH on male liver gene expression (Waxman et al., 1995). Indeed, mice with a global disruption of the Stat5b gene display a loss of male-specific liver gene expression and lose their male-characteristic body growth rate profile (Udy et al., 1997; Teglund et al., 1998). Of seven male-specific liver RNAs investigated (Holloway et al., 2006), all were down-regulated (class I genes; Table 2), whereas five of eight female-specific RNAs were up-regulated in male liver in the absence of STAT5b (class II genes; Table 2) (Holloway et al., 2006). These findings are supported by a genome-wide transcriptional profiling study, where 90% of 850 male-specific genes were down-regulated and 61% of 753 female-specific genes were up-regulated in male liver in the absence of STAT5b (Clodfelter et al., 2006). In contrast, 90% of the sex-dependent genes investigated were unaffected by the loss of STAT5b in female liver, where STAT5b activity is much lower than in male liver (Choi and Waxman, 1999), and where STAT5a is required for expression of a subset of female-specific genes (Clodfelter et al., 2007). Thus, in male liver, STAT5b maintains male liver gene expression through its stimulatory effects on male-specific genes and its inhibitory effects on female-specific genes. These effects of STAT5b are likely to involve a combination of direct and indirect regulatory mechanisms, as discussed below.
Fig. 2.
Liver STAT5 is activated in direct response to each plasma GH pulse in adult male rats. Plasma samples obtained from intact rats every 15 min were assayed for GH. Samples were collected from 8 to 11 AM (A) or from 8 AM to 1 PM (B), with the collection end times of each group of n = 4 rats corresponding to a peak (A) and a trough in plasma GH levels (B), as indicated. The rats were then killed (red arrow) and the livers were assayed for STAT5 DNA-binding activity (blue bar on right). Data shown are from Tannenbaum et al. (2001).
Potential Limitations of the Global STAT5b-Deficient Mouse Model
The near-global effect of Stat5b gene disruption on sex-specific liver gene expression could be a direct result of impaired GH signaling due to the loss of STAT5b per se. However, indirect mechanisms involving the neuroendocrine control of pituitary GH secretion could also explain the observed demasculinization of male liver in the absence of STAT5b. GH secretion by the anterior pituitary is controlled by the action of two hypothalamic hormones: somatostatin, which inhibits GH release, and GH-releasing hormone, which stimulates GH secretion (Hartman et al., 1993; Fodor et al., 2006). Somatostatin mRNA is down-regulated in the hypothalamus of STAT5b-deficient mice, suggesting a role for hypothalamic STAT5b in the feedback regulation of somatostatin neurons by GH (Bennett et al., 2005). Consequently, the inhibition of pituitary GH release by somatostatin could be impaired in STAT5b knockout mice in a manner that feminizes circulating GH patterns and liver gene expression. A second possibility is that the loss of sex-specific liver gene expression in STAT5b-deficient male liver is a consequence of the down-regulation of Igf1, a STAT5b-dependent gene (Davey et al., 2001), the deletion of which in liver increases circulating GH levels as a result of the loss of a feedback inhibitory mechanism that regulates pituitary GH release (Sjögren et al., 1999; Wallenius et al., 2001). Indeed, circulating GH levels are elevated in global STAT5b knockout mice (Udy et al., 1997), and this could contribute to the feminization of male liver in the absence of STAT5b.
The impact of STAT5b deficiency on liver Cyp expression has been investigated in hypophysectomized mice given exogenous pulses of GH to help distinguish direct effects of STAT5b deficiency in the liver from indirect effects that the loss of STAT5b in the hypothalamus (or decreased Igf1 expression in the liver) may have on liver gene expression as a result of changes in the hypothalamic regulation of pituitary GH secretion. Whereas GH pulse treatment of hypophysectomized mice restored male liver gene expression and male-characteristic body growth rates in wild-type mice, these responses were not seen in STAT5b knockout mice (Davey et al., 1999; Holloway et al., 2006). This indicates a requirement for STAT5b for GH pulses to stimulate sex-specific gene expression in male liver. In other studies, hepatocyte-specific deletion of Stat5b and the neighboring Stat5a gene using a Cre transgene under the control of the albumin promoter significantly decreased liver expression of male-specific P450 genes and other genes and concomitantly increased female-specific liver gene expression in male mouse liver (i.e., a pattern very similar to that of the global STAT5b knockout) (Holloway et al., 2007). Although plasma GH levels were elevated in individual hepatocyte STAT5a/STAT5b-deficient male mice, the maintenance of normal male body growth rates and the absence in these male mice of certain female-specific, continuous GH-inducible liver gene transcripts (e.g., Cyp3a16; Holloway et al., 2007) indicates that the loss of STAT5b, and not the increase in plasma GH levels, is responsible for the observed demasculinization of liver gene expression. Targeted disruption of the Stat5a gene alone has a greater impact on sex-specific gene expression in female liver than in male liver (Clodfelter et al., 2007; Holloway et al., 2007), supporting the conclusion that the loss of STAT5b per se is responsible for the feminization of male liver gene expression in these STAT5-deficient mouse models.
STAT5-Dependent Sex Differences in Postnatal Growth
In addition to the above-described affects of GH on liver gene expression, pituitary GH release imparts sex-differences to long bone growth and overall body growth rates. These sex differences become apparent at puberty, when the sex differences in plasma GH patterns first emerge (Pampori et al., 1991; Gabriel et al., 1992). The male-characteristic pubertal body growth spurt is greatly reduced in global Stat5b-disrupted mice, resulting in decreased growth (Udy et al., 1997; Teglund et al., 1998), similar to that seen in STAT5b-deficient humans (Hwa et al., 2005; Vidarsdottir et al., 2006) and certain models of liver STAT5 deficiency (Engblom et al., 2007). Other studies, however, suggest that normal postnatal growth requires STAT5b signaling in one or more extrahepatic tissues, such as bone and skeletal muscle (Klover and Hennighausen, 2007). Further insight into the role of STAT5 in postnatal growth is provided by mouse models in which the intracellular cytoplasmic tail of GH receptor (required for STAT5 signaling) has been deleted or the membrane-proximal “Box 1” sequence of GH receptor (which binds JAK2) has been mutated to abolish JAK2 binding and thereby eliminate GH signaling via STAT5 and other molecules (Rowland et al., 2005; Lichanska and Waters, 2008). The requirement of glucocorticoids for postnatal growth may also be explained by their role in coregulating a subset of STAT5b-dependent transcripts (including some sex-specific transcripts) via a glucocorticoid receptor-STAT5b transcriptional complex (Engblom et al., 2007).
Impact of Liver-Specific Hnf4α-Deletion on Sex-Specific Gene Expression
HNF4α (NR2A1) is a member of the nuclear receptor superfamily (Sladek, 1994; Gonzalez, 2008). It is essential for normal liver development (Lemaigre and Zaret, 2004) and regulates multiple liver functions, including lipid homeostasis, lipoprotein production, and bile acid biosynthesis (Hayhurst et al., 2001; Hanniman et al., 2006; Inoue et al., 2006; Miura et al., 2006). Many sex-specific hepatic genes require HNF4α for full expression, as evidenced by the functional binding sites for HNF4α found in the promoters of several sex-specific P450 genes, including mouse Cyp2a4, mouse Cyp3a16, and rat CYP2C12 (Yokomori et al., 1997; Sasaki et al., 1999; Nakayama et al., 2001). In humans, a single nucleotide polymorphism at a putative HNF4α binding site in the CYP2B6 gene has been linked to polymorphic gene expression (Lamba et al., 2003), and in primary human hepatocytes, HNF4α is required for the expression of CYP3A4, CYP3A5, and CYP2A6 (Jover et al., 2001).
Liver-specific deletion of the Hnf4a gene (Hayhurst et al., 2001) has a sex-dependent impact on gene expression in liver, with ∼1000 fewer genes responding to the loss of HNF4α in female compared with male mice (Holloway et al., 2008). The male-predominant impact of liver HNF4α deficiency is even more striking when sex-specific genes are considered (372 sex-specific genes are specifically affected by the loss of HNF4α in male mouse liver, versus only 61 sex-specific genes are specifically affected in female mouse liver). The similarity between the response of sex-specific liver genes to the loss of HNF4α compared with the loss of STAT5b in male liver is striking, with parallel responses seen for 65% of the sex-specific genes common to the wild-type mouse strains used in both knockout studies (Table 2) (Clodfelter et al., 2006; Holloway et al., 2008). Thus, in male mouse liver, STAT5b and HNF4α positively regulate many male-specific Cyp genes and at the same time suppress a subset of female-specific genes. Moreover, the impact of STAT5b deletion and HNF4α deletion on sex-specific gene expression is substantially reduced in female compared with male liver. Thus, STAT5b and HNF4α may coregulate sex-specific liver genes by mechanisms that are primarily active in male liver. STAT5b and HNF4α exhibit mutual signaling cross-talk (Park et al., 2006); STAT5b is able to enhance HNF4α activation of select male-specific liver P450 genes, including Cyp2d9 and CYP8B1 (Wiwi and Waxman, 2005). STAT5b and HNF4α binding sites are found in the promoter regions of several sex-specific Cyp genes, including Cyp2d9 and Cyp2a4 (Yokomori et al., 1997; Wiwi et al., 2004). Moreover, HNF6 (ONECUT1), a female-predominant transcription factor (Lahuna et al., 1997) that positively regulates the female-specific genes CYP2C12 and A1BG (Delesque-Touchard et al., 2000; Gardmo and Mode, 2006), shows increased 5′-regulatory region binding of STAT5b and HNF4α in liver cells stimulated with GH (Lahuna et al., 2000).
A substantial fraction (31%) of the liver-expressed genes that contain functional HNF4α binding sites within 10 kilobases of the transcription start site, as determined by chromatin immunoprecipitation (Odom et al., 2007), require HNF4α for expression in one or both sexes (Holloway et al., 2008). The set of HNF4α-bound genes is 5-fold enriched for genes that are positively regulated by HNF4α (Holloway et al., 2008), supporting the conclusion that positive regulation by HNF4α proceeds by a direct DNA-binding mechanism. HNF4α protein and DNA-binding activity (but not RNA levels) are ∼5-fold higher in male compared with female rat liver (C. A. Wiwi and D. J. Waxman, unpublished observations), which suggests a mechanism for the sex specificity that characterizes certain HNF4α-dependent genes.
Mechanisms Whereby STAT5b Regulates Sex-Specific Gene Expression
Given the stimulatory effects of liver STAT5b on male-specific gene expression established by the mouse knockout studies discussed above, STAT5b may directly trans-activate its male-specific gene targets (Fig. 3). This mechanism is likely to apply to those male-specific genes that are rapidly activated in liver in vivo in response to a single plasma GH pulse, such as the Mup genes (Wauthier and Waxman, 2008). Indeed, Mup genes bind STAT5 at multiple sites in male liver chromatin, as determined by chromatin immunoprecipitation, with no specific binding to these sites detectable in female liver (Laz et al., 2009). STAT5 binding to liver chromatin is dynamic; i.e., it is directly induced by GH and cycles on and off chromatin in response to each plasma GH pulse. Male-specific STAT5 binding in liver in vivo is observed at low-affinity STAT5 binding sites, including those associated with Mup genes (Laz et al., 2009). The persistent but low level of STAT5 typically found in female liver nuclei (Choi and Waxman, 1999) is too low for efficient binding to occur at these sites. In contrast, STAT5 binds to chromatin at similar levels in male and female liver at several high-affinity STAT5 sites implicated in the regulation of GH-responsive genes such as IGF1 and SOCS2 (Laz et al., 2009), which do not show marked sex specificity. Based on these findings, it can be predicted that male-specific genes that are directly regulated by STAT5b (e.g., Mup genes) will have low-affinity STAT5 binding sites. These may potentially include nonconsensus STAT5 sites (Soldaini et al., 2000), which could complicate efforts aimed at their discovery (Park and Waxman, 2001).
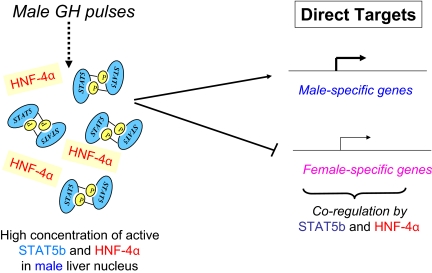
Fig. 3.
Model for direct regulation of sex-specific genes by STAT5b and HNF4α in male liver. In the model shown, GH pulse-activated STAT5 binds directly to and trans-activates its direct male-specific genes targets, which may include the family of male-specific Mup genes (Laz et al., 2009). HNF4α may also contribute to the expression of these genes. STAT5 and HNF4α may also directly bind to and repress female-specific genes, as shown. Some of the genes that serve as primary targets of STAT5 and HNF4α may also mediate the effects of these transcription factors on their downstream (i.e., indirect) targets, as shown in Fig. 4.
Two findings indicate that for a majority of liver male-specific genes, the requirement for STAT5b for full expression in male liver is likely to involve an indirect mechanism. First, most sex-specific genes are not activated when a single physiological plasma GH pulse is given to hypophysectomized animals (Wauthier and Waxman, 2008), even though liver STAT5 activation is rapid (within 5-10 min) under these conditions (Ram et al., 1996); second, continuous GH infusion in male mice feminizes liver gene expression slowly (over a period of days) (Holloway et al., 2006), whereas the down-regulation of STAT5 signaling occurs rapidly (within a few hours) (Fernández et al., 1998; Gebert et al., 1999). In one model of indirect regulation, a limited number of transcriptional regulators or other signaling molecules serve as the primary targets of GH pulse-activated STAT5b. These include transcriptional activators and transcriptional repressors, which regulate a larger set of secondary sex-specific target genes through a hierarchical network that includes both positive and negative regulatory mechanisms (Fig. 4). In an alternative formulation of this model, activation of some male-specific genes could require the coordinate binding of STAT5b and a STAT5b-dependent transcriptional activator. Several candidates for these primary transcriptional regulators have been identified based on their early response to a single plasma GH pulse given to hypophysectomized rats (Wauthier and Waxman, 2008). Liver expression of these primary response factors could be subject to postnatal developmental control that requires factors distinct from STAT5b. This, in turn, could help explain the finding that liver STAT5 alone is not sufficient to induce an adult male pattern of liver gene expression when it is activated precociously in the livers of prepubertal rats given exogenous GH pulses (Choi and Waxman, 2000). Indirect GH regulation of female-specific genes is also likely, as suggested by studies of the GH-regulated and female-predominant transcriptional activators HNF6 (Onecut1), HNF3α (Foxa1), and HNF3β (Foxa2), which can trans-activate female-specific genes, such as CYP2C12 and A1BG (Delesque-Touchard et al., 2000; Gardmo and Mode, 2006) and Cyp2b9 (Hashita et al., 2008). Finally, three nuclear factors are derepressed in STAT5b-deficient male liver and also HNF4α-deficient male liver; all three factors (Cux2/Cutl2, Tox, and Trim24) are female-specific transcriptional repressors that could contribute to the global down-regulation of male-specific genes seen in STAT5b-deficient male liver (Laz et al., 2007).
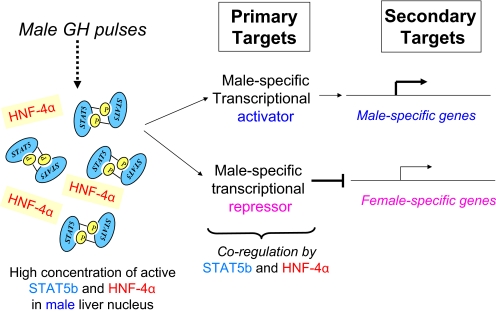
Fig. 4.
Model for indirect regulation of sex-specific genes by STAT5b and HNF4α in male liver. In the model shown, the effects of STAT5b and HNF4α on sex-specific liver gene expression are mediated indirectly, by male-specific transcriptional activators and repressors, which are primary targets of these two transcription factors. In an alternative formulation of this model (not shown), trans-activation of a secondary male-specific target gene could involve direct binding of STAT5b and the primary male-specific transcriptional activator. Several candidate primary target transcriptional regulators have been identified (Wauthier and Waxman, 2008).
Although STAT5b is not generally thought of as a transcriptional repressor, widespread derepression of female-specific genes is observed in both hypophysectomized and STAT5b-deficient male mouse liver (class II female genes; Table 2) (Clodfelter et al., 2006), as noted above. STAT5b could mediate the inhibitory effects of plasma GH pulses on these female-specific genes, and on class II male-specific genes in male liver (Wauthier and Waxman, 2008), in an indirect manner, as exemplified by its inhibition of IGF binding protein-1 expression through effects on the transcription factor FoxO1 (Ono et al., 2007). Twice as many genes were repressed by STAT5b as were induced in studies where adenoviral vectors were used to deliver a constitutively active form of STAT5b to the liver, suggesting that STAT5b is a major mediator of the rapid suppressive effects of GH (Vidal et al., 2007). It has been suggested that even the rapid transcriptional effects of GH in the liver largely proceed via STAT5b-independent pathways, given the lack of phylogenetically conserved STAT5b binding sites immediately upstream or within hepatic genes that are rapidly induced by GH treatment (Vidal et al., 2007). However, the involvement of far upstream STAT5 sites, not examined in that study but seen in the case of Igf1 (Wang and Jiang, 2005; Laz et al., 2009), cannot be excluded.
Hepatic STAT5a/STAT5b deficiency leads to aberrant activation of two other STAT family members, STAT1 and STAT3 (Cui et al., 2007), a response also seen in both male and female livers of global STAT5b knockout mice (Udy et al., 1997). Because each STAT protein regulates its own unique set of target genes, the activation of STAT1 and STAT3 in STAT5-deficient liver is likely to lead to changes in the expression of genes that are otherwise independent of STAT5b. Thus, some of the genes that are up-regulated in both male and female global STAT5b-deficient male mice (Clodfelter et al., 2006) may be induced due to the increased activity of STAT1 and STAT3, rather than the loss of inhibitory signaling by STAT5b per se (Hosui and Hennighausen, 2008).
Epigenetic Regulation of GH-Responsive Sex-Specific Genes
For many genes, a change in chromatin accessibility is an important, underlying mechanism of transcriptional control. Long-term silencing of gene expression by the conversion of euchromatin to heterochromatin involves epigenetic events, such as DNA CpG methylation, binding of sequence-specific repressors linked to recruitment of heterochromatin protein 1 and interactions with noncoding RNAs (Kwon and Workman, 2008; Delcuve et al., 2009) and plays a major role in tissue-specific gene expression. Support for a role of epigenetic factors in the regulation of sex-specific liver gene expression comes from the discovery of sex- and GH-dependent DNase I hypersensitive sites in the promoter regions of CYP2C11 and Slp, which correlate with the male-specific expression of these genes (Hemenway and Robins, 1987; Ström et al., 1994), and from studies of female-specific hypersensitivity sites in the case of CYP2C12 (Endo et al., 2005). These GH- and sex-regulated changes in chromatin accessibility could be functionally linked to the sexual dimorphism of gene expression in the liver and may be a general feature of sex-specific genes.
The slow feminization of gene expression in male liver after continuous GH infusion (Holloway et al., 2006) may involve large-scale chromatin remodeling that is ultimately dependent on either STAT5b or HNF4α. The silencing of male-specific genes after continuous GH treatment may require the local conversion of euchromatin to heterochromatin, whereas the de-repression of many female-specific genes that is seen in male liver deficient in STAT5b or HNF4α, or after continuous GH infusion, may require the conversion of heterochromatin to euchromatin (Fig. 5). The latter process could be facilitated by HNF3β (FOXA2), which is more highly expressed in female than male liver (Wiwi et al., 2004), and whose related family member, HNF3α (FOXA1), serves as a “pioneer factor” that binds to nucleosomal DNA at distant enhancers and facilitates recruitment of other tissue-specific factors (Lupien et al., 2008).
Fig. 5.
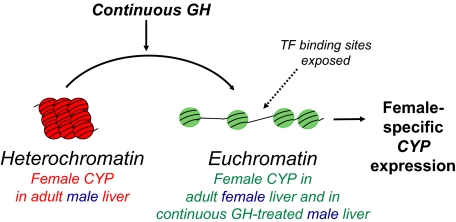
Epigenetic regulation of sex-specific P450 genes. Female-specific Cyp genes are proposed to be repressed in male liver, and male-specific Cyp genes are proposed to be repressed in female liver by packaging in heterochromatin. Continuous GH is proposed to activate female-specific genes, such as Cyp3a genes, by a mechanism that involves the local conversion of heterochromatin to euchromatin, which enables the binding of transcription factors (TF) that activate P450 gene expression. This process could involve loss of DNA CpG methylation and/or loss of chromatin marks associated with repressed chromatin, such as histone H3 lysine 27 trimethylation, which is typically found in genes in a compact chromatin structure and is associated with a stable, inactive heterochromatic state (Cheung and Lau, 2005).
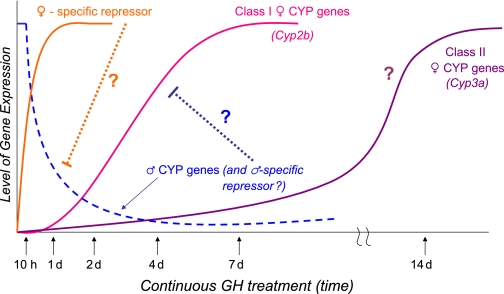
An unusually long period of continuous GH treatment (≥7 days) is required to induce hepatic expression of the female-specific genes Cyp3a16, Cyp3a41, and Cyp3a44 in adult male mouse liver (Fig. 6) (Holloway et al., 2006, 2007). These three female-specific genes can be induced in male liver by continuous GH treatment, even in the absence of STAT5a and STAT5b (Table 2) (Holloway et al., 2006, 2007), indicating a unique regulatory mechanism. This could involve STAT5-independent signaling pathways induced by GH, for example extracellular signal-regulated kinase/mitogen-activated protein kinase signaling (Ceseña et al., 2007), which requires a Src-like tyrosine kinase, rather than JAK2 signaling (Rowlinson et al., 2008). All three Cyp3a genes are expressed in both male and female liver before puberty, after which they are selectively down-regulated in male liver (Sakuma et al., 2002; Cheung et al., 2006). The silencing of these genes in male mouse liver at puberty could be associated with formation of a “permanent” closed heterochromatin structure; accordingly, their delayed responses to a change in plasma GH status may reflect molecular events required to reverse long-term silencing via CpG demethylation, the reversal of heterochromatin protein 1 binding (Kwon and Workman, 2008; Delcuve et al., 2009), and/or nucleosome displacement, processes that may be dependent on hepatocyte division associated with continued body growth and an increase in liver size.
Fig. 6.
Time course for continuous GH feminization of P450 gene expression in adult male liver. Model shown is based on data presented in Holloway et al. (2006); hypothetical regulatory interactions are indicated by question marks. Female-specific repressors (Laz et al., 2007), are rapidly induced in male liver by continuous GH treatment and are proposed to down-regulate male-specific genes. The latter genes are proposed to include one or more male-specific repressors, whose down-regulation leads to de-repression of female-specific genes, such as Cyp2b9 and Cyp2b10; these Cyp2b genes require several days of continuous GH treatment for induction (Holloway et al., 2006). A distinctly longer time frame (≥7 days) is required for derepression leading to induction of the female-specific Cyp3a genes, which may involve epigenetic mechanisms, as discussed in the text and diagrammed in Fig. 5.
Conclusion
Studies carried out in rat and mouse models have established that GH is the key hormonal factor that dictates sex differences in the expression of a large number of liver gene products, including many P450s and other DMEs. A similar mode of regulation may characterize CYP3A4 and other DMEs in humans, where clinically significant sex differences in metabolism are increasingly being recognized. GH is proposed to activate a complex hierarchical regulatory network of transcription factors that exert both stimulatory and inhibitory effects on sex-specific DMEs and other liver-expressed genes. The transcription factors STAT5b and HNF4α are both essential for the sexual dimorphism of an unexpectedly large number of liver-expressed genes. The actions of these factors are likely to be mediated through the actions of secondary target genes, including other transcription factors and downstream signaling molecules. Further studies are needed to identify and characterize these secondary regulators, the mechanisms by which they are regulated by GH and its sex-dependent plasma profiles, and their potential to contribute to sex-dependent chromatin remodeling and epigenetic events likely to be important in enforcing liver sex-specificity.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK33765].
ABBREVIATIONS: DME, drug-metabolizing enzyme; P450, cytochrome P450; GH, growth hormone; IGF, insulin-like growth factor; STAT, signal transducer and activator of transcription; JAK, Janus kinase; HNF, hepatic nuclear factor.
Footnotes
Although the term “gender differences” is often used in the medical literature to describe male-female differences, “sex” is the preferred term when describing biologically determined physiological, or pathophysiological, differences between male and female members of a species. “Gender” is a social construct that refers to an individual's self-representation as masculine or feminine, which, unlike “sex,” can be influenced by cultural factors (Gray, 2007).
References
- Agrawal AK and Shapiro BH (2000) Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J Pharmacol Exp Ther 292 228-237. [PubMed] [Google Scholar]
- Agrawal AK and Shapiro BH (2001) Intrinsic signals in the sexually dimorphic circulating growth hormone profiles of the rat. Mol Cell Endocrinol 173 167-181. [DOI] [PubMed] [Google Scholar]
- Amador-Noguez D, Zimmerman J, Venable S, and Darlington G (2005) Gender-specific alterations in gene expression and loss of liver sexual dimorphism in the long-lived Ames dwarf mice. Biochem Biophys Res Commun 332 1086-1100. [DOI] [PubMed] [Google Scholar]
- Améen C, Lindén D, Larsson BM, Mode A, Holmäng A, and Oscarsson J (2004) Effects of gender and GH secretory pattern on sterol regulatory element-binding protein-1c and its target genes in rat liver. Am J Physiol Endocrinol Metab 287 E1039-E1048. [DOI] [PubMed] [Google Scholar]
- Anderson GD (2005a) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44 989-1008. [DOI] [PubMed] [Google Scholar]
- Anderson GD (2005b) Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 14 19-29. [DOI] [PubMed] [Google Scholar]
- Austin KL, Mather LE, Philpot CR, and McDonald PJ (1980) Intersubject and dose-related variability after intravenous administration of erythromycin. Br J Clin Pharmacol 10 273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, McGuinness L, Gevers EF, Thomas GB, Robinson IC, Davey HW, and Luckman SM (2005) Hypothalamic STAT proteins: regulation of somatostatin neurones by growth hormone via STAT5b. J Neuroendocrinol 17 186-194. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Coley KC, Miller del D, Marder SR, Aravagiri M, Kirshner MA, Schneider LS, and Bies RR (2008) Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol 48 157-165. [DOI] [PubMed] [Google Scholar]
- Buckley DB and Klaassen CD (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35 121-127. [DOI] [PubMed] [Google Scholar]
- Ceseña TI, Cui TX, Piwien-Pilipuk G, Kaplani J, Calinescu AA, Huo JS, Iñiguez-Lluhí JA, Kwok R, and Schwartz J (2007) Multiple mechanisms of growth hormone-regulated gene transcription. Mol Genet Metab 90 126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Yu AM, Chen CS, Krausz KW, Byrd LG, Feigenbaum L, Edwards RJ, Waxman DJ, and Gonzalez FJ (2006) Growth hormone determines sexual dimorphism of hepatic cytochrome P450 3A4 expression in transgenic mice. J Pharmacol Exp Ther 316 1328-1334. [DOI] [PubMed] [Google Scholar]
- Cheung L, Andersen M, Gustavsson C, Odeberg J, Fernández-Pérez L, Norstedt G, and Tollet-Egnell P (2007) Hormonal and nutritional regulation of alternative CD36 transcripts in rat liver-a role for growth hormone in alternative exon usage. BMC molecular biology 8 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P and Lau P (2005) Epigenetic regulation by histone methylation and histone variants. Mol Endocrinol 19 563-573. [DOI] [PubMed] [Google Scholar]
- Choi HK and Waxman DJ (1999) Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology 140 5126-5135. [DOI] [PubMed] [Google Scholar]
- Choi HK and Waxman DJ (2000) Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology 141 3245-3255. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, and Bélanger A (2008) Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J Steroid Biochem Mol Biol 109 247-253. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, and Waxman DJ (2006) Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20 1333-1351. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, and Waxman DJ (2007) Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics 31 63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby HD, Gaskin JH, and Kitay JI (1973) Requirement of the pituitary gland for gonadal hormone effects on hepatic corticosteroid metabolism in rats and hamsters. Endocrinology 92 769-774. [DOI] [PubMed] [Google Scholar]
- Cotreau MM, von Moltke LL, and Greenblatt DJ (2005) The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet 44 33-60. [DOI] [PubMed] [Google Scholar]
- Court MH, Hao Q, Krishnaswamy S, Bekaii-Saab T, Al-Rohaimi A, von Moltke LL, and Greenblatt DJ (2004) UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of oxazepam glucuronidation by human liver. J Pharmacol Exp Ther 310 656-665. [DOI] [PubMed] [Google Scholar]
- Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, and Hennighausen L (2007) Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46 504-513. [DOI] [PubMed] [Google Scholar]
- Darnell JE Jr. (1997) STATs and gene regulation. Science 277 1630-1635. [DOI] [PubMed] [Google Scholar]
- Davey HW, Park SH, Grattan DR, McLachlan MJ, and Waxman DJ (1999) STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem 274 35331-35336. [DOI] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, and Grattan DR (2001) STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142 3836-3841. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Rastegar M, and Davie JR (2009) Epigenetic control. J Cell Physiol 219 243-250. [DOI] [PubMed] [Google Scholar]
- Delesque-Touchard N, Park SH, and Waxman DJ (2000) Synergistic action of hepatocyte nuclear factors 3 and 6 on CYP2C12 gene expression and suppression by growth hormone-activated STAT5b. Proposed model for female specific expression of CYP2C12 in adult rat liver. J Biol Chem 275 34173-34182. [DOI] [PubMed] [Google Scholar]
- Denef C (1974) Effect of hypophysectomy and pituitary implants at puberty on the sexual differentiation of testosterone metabolism in rat liver. Endocrinology 94 1577-1582. [DOI] [PubMed] [Google Scholar]
- Dhir RN, Dworakowski W, Thangavel C, and Shapiro BH (2006) Sexually dimorphic regulation of hepatic isoforms of human cytochrome p450 by growth hormone. J Pharmacol Exp Ther 316 87-94. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U, Miura J, Roh HK, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allqvist A, Jande M, Kim JW, et al. (2008) 4Beta-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics 18 201-208. [DOI] [PubMed] [Google Scholar]
- Edén S (1979) Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology 105 555-560. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M, Ingelman-Sundberg M, and Evans WE (2006) Pharmacogenomics and individualized drug therapy. Annu Rev Med 57 119-137. [DOI] [PubMed] [Google Scholar]
- Endo M, Takahashi Y, Sasaki Y, Saito T, and Kamataki T (2005) Novel gender-related regulation of CYP2C12 gene expression in rats. Mol Endocrinol 19 1181-1190. [DOI] [PubMed] [Google Scholar]
- Engblom D, Kornfeld JW, Schwake L, Tronche F, Reimann A, Beug H, Hennighausen L, Moriggl R, and Schütz G (2007) Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev 21 1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández L, Flores-Morales A, Lahuna O, Sliva D, Norstedt G, Haldosén LA, Mode A, and Gustafsson JA (1998) Desensitization of the growth hormone-induced Janus kinase 2 (Jak 2)/signal transducer and activator of transcription 5 (Stat5)-signaling pathway requires protein synthesis and phospholipase C. Endocrinology 139 1815-1824. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Acosta EP, and Strykowski JM (1994) Gender differences in human pharmacokinetics and pharmacodynamics. J Adolesc Health 15 619-629. [DOI] [PubMed] [Google Scholar]
- Fodor M, Kordon C, and Epelbaum J (2006) Anatomy of the hypophysiotropic somatostatinergic and growth hormone-releasing hormone system minireview. Neurochem Res 31 137-143. [DOI] [PubMed] [Google Scholar]
- Franconi F, Brunelleschi S, Steardo L, and Cuomo V (2007) Gender differences in drug responses. Pharmacol Res 55 81-95. [DOI] [PubMed] [Google Scholar]
- Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, and Wells JA (1992) Rational design of potent antagonists to the human growth hormone receptor. Science 256 1677-1680. [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Roncancio JR, and Ruiz NS (1992) Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology 56 619-625. [DOI] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, and Schibler U (2006) The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab 4 25-36. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, and Blaschke TF (2004) Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 44 499-523. [DOI] [PubMed] [Google Scholar]
- Gardmo C and Mode A (2006) In vivo transfection of rat liver discloses binding sites conveying GH-dependent and female-specific gene expression. J Mol Endocrinol 37 433-441. [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, and Waxman DJ (1999) Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol 13 213-227. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ (2008) Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab Pharmacokinet 23 2-7. [DOI] [PubMed] [Google Scholar]
- Gray ES, Woodin BR, and Stegeman JJ (1991) Sex differences in hepatic monooxygenases in winter flounder (Pseudopleuronectes americanus) and scup (Stenotomus chrysops) and regulation of P450 forms by estradiol. J Exp Zool 259 330-342. [DOI] [PubMed] [Google Scholar]
- Gray J (2007) Why can't a woman be more like a man? Clin Pharmacol Ther 82 15-17. [DOI] [PubMed] [Google Scholar]
- Grimley PM, Dong F, and Rui H (1999) Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev 10 131-157. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA and Stenberg A (1974) Masculinization of rat liver enzyme activities following hypophysectomy. Endocrinology 95 891-896. [DOI] [PubMed] [Google Scholar]
- Handschin C and Meyer UA (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55 649-673. [DOI] [PubMed] [Google Scholar]
- Hanniman EA, Lambert G, Inoue Y, Gonzalez FJ, and Sinal CJ (2006) Apolipoprotein A-IV is regulated by nutritional and metabolic stress: involvement of glucocorticoids, HNF-4 alpha, and PGC-1 alpha. J Lipid Res 47 2503-2514. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Veldhuis JD, and Thorner MO (1993) Normal control of growth hormone secretion. Horm Res 40 37-47. [DOI] [PubMed] [Google Scholar]
- Hashita T, Sakuma T, Akada M, Nakajima A, Yamahara H, Ito S, Takesako H, and Nemoto N (2008) Forkhead box A2-mediated regulation of female-predominant expression of the mouse Cyp2b9 gene. Drug Metab Dispos 36 1080-1087. [DOI] [PubMed] [Google Scholar]
- Hay Kraus BL, Greenblatt DJ, Venkatakrishnan K, and Court MH (2000) Evidence for propofol hydroxylation by cytochrome P4502B11 in canine liver microsomes: breed and gender differences. Xenobiotica 30 575-588. [DOI] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, and Gonzalez FJ (2001) Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21 1393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemenway C and Robins DM (1987) DNase I-hypersensitive sites associated with expression and hormonal regulation of mouse C4 and Slp genes. Proc Natl Acad Sci U S A 84 4816-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ and Wolf CR (1991) Evidence that the androgen receptor mediates sexual differentiation of mouse renal cytochrome P450 expression. Biochem J 278 499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J and Carter-Su C (2001) Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab 12 252-257. [DOI] [PubMed] [Google Scholar]
- Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, and Nakajima M (2007) Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos 35 1935-1941. [DOI] [PubMed] [Google Scholar]
- Hines RN, Koukouritaki SB, Poch MT, and Stephens MC (2008) Regulatory polymorphisms and their contribution to interindividual differences in the expression of enzymes influencing drug and toxicant disposition. Drug Metab Rev 40 263-301. [DOI] [PubMed] [Google Scholar]
- Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, and Waxman DJ (2007) Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148 1977-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway MG, Laz EV, and Waxman DJ (2006) Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4alpha. Mol Endocrinol 20 647-660. [DOI] [PubMed] [Google Scholar]
- Holloway MG, Miles GD, Dombkowski AA, and Waxman DJ (2008) Liver-specific hepatocyte nuclear factor-4alpha deficiency: greater impact on gene expression in male than in female mouse liver. Mol Endocrinol 22 1274-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosui A and Hennighausen L (2008) Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics 34 135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CM, Westerkam WR, and Stave GM (1992) Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol 44 275-283. [DOI] [PubMed] [Google Scholar]
- Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, and Rosenfeld RG (2005) Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol Metab 90 4260-4266. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Inagaki M, Kataoka T, Sekido I, Gill MA, and Nishida M (2002) A wide interindividual variability of urinary 6beta-hydroxycortisol to free cortisol in 487 healthy Japanese subjects in near basal condition. Ther Drug Monit 24 722-727. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M (2004) Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci 25 193-200. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Sim SC, Gomez A, and Rodriguez-Antona C (2007) Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116 496-526. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW, Inoue J, Xiang CC, Brownstein MJ, Eggertsen G, Björkhem I, et al. (2006) Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res 47 215-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, and Watkins PB (2002) Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283 E1008-E1015. [DOI] [PubMed] [Google Scholar]
- Jalouli M, Carlsson L, Améen C, Lindén D, Ljungberg A, Michalik L, Edén S, Wahli W, and Oscarsson J (2003) Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology 144 101-109. [DOI] [PubMed] [Google Scholar]
- Jana S and Paliwal J (2007) Molecular mechanisms of cytochrome p450 induction: potential for drug-drug interactions. Curr Protein Pept Sci 8 619-628. [DOI] [PubMed] [Google Scholar]
- Janeczko R, Waxman DJ, Le Blanc GA, Morville A, and Adesnik M (1990) Hormonal regulation of levels of the messenger RNA encoding hepatic P450 2c (IIC11), a constitutive male-specific form of cytochrome P450. Mol Endocrinol 4 295-303. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Edén S, and Isaksson O (1985) Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6 128-150. [DOI] [PubMed] [Google Scholar]
- Johnson D, al-Shawi R, and Bishop JO (1995) Sexual dimorphism and growth hormone induction of murine pheromone-binding proteins. J Mol Endocrinol 14 21-34. [DOI] [PubMed] [Google Scholar]
- Jover R, Bort R, Gómez-Lechón MJ, and Castell JV (2001) Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. Hepatology 33 668-675. [DOI] [PubMed] [Google Scholar]
- Jürgens G, Lange KH, Reuther LØ, Rasmussen BB, Brøsen K, and Christensen HR (2002) Effect of growth hormone on hepatic cytochrome P450 activity in healthy elderly men. Clin Pharmacol Ther 71 162-168. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Kramer WG, Wideman C, Flechner SM, Lorber MI, and Van Buren CT (1986) Demographic factors affecting the pharmacokinetics of cyclosporine estimated by radioimmunoassay. Transplantation 41 459-464. [DOI] [PubMed] [Google Scholar]
- Kato R (1974) Sex-related differences in drug metabolism. Drug Metab Rev 3 1-32. [DOI] [PubMed] [Google Scholar]
- Kato R and Onoda K (1970) Studies on the regulation of the activity of drug oxidation in rat liver microsomes by androgen and estrogen. Biochem Pharmacol 19 1649-1660. [DOI] [PubMed] [Google Scholar]
- Kennedy M (2008) Hormonal regulation of hepatic drug-metabolizing enzyme activity during adolescence. Clin Pharmacol Ther 84 662-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK and Novak RF (2007) The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther 113 88-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Liu L, and Dunn RT 2nd (1998) Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem Biol Interact 109 299-313. [DOI] [PubMed] [Google Scholar]
- Klover P and Hennighausen L (2007) Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148 1489-1497. [DOI] [PubMed] [Google Scholar]
- Knight TR, Choudhuri S, and Klaassen CD (2007) Constitutive mRNA expression of various glutathione S-transferase isoforms in different tissues of mice. Toxicol Sci 100 513-524. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Duanmu Z, Fang HL, and Runge-Morris M (2008) Age- and sex-dependent expression of multiple murine hepatic hydroxysteroid sulfotransferase (SULT2A) genes. Biochem Pharmacol 76 1036-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RE, Greiner JW, Rumbaugh RC, Sweeney TD, and Colby HD (1978) Relation of the gonadal hormones to growth hormone actions on hepatic drug metabolism in rats. J Pharmacol Exp Ther 204 247-254. [PubMed] [Google Scholar]
- Krecic-Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, and Schwartz JB (2000) Race and sex influence clearance of nifedipine: results of a population study. Clin Pharmacol Ther 68 130-142. [DOI] [PubMed] [Google Scholar]
- Kwon SH and Workman JL (2008) The heterochromatin protein 1 (HP1) family: put away a bias toward HP1. Mol Cells 26 217-227. [PubMed] [Google Scholar]
- Lahuna O, Fernandez L, Karlsson H, Maiter D, Lemaigre FP, Rousseau GG, Gustafsson J, and Mode A (1997) Expression of hepatocyte nuclear factor 6 in rat liver is sex-dependent and regulated by growth hormone. Proc Natl Acad Sci U S A 94 12309-12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahuna O, Rastegar M, Maiter D, Thissen JP, Lemaigre FP, and Rousseau GG (2000) Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol Endocrinol 14 285-294. [DOI] [PubMed] [Google Scholar]
- Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, Fackenthal JD, Rogan PK, Ring B, Wrighton SA, et al. (2003) Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther 307 906-922. [DOI] [PubMed] [Google Scholar]
- Lax ER, Hoff HG, Ghraf R, Schröder E, and Schriefers H (1974) The role of the hypophysis in the regulation of sex differences in the activities of enzymes involved in hepatic steroid hormone metabolism. Hoppe Seylers Z Physiol Chem 355 1325-1331. [DOI] [PubMed] [Google Scholar]
- Laz EV, Holloway MG, Chen CS, and Waxman DJ (2007) Characterization of three growth hormone-responsive transcription factors preferentially expressed in adult female liver. Endocrinology 148 3327-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laz EV, Sugathan A and Waxman DJ (2009) Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low but not high affinity STAT5 sites. Mol Endocrinol doi: 10.1210/me.2008-0449. [DOI] [PMC free article] [PubMed]
- Lee SJ, Wang-Buhler JL, Cok I, Yu TS, Yang YH, Miranda CL, Lech J, and Buhler DR (1998) Cloning, sequencing, and tissue expression of CYP3A27, a new member of the CYP3A subfamily from embryonic and adult rainbow trout livers. Arch Biochem Biophys 360 53-61. [DOI] [PubMed] [Google Scholar]
- Lemaigre F and Zaret KS (2004) Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev 14 582-590. [DOI] [PubMed] [Google Scholar]
- Levi F and Schibler U (2007) Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47 593-628. [DOI] [PubMed] [Google Scholar]
- Lichanska AM and Waters MJ (2008) How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet 24 41-47. [DOI] [PubMed] [Google Scholar]
- Liddle C, Goodwin BJ, George J, Tapner M, and Farrell GC (1998) Separate and interactive regulation of cytochrome P450 3A4 by triiodothyronine, dexamethasone, and growth hormone in cultured hepatocytes. J Clin Endocrinol Metab 83 2411-2416. [DOI] [PubMed] [Google Scholar]
- Lin JH, Chiba M, Chen IW, Nishime JA, and Vastag KJ (1996) Sex-dependent pharmacokinetics of indinavir: in vivo and in vitro evidence. Drug Metab Dispos 24 1298-1306. [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, and Hennighausen L (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11 179-186. [DOI] [PubMed] [Google Scholar]
- Löfgren S, Baldwin RM, Hiratsuka M, Lindqvist A, Carlberg A, Sim SC, Schülke M, Snait M, Edenro A, Fransson-Steen R, et al. (2008) Generation of mice transgenic for human CYP2C18 and CYP2C19: characterization of the sexually dimorphic gene and enzyme expression. Drug Metab Dispos 36 955-962. [DOI] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, and Brown M (2008) FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132 958-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGeoch C, Morgan ET, and Gustafsson JA (1985) Hypothalamo-pituitary regulation of cytochrome P-450(15) beta apoprotein levels in rat liver. Endocrinology 117 2085-2092. [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, and Shapiro BH (1991) Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131 395-399. [DOI] [PubMed] [Google Scholar]
- MacLeod SM, Giles HG, Bengert B, Liu FF, and Sellers EM (1979) Age- and gender-related differences in diazepam pharmacokinetics. J Clin Pharmacol 19 15-19. [DOI] [PubMed] [Google Scholar]
- Mader RM (2006) Gender specific tumour pharmacology-from kinetics to genetics. Wien Med Wochenschr 156 545-548. [DOI] [PubMed] [Google Scholar]
- Mann HJ (2006) Drug-associated disease: cytochrome P450 interactions. Crit Care Clin 22 329-345, vii. [DOI] [PubMed] [Google Scholar]
- McClellan-Green PD, Linko P, Yeowell HN, and Goldstein JA (1989) Hormonal regulation of male-specific rat hepatic cytochrome P-450g (P-450IIC13) by androgens and the pituitary. J Biol Chem 264 18960-18965. [PubMed] [Google Scholar]
- Miners JO, Attwood J, and Birkett DJ (1983) Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol 16 503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, et al. (2006) Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem 281 5246-5257. [DOI] [PubMed] [Google Scholar]
- Mode A and Gustafsson JA (2006) Sex and the liver - a journey through five decades. Drug Metab Rev 38 197-207. [DOI] [PubMed] [Google Scholar]
- Mode A, Gustafsson JA, Jansson JO, Edén S, and Isaksson O (1982) Association between plasma level of growth hormone and sex differentiation of hepatic steroid metabolism in the rat. Endocrinology 111 1692-1697. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, and Morris ME (2006) Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro 20 187-210. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, et al. (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36 205-216. [DOI] [PubMed] [Google Scholar]
- Morgan ET, MacGeoch C, and Gustafsson JA (1985) Hormonal and developmental regulation of expression of the hepatic microsomal steroid 16 alpha-hydroxylase cytochrome P-450 apoprotein in the rat. J Biol Chem 260 11895-11898. [PubMed] [Google Scholar]
- Murray M (2007) Role of signalling systems in the effects of dietary factors on the expression of mammalian CYPs. Expert Opin Drug Metab Toxicol 3 185-196. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Sudo Y, Sasaki Y, Iwata H, Takahashi M, and Kamataki T (2001) Studies on transcriptional regulation of Cyp3a16 gene in mouse livers by application of direct DNA injection method. Biochem Biophys Res Commun 287 820-824. [DOI] [PubMed] [Google Scholar]
- Nicholas JS and Barron DH (1932) The use of sodium amytal in the production of anesthesia in the rat. J Pharmacol Exp Ther 46 125-129. [Google Scholar]
- Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, and Fraenkel E (2007) Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet 39 730-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I (2005) Sex differences in the pharmacologic effects of antiretroviral drugs: potential roles of drug transporters and phase 1 and 2 metabolizing enzymes. Top HIV Med 13 79-83. [PubMed] [Google Scholar]
- Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, and Rotwein P (2007) Signal transducer and activator of transcription (Stat) 5b-mediated inhibition of insulin-like growth factor binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol 21 1443-1457. [DOI] [PubMed] [Google Scholar]
- Oscarsson J, Olofsson SO, Vikman K, and Edén S (1991) Growth hormone regulation of serum lipoproteins in the rat: different growth hormone regulatory principles for apolipoprotein (apo) B and the sexually dimorphic apo E concentrations. Metabolism 40 1191-1198. [DOI] [PubMed] [Google Scholar]
- Palva ES (1985) Gender-related differences in diazepam effects on performance. Med Biol 63 92-95. [PubMed] [Google Scholar]
- Pampori NA, Agrawal AK, and Shapiro BH (1991) Renaturalizing the sexually dimorphic profiles of circulating growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 124 283-289. [DOI] [PubMed] [Google Scholar]
- Pampori NA and Shapiro BH (1993) Sexual dimorphism in avian hepatic monooxygenases. Biochem Pharmacol 46 885-890. [DOI] [PubMed] [Google Scholar]
- Pampori NA and Shapiro BH (1996) Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile. Mol Pharmacol 50 1148-1156. [PubMed] [Google Scholar]
- Pampori NA and Shapiro BH (1999) Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology 140 1245-1254. [DOI] [PubMed] [Google Scholar]
- Park SH and Waxman DJ (2001) Inhibitory cross-talk between STAT5b and liver nuclear factor HNF3beta. Impact on the regulation of growth hormone pulse-stimulated, male-specific liver cytochrome P-450 gene expression. J Biol Chem 276 43031-43039. [DOI] [PubMed] [Google Scholar]
- Park SH, Wiwi CA, and Waxman DJ (2006) Signalling cross-talk between hepatocyte nuclear factor 4alpha and growth-hormone-activated STAT5b. Biochem J 397 159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, and Carroll KM (2004) The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol 199 193-209. [DOI] [PubMed] [Google Scholar]
- Rademaker M (2001) Do women have more adverse drug reactions? Am J Clin Dermatol 2 349-351. [DOI] [PubMed] [Google Scholar]
- Ram PA, Park SH, Choi HK, and Waxman DJ (1996) Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem 271 5929-5940. [DOI] [PubMed] [Google Scholar]
- Ram PA and Waxman DJ (1999) SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274 35553-35561. [DOI] [PubMed] [Google Scholar]
- Ram PA and Waxman DJ (2000) Role of the cytokine-inducible SH2 protein CIS in desensitization of STAT5b signaling by continuous growth hormone. J Biol Chem 275 39487-39496. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Marcus C, and Perdew GH (2005) Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin Drug Metab Toxicol 1 9-21. [DOI] [PubMed] [Google Scholar]
- Roberts RK, Desmond PV, Wilkinson GR, and Schenker S (1979) Disposition of chlordiazepoxide: sex differences and effects of oral contraceptives. Clin Pharmacol Ther 25 826-831. [DOI] [PubMed] [Google Scholar]
- Robertson JA, Haldosén LA, Wood TJ, Steed MK, and Gustafsson JA (1990) Growth hormone pretranslationally regulates the sexually dimorphic expression of the prolactin receptor gene in rat liver. Mol Endocrinol 4 1235-1239. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, Boussahmain C, Cormier KS, and Fox JG (2007) Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res 67 11536-11546. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, and Hwa V (2007) Defects in growth hormone receptor signaling. Trends Endocrinol Metab 18 134-141. [DOI] [PubMed] [Google Scholar]
- Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, and Waters MJ (2005) In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25 66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, Millard K, Palethorpe K, Nielsen K, Clyde-Smith J, et al. (2008) An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat Cell Biol 10 740-747. [DOI] [PubMed] [Google Scholar]
- Rudling M, Norstedt G, Olivecrona H, Reihnér E, Gustafsson JA, and Angelin B (1992) Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc Natl Acad Sci U S A 89 6983-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh RC and Colby HD (1980) Is growth hormone the pituitary feminizing factor mediating the actions of estradiol on hepatic drug and steroid metabolism? Endocrinology 107 719-724. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Endo Y, Mashino M, Kuroiwa M, Ohara A, Jarukamjorn K, and Nemoto N (2002) Regulation of the expression of two female-predominant CYP3A mRNAs (CYP3A41 and CYP3A44) in mouse liver by sex and growth hormones. Arch Biochem Biophys 404 234-242. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Masaki K, Itoh S, Yokoi T, and Kamataki T (1994) Sex-related differences in the expression of cytochrome P450 in hamsters: cDNA cloning and examination of the expression of three distinct CYP2C cDNAs. Mol Pharmacol 45 228-236. [PubMed] [Google Scholar]
- Sasaki Y, Takahashi Y, Nakayama K, and Kamataki T (1999) Cooperative regulation of CYP2C12 gene expression by STAT5 and liver-specific factors in female rats. J Biol Chem 274 37117-37124. [DOI] [PubMed] [Google Scholar]
- Scandlyn MJ, Stuart EC, and Rosengren RJ (2008) Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol 4 413-424. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Baumann F, Hanschmann H, Geissler F, and Preiss R (2001) Gender difference in ifosfamide metabolism by human liver microsomes. Eur J Drug Metab Pharmacokinet 26 193-200. [DOI] [PubMed] [Google Scholar]
- Schwartz JB (2007) The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82 87-96. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK, and Pampori NA (1995) Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol 27 9-20. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, and Yao DF (2007) Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 13 4295-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinues B, Fanlo A, Mayayo E, Carcas C, Vicente J, Arenaz I, and Cebollada A (2008) CYP2A6 activity in a healthy Spanish population: effect of age, sex, smoking, and oral contraceptives. Hum Exp Toxicol 27 367-372. [DOI] [PubMed] [Google Scholar]
- Sinz M, Wallace G, and Sahi J (2008) Current industrial practices in assessing CYP450 enzyme induction: preclinical and clinical. Aaps J 10 391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, et al. (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A 96 7088-7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skett P (1988) Biochemical basis of sex differences in drug metabolism. Pharmacol Ther 38 269-304. [DOI] [PubMed] [Google Scholar]
- Sladek FM (1994) Orphan receptor HNF-4 and liver-specific gene expression. Receptor 4 64. [PubMed] [Google Scholar]
- Soldaini E, John S, Moro S, Bollenbacher J, Schindler U, and Leonard WJ (2000) DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol 20 389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WC and Melner MH (2000) Steroid transformation enzymes as critical regulators of steroid action in vivo. Endocrinology 141 1587-1589. [DOI] [PubMed] [Google Scholar]
- Srivastava PK and Waxman DJ (1993) Sex-dependent expression and growth hormone regulation of class alpha and class mu glutathione S-transferase mRNAs in adult rat liver. Biochem J 294 159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström A, Eguchi H, Mode A, Legraverend C, Tollet P, Strömstedt PE, and Gustafsson JA (1994) Characterization of the proximal promoter and two silencer elements in the CYP2C11 gene expressed in rat liver. DNA Cell Biol 13 805-819. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Yokomori N, Korach KS, and Negishi M (1999) Developmental action of estrogen receptor-alpha feminizes the growth hormone-Stat5b pathway and expression of Cyp2a4 and Cyp2d9 genes in mouse liver. Mol Pharmacol 56 473-477. [DOI] [PubMed] [Google Scholar]
- Sundseth SS and Waxman DJ (1992) Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J Biol Chem 267 3915-3921. [PubMed] [Google Scholar]
- Szyf M (2007) The dynamic epigenome and its implications in toxicology. Toxicol Sci 100 7-23. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Nakamura N, Ikezuki Y, Takai Y, Yano T, and Taketani Y (2004) Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem Biophys Res Commun 325 549-554. [DOI] [PubMed] [Google Scholar]
- Tanaka E (1999) Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24 339-346. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Choi HK, Gurd W, and Waxman DJ (2001) Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology 142 4599-4606. [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS and Martin JB (1976) Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98 562-570. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, and Ihle JN (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93 841-850. [DOI] [PubMed] [Google Scholar]
- Teixeira J and Gil G (1991) Cloning, expression, and regulation of lithocholic acid 6 beta-hydroxylase. J Biol Chem 266 21030-21036. [PubMed] [Google Scholar]
- Thangavel C, Dworakowski W, and Shapiro BH (2006) Inducibility of male-specific isoforms of cytochrome p450 by sex-dependent growth hormone profiles in hepatocyte cultures from male but not female rats. Drug Metab Dispos 34 410-419. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Garcia MC, and Shapiro BH (2004) Intrinsic sex differences determine expression of growth hormone-regulated female cytochrome P450s. Mol Cell Endocrinol 220 31-39. [DOI] [PubMed] [Google Scholar]
- Thangavel C and Shapiro BH (2007) A molecular basis for the sexually dimorphic response to growth hormone. Endocrinology 148 2894-2903. [DOI] [PubMed] [Google Scholar]
- Timsit YE and Negishi M (2007) CAR and PXR: the xenobiotic-sensing receptors. Steroids 72 231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, and Davey HW (1997) Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A 94 7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, and Kim RB (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47 566-578. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD (1998) Neuroendocrine control of pulsatile growth hormone release in the human: relationship with gender. Growth Horm IGF Res 8 (Suppl B): 49-59. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD and Bowers CY (2003) Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest 26 799-813. [DOI] [PubMed] [Google Scholar]
- Vidal OM, Merino R, Rico-Bautista E, Fernandez-Perez L, Chia DJ, Woelfle J, Ono M, Lenhard B, Norstedt G, Rotwein P, et al. (2007) In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol Endocrinol 21 293-311. [DOI] [PubMed] [Google Scholar]
- Vidarsdottir S, Walenkamp MJ, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, White S, Breuning MH, Roelfsema F, Kruithof MF, et al. (2006) Clinical and biochemical characteristics of a male patient with a novel homozygous STAT5b mutation. J Clin Endocrinol Metab 91 3482-3485. [DOI] [PubMed] [Google Scholar]
- Wallenius K, Sjögren K, Peng XD, Park S, Wallenius V, Liu JL, Umaerus M, Wennbo H, Isaksson O, Frohman L, et al. (2001) Liver-derived IGF-I regulates GH secretion at the pituitary level in mice. Endocrinology 142 4762-4770. [DOI] [PubMed] [Google Scholar]
- Wang J and Huang Y (2007) Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr Drug Discov Technol 4 59-68. [DOI] [PubMed] [Google Scholar]
- Wang Y and Jiang H (2005) Identification of a distal STAT5-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem 280 10955-10963. [DOI] [PubMed] [Google Scholar]
- Watkins PB, Turgeon DK, Jaffe CA, Ho PJ, and Barkan AL (1993) Pulsation frequency of growth hormone may mediate gender differences in CYP3A activity in man (Abstract). Clin Res 41 13219. [Google Scholar]
- Wauthier V and Waxman DJ (2008) Sex-specific early growth hormone response genes in rat liver. Mol Endocrinol 22 1962-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ (1988) Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem Pharmacol 37 71-84. [DOI] [PubMed] [Google Scholar]
- Waxman DJ (1999) P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys 369 11-23. [DOI] [PubMed] [Google Scholar]
- Waxman DJ and Azaroff L (1992) Phenobarbital induction of cytochrome P-450 gene expression. Biochem J 281 577-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, LeBlanc GA, Morrissey JJ, Staunton J, and Lapenson DP (1988) Adult male-specific and neonatally programmed rat hepatic P-450 forms RLM2 and 2a are not dependent on pulsatile plasma growth hormone for expression. J Biol Chem 263 11396-11406. [PubMed] [Google Scholar]
- Waxman DJ and O'Connor C (2006) Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20 2613-2629. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, and Shapiro BH (1991) Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A 88 6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Ram PA, Park SH, and Choi HK (1995) Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem 270 13262-13270. [DOI] [PubMed] [Google Scholar]
- Wilson JT (1973) Growth hormone modulation of liver drug metabolic enzyme activity in the rat. I. Effect of the hormone on the content and rate of reduction of microsomal cytochrome P-450. Biochem Pharmacol 22 1717-1728. [DOI] [PubMed] [Google Scholar]
- Wilson JT and Bass A (1973) Growth hormone modulation of liver drug metabolic enzyme activity in the rat. II. Specificity of the hormone effect. Proc Soc Exp Biol Med 143 978-985. [DOI] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, and Waxman DJ (2004) Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrinol 18 1975-1987. [DOI] [PubMed] [Google Scholar]
- Wiwi CA and Waxman DJ (2005) Role of hepatocyte nuclear factors in transcriptional regulation of male-specific CYP2A2. J Biol Chem 280 3259-3268. [DOI] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, and Zanger UM (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38 978-988. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y, Shimada M, Murayama N, Kawano S, and Kato R (1986) The regulation by growth hormone of microsomal testosterone 6 beta-hydroxylase in male rat livers. J Biochem 100 1095-1097. [DOI] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, and Lusis AJ (2006) Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori N, Nishio K, Aida K, and Negishi M (1997) Transcriptional regulation by HNF-4 of the steroid 15alpha-hydroxylase P450 (Cyp2a-4) gene in mouse liver. J Steroid Biochem Mol Biol 62 307-314. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nimura Y, Nagino M, Bland KI, and Chaudry IH (2005) Current understanding of gender dimorphism in hepatic pathophysiology. J Surg Res 128 147-156. [DOI] [PubMed] [Google Scholar]
- You L (2004) Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem Biol Interact 147 233-246. [DOI] [PubMed] [Google Scholar]