Abstract
Nicotinic acetylcholine receptors are implicated in several neuropsychiatric disorders, including nicotine addiction, Alzheimer's, schizophrenia, and depression. Therefore, they represent a critical molecular target for drug development and targeted therapeutic intervention. Understanding the molecular mechanisms by which allosteric modulators enhance activation of these receptors is crucial to the development of new drugs. We used the substituted cysteine accessibility method to study conformational changes induced by the positive allosteric modulator N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596) in the extracellular ligand binding domain of α7 nicotinic receptors carrying the L247T mutation. PNU-120596 caused changes in cysteine accessibility at the inner beta sheet, transition zone, and agonist binding site. These changes in accessibility are similar to but not identical to those caused by ACh alone. In particular, PNU-120596 induced changes in MTSEA accessibility at N170C (in the transition zone) that were substantially different from those evoked by acetylcholine (ACh). We found that PNU-120596 induced changes at position E172C in the absence of allosteric modulation. We identified a cysteine mutation of the agonist binding site (W148C) that exhibited an unexpected phenotype in which PNU-120596 acts as a full agonist. In this mutant, ACh-evoked currents were more sensitive to thiol modification than PNU-evoked currents, suggesting that PNU-120596 does not bind at unoccupied agonist-binding sites. Our results provide evidence that binding sites for PNU-120596 are not in the agonist-binding sites and demonstrate that positive allosteric modulators such as PNU-120596 enhance agonist-evoked gating of nicotinic receptors by eliciting conformational effects that are similar but nonidentical to the gating conformations promoted by ACh.
Nicotinic acetylcholine receptors (nAChRs) are the prototypical member of the Cys-loop family of ligand-gated ion channels that also includes GABAA, serotonin type 3 (5-HT3), and glycine receptors. This family of receptors assembles as heteromeric or homomeric pentamers around a central pore (Karlin, 2002). Each subunit contains an extracellular ligand-binding domain (LBD), an α-helical transmembrane domain (TMD), a transition zone that couples the LBD to the TMD, and an intracellular domain (Gay and Yakel, 2007).
Neuronal nAChRs are expressed diffusely throughout most of the central nervous system; α7-containing receptors show the highest levels of expression (Orr-Urtreger et al., 1997). Of the neuronal nicotinic receptors, the homomeric α7 receptor is implicated in neurological diseases such as schizophrenia, Alzheimer's Disease, and anxiety disorders (Gotti and Clementi, 2004). Therefore, the α7 nicotinic receptor represents an important therapeutic target.
Over the last several years, there has been success in developing synthetic positive allosteric modulators (PAMs) for α7 nAChRs, including PNU-120596 (Bertrand and Gopalakrishnan, 2007). These compounds are predicted to bind away from the orthosteric agonist binding sites and enhance gating of the receptor in the presence of agonists. PNU-120596 is part of a growing class of PAMs that can reopen α7 receptors from the desensitized state and slow additional desensitization, designated as type II modulators (Grønlien et al., 2007). By eliminating the desensitized state, type II PAMs exert a much greater effect on α7 receptor activation than agonists or PAMs that do not alter desensitization (type I modulators).
In animal models, PNU-120596 can partially restore auditory gating deficits (Hurst et al., 2005), a common symptom of schizophrenia. Understanding the molecular mechanisms and structural determinants of PAM action could lead to the development of drugs for the treatment of a wide variety of neuropsychiatric disorders. For example, structural elements from cytisine and morphine guided the development of varenicline, a α4β2 partial agonist and α7 agonist that reduces drug-seeking behavior and consumption of nicotine (Mihalak et al., 2006).
To understand how different nAChR subtypes contribute to disease states, it is crucial to understand the molecular mechanisms by which these receptors couple the binding of agonists and PAMs to opening of the channel. Benzodiazepines, the archetypal positive allosteric modulators of GABAA receptors, induce conformational changes in the ligand-binding domain of GABAA receptors (Sharkey and Czajkowski, 2008). We have found that agonists of α7 receptors induce structural transitions in the LBD, as measured by the substituted cysteine accessibility method (SCAM) (Lyford et al., 2003; McLaughlin et al., 2006, 2007). Based on the existing data, we hypothesize that PAMs and agonists cause similar but nonidentical conformational changes.
Here, we used SCAM to compare changes in cysteine accessibility caused by PNU-120596 and ACh. We found that PNU-120596 induced conformational changes in the inner β sheet, transition zone, and orthosteric site that were similar to but not identical to those induced by ACh. We have identified mutations that either eliminate or enhance allosteric modulation to the point of full agonism. Finally, we present evidence that PNU-120596 does not bind at the agonist-binding site.
Materials and Methods
Reagents. Female Xenopus laevis frogs were obtained from Xenopus One (Dexter, MI) or Xenopus Express (Brooksville, FL). Methanethiosulfonate (MTS) chemicals were obtained from Toronto Research Chemicals (Toronto, ON, Canada). PNU-120596 was obtained from Tocris Bioscience (Ellisville, MO). The QuikChange site-directed mutagenesis kit was obtained from Stratagene (La Jolla, CA) and the mMessage mMachine in vitro RNA transcription kit was obtained from Ambion (Austin, TX). All other reagents for molecular biology, oocyte dissection, and electrophysiological recordings were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Molecular Biology. The chick α7 nAChR was expressed in the pAMV vector under the control of the T7 promoter. Mutations were introduced into C115A/L247T receptors using the QuikChange site-directed mutagenesis kit according to the manufacturer's instructions, and were verified by DNA sequencing. All receptors contained a cysteine-to-alanine mutation at position 115. Cys115 is the only unpaired cysteine in the LBD, and the C115A mutation simplifies the interpretation of thiol modification experiments without affecting responses to ACh (McLaughlin et al., 2006) or PNU-120596. The utility of the L247T mutation is described under Results. Capped cRNA transcripts were made as described previously (Lyford et al., 2003).
Construct Expression in X. laevis oocytes. X. laevis oocytes were surgically removed as described previously (Lyford et al., 2003). The oocytes were injected with 20 ng of α7 nAChR cRNA and were incubated for 2 to 7 days in ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM Na-HEPES, pH 7.5) plus 50 mg/ml gentamicin and 0.55 mg/ml sodium pyruvate.
Some mutants, displaying a peak current response to maximal ACh of less than 100 nA, were coexpressed with human RIC-3 protein (resistant to inhibitors of cholinesterase) in a 1:1 (w/w) cRNA ratio (Lansdell et al., 2005). Dose-response curves from several mutant α7 receptors were generated with and without coexpression of RIC-3. The data suggest that RIC-3 substantially increased the peak current amplitude without major effects on the ACh EC50 values (Supplemental Table 1).
Two-Electrode Voltage Clamp of X. laevis oocytes. Oocytes were superfused with ESLC (96 mM NaCl, 2 mM KCl, 10 mM MgCl2, 0.1 mM CaCl2, and 10 mM HEPES-NaOH, pH 7.5), a low-Ca2+ solution that minimizes currents through Ca2+-activated Cl- channels. For each mutant α7 nAChR, a 5 to 7 point dose-response curve was generated to ACh alone or with the addition of 1 μM PNU-120596 (in ESLC). Dose responses for ACh with and without PNU-120596 were obtained from the same oocytes. Unless otherwise noted, PNU-120596 was preapplied for 30 s and then coapplied with ACh. For some mutant receptors, a dose-response curve was generated for the allosteric modulator in the presence of an EC30-50 dose of ACh. All dose response curves were fit to a three-parameter Hill equation using SigmaPlot 9.0 (Systat Software, San Jose, CA). Data were reported as the average ± S.E.M. Two-electrode voltage clamp was performed as described previously (McLaughlin et al., 2007). Solutions were applied by gravity perfusion with a flow rate of 3 to 5 ml/min. Oocytes were superfused with ESLC for at least 2 min between all drug applications, and current amplitudes returned to baseline.
To eliminate complications of run-up and run-down of current over the course of an experiment, all oocytes were initially treated with a maximal ACh dose 3 to 4 times consecutively. Oocytes were discarded if the response to the maximal ACh dose varied by more than ± 10%.
Substituted Cysteine Accessibility Method. MTS reagents were made fresh daily in distilled H2O and stored on ice. Just before use, the MTS reagents were diluted to the appropriate concentration in ESLC and were applied immediately to the oocytes. 2-Aminoethyl methanethiosulfonate (MTSEA) was our test compound to screen for reactivity at introduced thiols. We compared the ACh dose response curves of each mutant before and after application of a high concentration of MTSEA (0.5-10 mM for 0.5-4 min). For mutants that showed a functional effect of MTSEA, a limiting concentration of MTSEA (0.1-100 μM), yielding 20 to 50% of the maximal MTSEA effect, was determined empirically. To measure modification rates, the limiting concentration of MTSEA was applied repeatedly for 15 to 30 s. In experiments with PNU-120596, 1 μM was preapplied for 30 s and then coapplied with the limiting concentration of MTSEA. In experiments with agonists, an EC100 (a maximally effective concentration) was coapplied with the limiting concentration of MTSEA. In all experimental conditions, the functional effect of MTSEA modification was tested with a ∼EC50 concentration of ACh. Each application of ACh was applied until a peak current amplitude was obtained (∼15 s), and then the perfusion was switched back to ESLC (wash buffer). At the end of each experiment, a maximal dose of MTSEA was applied (0.5-10 mM) to measure the effect when all accessible thiols were modified.
Normalized current amplitudes ({It - I∞}/{Izero - I∞}, where It is the current amplitude after the cumulative time of MTSEA exposure, I∞ is the current amplitude after the final maximal dose of MTSEA, and Izero is the initial current amplitude before modification) were fit to a single exponential decay (Pascual and Karlin, 1998) using SigmaPlot 9.0. The pseudo first-order rate constant was determined and was divided by the MTSEA concentration to give the second-order rate constant (molar-1 seconds-1).
Statistical Analysis. Hill equation parameters and second-order rate constants were analyzed by one-way analysis of variance with Tukey's post hoc test (SigmaStat 3.0; Systat Software, San Jose, CA). P values of <0.05 were interpreted to indicate significant differences.
Structural Models of the α7 Nicotinic Receptor. A model of the chick α7 nicotinic receptor extracellular domain, based on the coordinates of the Lymnea stagnalis ACh Binding Protein (Brejc et al., 2001) was constructed as described previously (Lyford et al., 2003; McLaughlin et al., 2006). Images of the model were generated with Pymol (DeLano Scientific, South San Francisco, CA). For reference, Leu247 is located in the transmembrane domain, in the pore-lining M2 helix, approximately a third of the pore's length from the intracellular end (Revah et al., 1991; Unwin, 2005).
Results
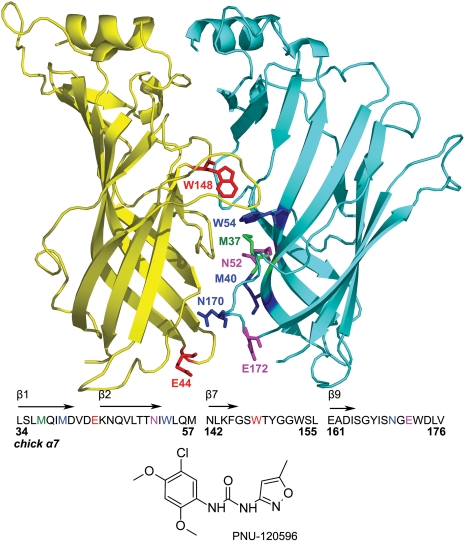
Effect of Mutations on Positive Allosteric Modulation by PNU-120596. Figure 1 shows our homology model of the α7 nAChR derived from the structure of the L. stagnalis Acetylcholine Binding Protein (Brejc et al., 2001) and the regions of interest targeted in this study. Trp148 and Trp54 are located in the agonist-binding “pocket” (the orthosteric site). Met37, Asn52, and Met40 are located at the interface between subunits (the inner β sheet), “below” the agonist binding pocket. These residues were previously shown to be good reporters of agonist-induced conformational changes (McLaughlin et al., 2007). Glu44, Asn170, and Glu172 are located in the “transition zone” that couples the LBD to the TMD.
Fig. 1.
Homology model of the extracellular domain of α7 nAChR. A ribbon cartoon displaying two subunits of the pentameric receptor, viewed from the outside. The primary ACh-binding subunit is shown in yellow and the complimentary subunit is in cyan. Residues of interest are shown as sticks and are labeled on one of the two subunits for clarity. Met37, Met40, and Asn52 are part of the inner β sheet (β1, β2, β6). Glu44, Asn170, and Glu172 are part of the transition zone (loops 2 and 9). Trp54 and Trp148 are part of the orthosteric (agonist-binding) site (β6, and behind the C loop). The residues of interest and the surrounding amino acid sequence is shown beneath the cartoon. The structure of PNU-120596 is shown below the chick α7 nAChR amino acid sequence.
All mutants in this study contained the well characterized leucine 247-to-threonine (L247T or L9′T) mutation. L247T-containing α7 receptors have conductance and ion selectivity that are similar to those of the wild-type receptor but are more sensitive to acetylcholine and exhibit slower macroscopic desensitization (Revah et al., 1991). L247T is a good model system for our studies, because its large current amplitudes allow us to measure modification rates for cysteine substitutions with decreased functional expression levels. All mutants also contained the C115A mutation, in which the single unpaired cysteine in the LBD is mutated to an alanine. This mutation, which has no effect on activation kinetics, ligand sensitivity, or ion permeation, simplifies interpretation of cysteine modification experiments (McLaughlin et al., 2006).
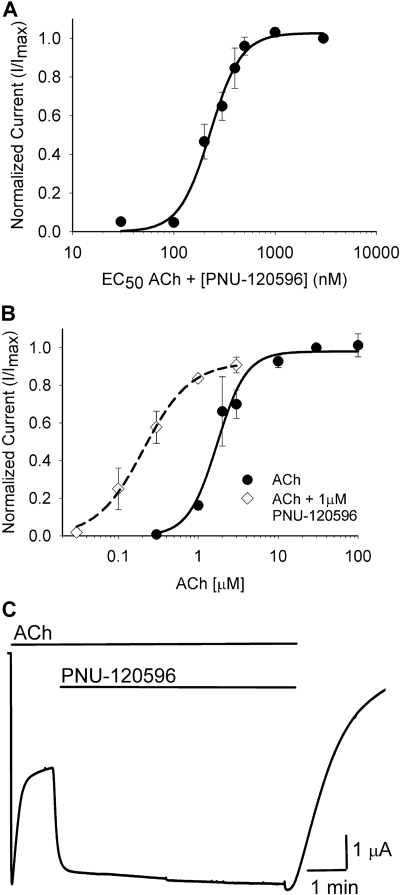
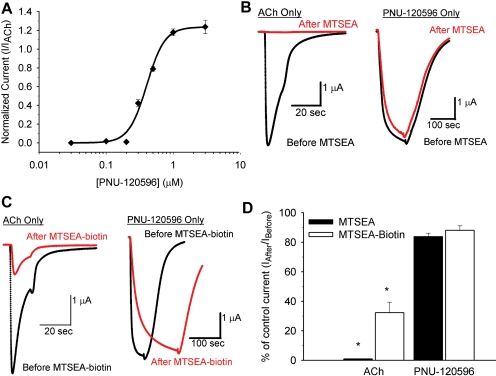
We first examined whether PNU-120596 acts as a positive allosteric modulator of C115A/L247T α7 receptors. PNU-120596 retained its modulatory effect in the C115A/L247T background, as measured by the ability to enhance ACh-evoked currents in a dose-dependent manner (Fig. 2A). The concentration of PNU-120596 that elicited a half-maximal modulation (when applied with an EC30-50 concentration of ACh) was 257 ± 22 nM (Fig. 2A), a value that is similar to that reported for wild-type α7 receptors (216 ± 64 nM; Hurst et al., 2005). This suggests that the C115A/L247T mutations do not significantly alter the affinity of PNU-120596 for the α7 nicotinic receptor.
Fig. 2.
PNU-120596 is a positive allosteric modulator of C115A/L247T α7 receptor and elicits opening from a partially desensitized state. A, PNU-120596, at the concentrations shown, was applied with an ∼EC50 dose of ACh (2-3 μM). Positive allosteric modulation was observed as a significant enhancement of ACh-evoked current (189 ± 18%, n = 5). The EC50 for PNU-120596 was 257 ± 22 nM (n = 5). B, ACh was applied with an EC100 dose of PNU-120596 (1 μM). Positive allosteric modulation was observed as a significant reduction in the EC50 for ACh (Table 1). Data are fit to the Hill equation and are the mean value ± S.E.M, normalized to the maximal value of the Hill equation fit of each data set. C, a representative trace showing that an EC100 dose of PNU-120596 (1 μM) is sufficient to reactivate partially desensitized C115A/L247T receptors (n = 5). Coapplication of ACh and PNU-120596 is also sufficient to completely inhibit desensitization, and responses are reversible when both compounds are washed out.
PNU-120596 (1 μM) caused a left-shift of the ACh dose-response curve of C115A/L247T α7 receptors and a significant decrease in the EC50 for ACh (Fig. 2B, Table 1). In contrast to the responses of wild-type α7 receptors (Hurst et al., 2005), PNU-120596 did not cause a significant change in the current amplitudes of C115A/L247T α7 receptors evoked at maximal ACh concentrations. We speculate that the L247T mutation increased the “gating constant” of the receptors (Colquhoun, 1998), lowering the ACh EC50 compared with wild-type receptors. For receptors with a low gating constant, such as wild-type α7 receptors, a PAM could increase the maximal response, decrease the EC50, or both. In contrast, for receptors with a high gating constant, such as L247T-containing α7 receptors, we expect PAMs to affect EC50 alone, because the maximal response to agonist is already near 1 (Colquhoun, 1998).
TABLE 1.
Summary of ACh dose response data
| Mutant | ACh EC50 | Imax | Hill Coefficient | n | ACh EC50 + 1 μM PNU-120596 | n | Effecta |
|---|---|---|---|---|---|---|---|
| μM | μA | μM | |||||
| C115A/L247T background | 2.4 ± 0.23 | 6.4 ± 0.64 | 2.8 ± 0.25 | 10 | 0.22 ± 0.09 | 3 | PAM |
| Inner β sheet | |||||||
| M37C | 4.5 ± 1.30 | 0.4 ± 0.12 | 1.3 ± 0.10 | 10 | 1.6 ± 0.18 | 7 | N.E. |
| M40C | 6.7 ± 0.70 | 2.2 ± 0.47 | 1.4 ± 0.11 | 16 | 0.58 ± 0.17 | 4 | PAM |
| N52C | 0.79 ± 0.20 | 0.31 ± 0.08 | 0.68 ± 0.07 | 13 | N.D.b | ||
| Transition zone | |||||||
| E44C | 7.2 ± 0.56 | 4.6 ± 0.39 | 2.6 ± 0.31 | 10 | 2.5 ± 0.37 | 3 | PAM |
| N170Cc | 12 ± 1.4 | 1.3 ± 0.24 | 1.6 ± 0.12 | 15 | 2.6 ± 0.56 | 3 | PAM |
| E172Cc | 30 ± 2.0 | 0.74 ± 0.11 | 2.4 ± 0.10 | 16 | 41 ± 5.3 | 3 | N.E. |
| Agonist-binding site | |||||||
| W54C | 88 ± 9.7 | 2.3 ± 0.40 | 1.6 ± 0.04 | 12 | 15 ± 5.1 | 5 | PAM |
| W148Cc | 205 ± 28 | 3.5 ± 0.52 | 1.5 ± 0.06 | 11 | N.D.b |
N.E., no effect; N.D., not determined.
Based on statistically significant differences between ACh EC50 with and without 1 μM PNU-120596 (P < 0.05).
Value not determined because of agonism observed with PNU-120596.
Coexpression with human RIC-3 (see Materials and Methods).
A unique feature of PNU-120596 is the ability to reactivate desensitized α7 receptors in the presence of agonist, a feature defined as type II modulation (Hurst et al., 2005; Grønlien et al., 2007). Therefore, we determined whether PNU-120596 could reactivate C115A/L247T α7 receptors after slow desensitization. A representative trace is shown in Fig. 2C. As expected, C115A/L247T receptors showed partial desensitization during continuous application of an EC100 concentration of ACh (100 μM, a maximally effective concentration), reaching a plateau at 48.2 ± 0.02% desensitization by 87.5 ± 7.0 s (n = 5). Then, application of an EC100 concentration of PNU-120596 (1 μM) after partial desensitization reactivated the C115A/L247T receptors (n = 5), consistent with type II modulation. The continuous application of ACh and PNU-120596 completely blocked slow desensitization of C115A/L247T receptors, as observed in wild-type α7 receptors (Grønlien et al., 2007). The ability of PNU-120596 to reactivate partially desensitized C115A/L247T receptors and prevent subsequent desensitization did not depend on the ACh concentration (data not shown). We conclude that the C115A/L247T mutations do not alter the affinity or kinetics of PNU-120596 (Fig. 2, A and C), only the ability to enhance peak current amplitude (Fig. 2B), which we attribute to enhanced gating of the C115A/L247T receptors. This interpretation is supported by macroscopic and single-channel analysis, showing that effects on apparent desensitization by mutations at L9′ in other Cys-loop receptors can be explained by increases in mean open time alone (Filatov and White, 1995; Bianchi and Macdonald, 2001). Therefore, α7 receptors containing the C115A/L247T mutation are a reasonable model to examine conformational transitions underlying allosteric modulation by PNU-120596.
For all of the cysteine mutations used in this study, we generated ACh concentration-response curves to probe for possible deleterious effects of the individually introduced cysteines on channel function. Most cysteine mutations generated ACh EC50 values that were not significantly different from the parent C115A/L247T receptor (Table 1), suggesting that the introduced mutations were well tolerated. Two mutations at the orthosteric site (W54C and W148C) significantly increased the ACh EC50 compared with the parent C115A/L247T receptor, as expected for residues required for the binding of agonists or in close proximity to the binding site (Brejc et al., 2001). We found that two mutants (N52C and W148C) were unexpectedly activated by PNU-120596 alone. The EC50 values for PNU-120596 activation were 340 ± 20 nM (n = 3) and 450 ± 10 nM (n = 3) for N52C and W148C mutant receptors, respectively. Our interpretation is that the N52C and W148C mutations enhance the ability of PNU-120596 to induce conformational changes such that it can directly gate these mutant receptors.
Because a binding site for divalent cation modulators has been proposed in the transition zone (Galzi et al., 1996), we also introduced mutations at positions Glu44 and Glu172 to test whether the putative divalent cation binding site is required for modulation by PNU-120596. We found that one mutation in the transition zone (E172C) and one mutation in the inner β sheet (M37C) eliminated positive allosteric modulation by PNU-120596 (Table 1). These data provide evidence that Met37 and Glu172 are required for binding of PNU-120596 and/or PNU-120596-induced changes in gating.
PNU-120596 Causes Structural Transitions within the Extracellular Ligand Binding Domain Similar to Those Caused by Acetylcholine. To explore conformational changes in α7 receptors evoked by PNU-120596, we examined PNU-120596-dependent changes in the rate of MTSEA modification of cysteines introduced in the LBD. The ability of ligands to alter the rate of MTS modification of introduced thiols is interpreted as 1) steric interference between the ligand and the MTS reagent, 2) a conformational change of the introduced thiol induced by that ligand that changes the surface accessibility of the thiol, and/or 3) a conformational change induced by the ligand in the environment near the introduced thiol that alters its local electrostatic environment (Akabas et al., 1992; McLaughlin et al., 2007).
It is important to note that SCAM measures the time-averaged conformational transition of the receptor, including closed, open, desensitized, and multiple intermediate states. Because the L9′T mutation increases the open time of α7 receptors (Revah et al., 1991; Filatov and White, 1995), we assume that the conformational changes measured in the presence of ACh are dominated by those relating to activation over those relating to desensitization. However, we cannot rule out the effects of our introduced cysteines and L247T on the conformational pathways associated with desensitization.
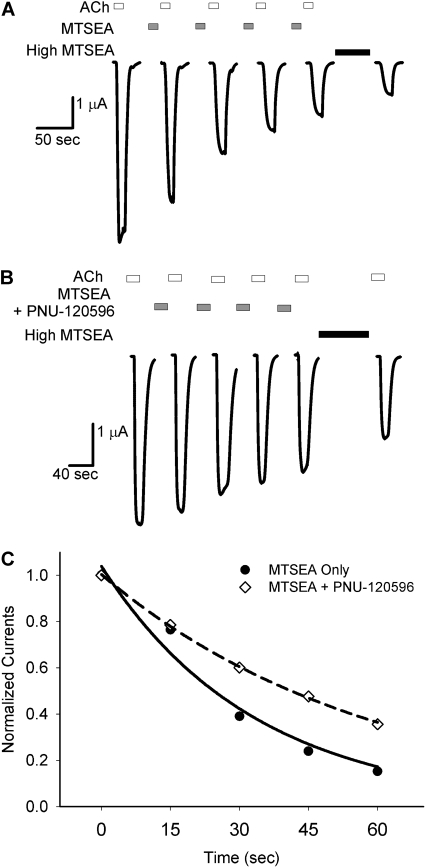
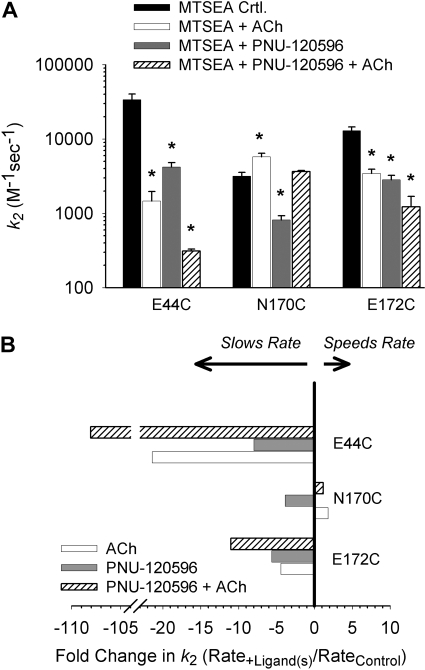
Fig. 3 shows an example of the protocol used to measure the thiol modification rate (E44C). Fig. 3A shows an experiment from a single oocyte in which a submaximal concentration of MTSEA (1 μM) was applied between test applications of ACh (see Materials and Methods). The effect of covalent modification was a decrease in ACh-evoked currents. Fig. 3B shows an experiment in which 1 μM PNU-120596 was preapplied and then coapplied with MTSEA (1 μM) between test applications of ACh. For clarity, only the ACh-evoked currents that were used to determine the rate of modification are shown. For each experiment, peak ACh-evoked current amplitudes were plotted versus the cumulative time of exposure to MTSEA (Fig. 3C). Pseudo-first-order rate constants obtained from the fits of the data to a single exponential equation were divided by the concentration of MTSEA to yield the second-order rate constants (k2) shown in Table 2.
Fig. 3.
PNU-120596 slows the rate of MTSEA modification at E44C. A, successive ACh-evoked currents before and after the addition of a submaximal concentration of MTSEA (1 μM, 15-s exposures). After four successive applications, the endpoint of MTSEA modification was determined by a prolonged application of 500 μM MTSEA for 60 s. Washes of 1 to 2 min between drug applications are not shown. B. the same protocol as A, with a 30-s pre-exposure to 1 μM PNU-120596 followed by coapplication of 1 μM PNU-120596 with MTSEA. C, normalized peak current amplitudes from a single experiment are plotted against cumulative MTSEA exposure. The calculated pseudo-first-order rate constants from these experiments are 0.0299 s-1 for control (A), and 0.0169 s-1 in the presence of PNU-120596 (B). Second-order rate constants (k2) are calculated from these values and are displayed in Table 2.
TABLE 2.
Second-order rate constants for MTSEA modification of receptors carrying Cys substitutions in the extracellular domain of α7 nAChR Numbers in parentheses represent n.
| Mutant | Control | MTSEA + ACh | MTSEA + PNU-120596 | MTSEA + ACh + PNU-120596 |
|---|---|---|---|---|
| M−1s−1 | ||||
| Inner β sheet | ||||
| M37C | 2470 ± 310 (12) | 333 ± 152* (6) | 271 ± 104* (7) | |
| M40C | 16,100 ± 2370 (15) | 2090 ± 748* (5) | 3030 ± 313* (5) | |
| N52C | 250 ± 68 (8) | 1320 ± 238* (9) | 390 ± 118† (4) | |
| Transition zone | ||||
| E44C | 33,700 ± 6540 (8) | 1460 ± 505* (8) | 4180 ± 637* (5) | 312 ± 19* (5) |
| N170C | 3160 ± 392 (7) | 5730 ± 682* (7) | 818 ± 114*† (10) | 3660 ± 115 (4) |
| E172C | 12,900 ± 1560 (9) | 3450 ± 470* (6) | 2830 ± 410* (7) | 1710 ± 310* (7) |
| Agonist-binding site | ||||
| W54C | 9500 ± 1330 (6) | 4030 ± 770* (5) | 6080 ± 920 (4) | |
| W148C | 45,900 ± 7640 (9) | 3010 ± 1100* (6) | 8830 ± 1320* (6) |
Statistically different from control (P < 0.05).
Statistically different from + ACh (P < 0.05).
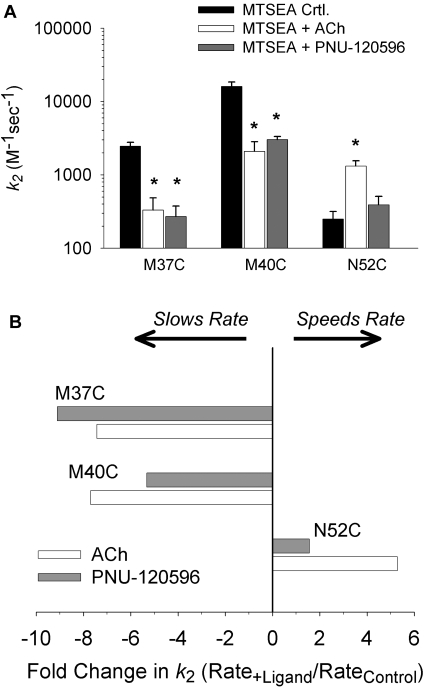
Using this protocol, we determined the second-order rate constants for modification of three residues in the inner β sheet (M37C, M40C, N52C). Fig. 4A shows the mean second-order rate constants measured in the presence of MTSEA alone (control), MTSEA plus ACh, and MTSEA plus PNU-120596. PNU-120596 and ACh each caused a 7- to 9-fold reduction in the modification rate of M37C and a 5- to 7-fold reduction in the modification rate of M40C (Fig. 4B). The modification rates in the presence of ACh or PNU-120596 were significantly different from control but were not significantly different from each other. Thus, without activating these receptors, PNU-120596 caused changes in the accessibility or electrostatic environment of M37C and M40C that were similar to those caused by ACh (McLaughlin et al., 2007). PNU-120596 decreased the rate of modification of M37C, even though receptors containing this mutation were not positively modulated by PNU-120596 (Table 1). This result demonstrates that PNU-120596 can elicit conformational changes in the inner β sheet in the absence of a modulatory effect. Because Met37 or Met40 are not part of the agonist binding site, it is unlikely that steric interference between ligand and MTSEA is responsible for the decreased modification rates observed at these positions. In contrast, PNU-120596 did not cause a difference in the rate of modification of N52C (Fig. 4, A and B). Although, ACh increased the rate of MTSEA modification of N52C (McLaughlin et al., 2007), PNU-120596 did not cause a significant change. Thus, PNU-120596 caused some but not all of the changes in thiol accessibility in the inner β-sheet caused by ACh. To verify that the conformational changes studied in L247T α7 receptors were similar to those in wild-type α7 receptors (WT), we also studied cysteine accessibility of M40C constructed in the WT background. The rates of MTSEA modification of M40C/WT, with and without ACh, were similar to those measured in the C115A/L247T background (Supplemental Fig. 1). This suggests that the changes in accessibility that we measure are independent of the C115A/L247T mutation.
Fig. 4.
PNU-120596 differentially alters the rate of MTSEA modification at inner β sheet residues. A, mean second-order modification rate constants (k2), calculated using the protocol outlined in Fig. 3, at three residues in the inner β sheet, M37C, M40C, and N52C. Mean values for MTSEA alone (control), MTSEA plus ACh, and MTSEA plus PNU-120596 are shown, plotted on a log scale. PNU-120596 slows the rate of modification at M37C and M40C compared with control but has no effect at N52C. *, rate was significantly different from control (P < 0.05). B, a plot of the second-order rate constant ratios. The average rate from each experimental condition is divided by the average control rate for each residue. Positive values represent an acceleration of the rate of modification, and negative values represent a reduction in the rate of modification.
Next, we determined the second order rate constants for the modification of three residues in the transition zone (E44C, N170C, and E172C). Fig. 5A shows the mean second-order rate constants measured in the presence of MTSEA alone (control), MTSEA plus ACh, and MTSEA plus PNU-120596. We observed differential effects of PNU-120596 in all three mutant receptors. PNU-120596 and ACh each decreased the rate of MTSEA modification of E44C by ∼8- and ∼21-fold, respectively (Fig. 5, A and B). PNU-120596 and ACh both decreased the rate of modification of E172C by 2- to 5-fold, even though receptors containing this mutation were not positively modulated by PNU-120596 (Table 1). This result demonstrates that PNU-120596 can elicit conformational changes in the transition zone in the absence of a modulatory effect. The rate of MTSEA modification of N170C was altered differently by PNU-120596 and ACh. PNU-120596 significantly decreased the rate of modification, whereas ACh increased the rate of modification. Overall, these data show that PNU-120596 induces conformational changes at Glu44 and Glu172 that are similar to those induced by ACh, but PNU-120596-induced changes at Asn170 are different from those induced by ACh.
Fig. 5.
PNU-120596 differentially alters the rate of MTSEA modification at residues in the transition zone. A, mean second-order modification rate constants (k2) of at three residues in the transition zone, E44C, N170C, and E172C. PNU-120596 significantly reduces the rate of modification at E44C and N170C. PNU-120596 significantly reduces the rate of modification at E172C, despite the lack of positive allosteric modulation at this residue (Table 1). At N170C, the rate of modification in the presence of PNU-120596 is significantly slower than in the presence of ACh. *, rate was significantly different from control (P < 0.05). B, a plot of the second-order rate constant ratios. Note that PNU-120596 slows the rate of modification of N170C whereas ACh increases the rate of modification.
We also measured the combined effects of ACh plus PNU-120596 on MTSEA modification at introduced cysteines in the transition zone (E44C, N170C, and E172C). At E44C and E172C, the combination of PNU-120596 and ACh caused a larger effect than that caused by either ACh or PNU-120596 alone (Fig. 5, Table 2). At N170C, PNU-120596 and ACh had opposite effects on the rate of MTSEA modification. The simultaneous application of both reagents gave a rate of MTSEA modification that was not significantly different from control. In this respect, combined effects of PNU-120596 and ACh led to a net cancelation of each individual effect.
Because the vestibule of the LBD of nicotinic receptors is predicted to be highly electronegative (Unwin, 2005), we also examined the rates of MTS modification using anionic MTS reagents (Supplemental Data Figs. 2-4). We found that MTSEA modified E44C much faster than MTSCE and MTSES (Supplemental Data Fig. 3), even after accounting for the lower intrinsic rates of reaction by MTSCE and MTSES (Supplemental Fig. 2). In addition, the effect of ACh on the rate of MTSEA modification was much greater than the effect of ACh on the rate of MTSCE modification. Modification of M40C by either MTSES or MTSCE was too slow to measure (Supplemental Fig. 4). These results provide additional evidence for a highly negative electrostatic environment lining the vestibule of AChRs and suggest that changes in the electrostatic environment affect modification rates at residues in the inner β sheet and transition zone.
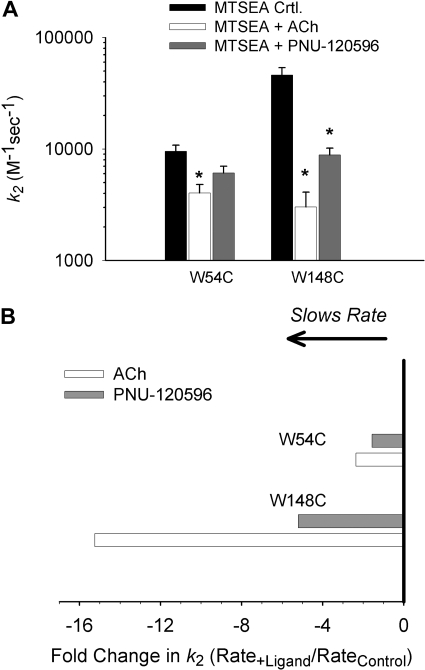
Fig. 6 shows second-order rate constants of MTSEA modification of two residues in the orthosteric agonist binding site (W54C and W148C). ACh slowed the rate of modification of W54C, which agrees with previously published results on wild-type α7 receptors (Gay et al., 2008). However, PNU-120596 did not significantly reduce the rate of modification at W54C (Fig. 6A). ACh and PNU-120596, acting as agonists (Table 1), both significantly slowed the rate of modification of W148C (Fig. 6A). Because the aromatic side chains of Trp54 and Trp148 are both known to be part of the ligand-binding site, it is likely that steric occlusion by ACh is at least partially responsible for the slowing of modification of these Cys mutants. Slowing of modification of W148C by PNU-120596 could also be explained by steric hindrance, if PNU-120596 binds at unoccupied agonist binding sites, analogous to the binding site for benzodiazepines at the α-γ subunit interface of GABAA receptors (Günther et al., 1995; Amin et al., 1997). Acetylcholine (Mr 146) contains a positively charged choline group that makes π-cation interactions with the agonist-binding site (Zhong et al., 1998). PNU-120596 (Mr 312) is a urea analog flanked by isoxazole and chlorodimethoxyphenyl groups (Hurst et al., 2005). Recent evidence, however, suggests that PAMs of α7 receptors bind to sites in the TMD (Bertrand et al., 2008; Young et al., 2008), and thus slowing of W148C modification by PNU-120596, could be explained by an allosteric effect at the ligand-binding pocket, perhaps including partial closure of loop C.
Fig. 6.
PNU-120596 slows the rate of MTSEA modification at W148C but not W54C in the orthosteric site. A, mean second-order rate constants (k2) were calculated at two residues in the transition zone, W54C and W148C. *, rate was significantly different from control (P < 0.05). B, a plot of the second-order rate constant ratios.
To distinguish between these possibilities we took advantage of an unexpected observation: introduction of W148C in the C115A/L247T parent receptor converted PNU-120596 from a positive allosteric modulator to a full agonist (Table 1 and Fig. 7A). The phenotype of this receptor is useful because it allows us to compare the effects of covalent modification with MTS reagents on either ACh- or PNU-120596-evoked gating at the well defined agonist-binding site. If PNU-120596 activates the receptors via binding to the orthosteric agonist binding site, we expect that covalent modification of W148C in the agonist binding pocket to disrupt activation by both ACh and PNU-120596 similarly via steric effects on ligand binding. If PNU-120596 does not activate W148C mutants by binding at the agonist binding site, then PNU-evoked currents should be less sensitive to covalent modification of W148C than ACh-evoked currents. For these experiments, we used two MTS reagents of different size and charge to determine moiety-dependent affects on ACh and PNU-evoked current at W148C. MTSEA adds a positively charged ethyl amine to thiols at physiological pH and is comparatively small. MTSEA-biotin adds a neutral, bulky ring structure to thiols and is comparatively large.
Fig. 7.
Covalent modification of W148C in the orthosteric site does not affect agonism by PNU-120596. A, PNU-120596 acts as a full agonist of W148C/C115A/L247T α7 receptors. Hill parameter fits for activation by ACh are in Table 1. For PNU-120596, the EC50 is 0.45 ± 0.01 μM, Imax is 3.87 ± 0.65 μA, and Hill coefficient = 6.79 ± 0.81 (n = 3). PNU-evoked currents are normalized to peak ACh-evoked current (I/IACh). B, representative traces for 3 mM ACh and 3 μM PNU-120596 shown before (black) and after (red) application of 100 μM MTSEA for 60 s. C, representative traces for 3 mM ACh and 3 μM PNU-120596 shown before (black) and after (red) application of 4 μM MTSEA-biotin for 60 s. D, summary data for the effects of covalent modification of W148C with MTSEA (n = 8) and MTSEA-biotin (n = 10) on ACh and PNU-evoked currents. Bars represent the percentage of ACh or PNU-evoked current after MTS modification relative to the control currents before modification (Iafter/Ibefore). Both MTSEA and MTSEA-biotin caused a significantly greater reduction in ACh-evoked currents than PNU-evoked currents (* P < 0.01).
Fig. 7B shows representative ACh- and PNU-120596-evoked currents before and after modification of W148C by MTSEA (100 μM for 60 s). This exposure to MTSEA was sufficient to completely eliminate ACh-evoked current, but it had no effect on currents evoked by PNU-120596. If we assume that modification of a binding site residue interferes with activation by steric interference with ligand, then this result implies that PNU-120596 activates receptors via a site distinct from the orthosteric site. However, an alternative explanation is that the two ligands interact with a different set of “contact points” in the binding pocket, and those for PNU-120596 are removed from the ethyl amine adduct at Trp148. To test this, we examined the effect of MTSEA-biotin, which modifies the thiol with a bulky ring structure. Fig. 7C shows representative ACh- and PNU-120596-evoked currents before and after modification of W148C by MTSEA-biotin (4 μM for 60 s). This exposure of MTSEA-biotin was sufficient to reduce ACh-evoked current by 70% but had no significant effect on the magnitude of currents evoked by PNU-120596. Modification of the agonist binding site by MTSEA-biotin slowed the kinetics of PNU-120596-dependent activation (Fig. 7C), suggesting that there are allosteric conformational changes at the agonist binding site during activation by PNU-120596. The results from multiple experiments are summarized in Fig. 7D. Covalent modification of W148C by MTSEA and MTSEA-biotin had a significantly greater effect on ACh-evoked current than on PNU-120596-evoked currents (P < 0.01). This observation provides evidence that PNU-120596, although behaving as an agonist, does not interact with the agonist-binding site of α7 receptors. Our interpretation is that PNU-120596 induces conformational changes at the agonist-binding site through allosteric mechanisms, rather then steric occlusion, because occlusion is predicted to impair both ACh and PNU-evoked currents similarly.
Discussion
In this study, we provide evidence that the positive allosteric modulator PNU-120596 causes conformational changes in the LBD of α7 nicotinic receptors that partially overlap with those caused by ACh. We focused on mapping the structural transitions of PAMs in three regions of the LBD: 1) The inner β sheet, 2) the transition zone, and 3) the orthosteric site (Fig. 1). A homology model of the LBD of the α7 receptor, based on the structure of ACh binding protein, was used to guide our experiments (Brejc et al., 2001; Lyford et al., 2003).
The inner β sheet, composed of the β1, β2, and β6 strands, resides at the interface between two subunits (Fig. 1). In this study, we found that PNU-120596 caused reductions in the rate of modification of M37C and M40C that were similar to those caused by ACh (Fig. 4, Table 2; McLaughlin et al., 2007). The similarity in the effects on cysteine modification at M37C and M40C suggests that PAMs and agonists induce similar conformational changes in the LBD of α7 receptors. We have provided the first evidence that PAMs of α7 receptors enhance gating by causing some of the same structural transitions in the LBD as ACh.
The transition zone, composed of loops from the LBD (loop 2, loop 9, and the Cys loop) and the TMD (pre-M1 sequence and M2-M3 linker), is positioned to convey structural rearrangements caused by agonist binding in the LBD to the channel gate in the TMD (Bouzat et al., 2004). Therefore, we examined the effect of PNU-120596 on the rate of MTSEA modification at three reporter residues within the transition zone (E44C, N170C, and E172C), which is also a proposed site of modulation by divalent cations (Galzi et al., 1996). PNU-120596 reduced the rate of MTSEA modification at all three positions (Fig. 5, Table 2). At N170C, PNU-120596 decreased the accessibility of the substituted cysteine, whereas ACh caused an increase. The application of both compounds offset each other at N170C. Overall, the changes in cysteine accessibility in the transition zone caused by ACh and PNU-120596 were similar but not identical.
It is noteworthy that positive modulation of ACh-evoked currents by PNU-120596 was lost in the E172C mutant (Table 1), but PNU-120596 still caused a decrease in thiol accessibility at this position (Fig. 5). One explanation is that the PNU-120596 binding site includes Glu172, and the introduced cysteine (E172C) eliminates allosteric modulation but not binding. In this scenario, the observed reduction in the rate of MTSEA modification at E172C is due to physical occlusion by PNU-120596 of the substituted cysteine at the putative binding site. An alternative explanation is that PNU-120596 binds to a site outside of the transition zone, away from Glu172, and the observed reduction in the rate of E172C modification is due to conformational changes induced there. In this scenario, the binding of PNU-120596 is unaffected and can still induce conformational changes, but the electrostatic coupling within the transition zone (Xiu et al., 2005) is sufficiently disrupted by the cysteine mutation that the induced conformational changes no longer enhance receptor gating.
Fiinally, we examined the effect of ACh and PNU-120596 on the rate of MTSEA modification at two residues in the orthosteric site (Trp54 and Trp148) (Fig. 6). Viewed from a structural perspective, these residues occupy two distinct locations within the agonist binding site. Trp148 is part of the principal subunit (Fig. 1, yellow subunit). It lines the back wall of the agonist binding site and makes contact with agonists and competitive antagonists (Celie et al., 2004; Hansen et al., 2005). Trp54 is part of the complimentary subunit and sits on the edge of the agonist binding site (Fig. 1, cyan subunit) (Brejc et al., 2001). ACh reduced the rate of MTSEA modification at these positions. In our interpretation, ACh reduces covalent modification at W148C by physically blocking access to the introduced cysteine. On the other hand, because carbamylcholine does not contact Trp54 (Celie et al., 2004), we hypothesize that ACh induces a short-range conformational change that makes W54C less accessible to covalent modification. The ACh-dependent effect at W54C agrees with previously published work on wild-type α7 receptors (Gay et al., 2008). PNU-120596 does not significantly affect the rate of MTSEA modification at W54C, but reduced the rate of MTSEA modification at W148C, suggesting that PNU-120596 induces allosteric conformational changes in the center of the agonist binding pocket, but not on the periphery.
We found unexpectedly that PNU-120596 was a full agonist of W148C receptor in C115A/L247T-containing α7 receptors (Fig. 7A). One explanation is that the introduced cysteine allowed partial closure of the C-loop and lowered the activation energy sufficiently to allow PNU-120596 to activate the receptor and at the same time increased energetic barriers to ACh binding. The C-loop is a dynamic and flexible region that acts as a hinge of the orthosteric site (Hansen et al., 2005; Venkatachalan and Czajkowski, 2008). The EC50 for ACh-dependent activation of the W148C mutant receptor was increased ∼100-fold (Table 1), as expected for a receptor with a mutation of an important aromatic residue of the agonist-binding pocket (Brejc et al., 2001). We took advantage of this phenotype to test whether PNU-120596's ability to alter cysteine accessibility was due to allostery or steric occlusion. Because ACh-evoked currents are more sensitive to covalent modification by different MTS reagents than PNU-evoked currents (Fig. 7), we conclude that PNU-120596 induces conformational changes at this position through an allosteric mechanism. A chemically related PAM, NS1738, does not affect equilibrium binding of 125I-α-bungarotoxin, also suggesting that it does not interact with the agonist binding site (Timmermann et al., 2007). Recent work with chimeric α7 nAChR/5-HT3 receptors and mutagenic studies suggests a binding site in the transmembrane domain for PNU-120596 and LY-2087101 (Bertrand et al., 2008; Young et al., 2008). These data suggest that PNU-120596 and other modulators of α7 receptors bind at a conserved site within the transmembrane domain and cause conformational changes in the LBD to enhance gating of the receptor.
In the Monod/Wyman/Changeux model of allostery, positive allosteric modulators of ligand-gated ion channels enhance activation by stabilizing the protein in the open state (Bertrand and Gopalakrishnan, 2007). Our results provide the first evidence that that PNU-120596 promotes activation of α7 receptors by causing some (but not all) of the same conformational changes in the LBD associated with agonists. We have previously shown that permeable divalent cations, which do not alter desensitization, also induce conformational changes in the LBD that are similar to those induced by agonists (McLaughlin et al., 2009). Our work adds to a growing body of literature of both convergent and divergent conformational changes during gating of Cys-loop receptors (Chang and Weiss, 2002; Pless et al., 2007; Sharkey and Czajkowski, 2008; Zhang et al., 2009).
Recent work suggests that the mechanisms of closed-to-open conformational changes induced by different agonists and of unliganded receptors are completely conserved regardless of the agonist applied; only the kinetics of the C-O transitions are affected (Purohit and Auerbach, 2009). Although most of our data with PNU-120596 agrees with this idea, modulation and rate measurements for the N170C mutant seem to be an exception. For this mutant, PNU-120596 slows the rate of modification, whereas ACh increases it (Fig. 5). Thus, each ligand stabilizes a population of conformational intermediates that are distinct from each other and from the resting unliganded state(s). A model in which C-O transitions occur via a single pathway would predict that PNU-120596 and ACh would cause similar changes in accessibility at all residues. If there is a single path, then the observation that PNU-120596 causes a change in accessibility of N170C opposite to that caused by ACh suggests that the receptor would be stabilized in closed intermediate states further away from the open state, and PNU-120596 would be unable to enhance the conserved C-O transition. But PNU-120596 still acts as a positive allosteric modulator of N170C receptors, suggesting that PNU-120596 induces conformational changes along alternative pathways that lower energetic barriers to activation and lead to positive modulation. The differences between the conformational intermediates induced by PNU-120596 and those induced by ACh are likely to be subtle, because they are not as apparent at other cysteine mutants.
In conclusion, we have shown that PNU-120596 and ACh induce a set of overlapping structural transitions in the extracellular ligand-binding domain. Our results indicate that PAMs such as PNU-120596 enhance gating of ligand-gated ion channels by inducing some of the same structural transitions caused by agonists. In addition, we have identified mutations in the transition zone that eliminate modulation of α7 nicotinic receptors by PNU-120596 via decoupling between the LBD and TMD. We have provided evidence that PNU-120596 does not bind to unoccupied agonist-binding site(s). The PAM-induced changes in receptor kinetics, although not usually sufficient to activate receptors, would lower the energy barriers to agonist-induced activation by both enhancing the agonist-evoked C-O transitions as well as alternate transitions. This process would “prime” the receptors to undergo a gating transition, allowing more of the energy of agonist binding to drive changes in the conformational equilibrium toward activation (Jackson, 1989).
Supplementary Material
Acknowledgments
We thank Drs. William Green and Millet Treinin for the human RIC-3 clone.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA017882].
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor; TMD, transmembrane domain; PAM, positive allosteric modulator; PNU-120596, N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea; LBD, ligand binding domain; SCAM, substituted cysteine accessibility method; ESLC, extracellular solution, low calcium; MTS, methanethiosulfonate; MTSEA, 2-aminoethylmethanethiosulfonate; ACh, acetylcholine; C-O, closed-open gating transition; WT, wild type.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Akabas MH, Stauffer DA, Xu M, and Karlin A (1992) Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science 258 307-310. [DOI] [PubMed] [Google Scholar]
- Amin J, Brooks-Kayal A, and Weiss DS (1997) Two tyrosine residues on the α subunit are crucial for benzodiazepine binding and allosteric modulation of γ-aminobutyric acidA receptors. Mol Pharmacol 51 833-841. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, and Gopalakrishnan M (2008) Positive allosteric modulation of the α7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2-M3 segment. Mol Pharmacol 74 1407-1416. [DOI] [PubMed] [Google Scholar]
- Bertrand D and Gopalakrishnan M (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 74 1155-1163. [DOI] [PubMed] [Google Scholar]
- Bianchi MT and Macdonald RL (2001) Mutation of the 9′ leucine in the GABA(A) receptor gamma2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacology 41 737-744. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, and Sine SM (2004) Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430 896-900. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, and Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411 269-276. [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, and Sixma TK (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41 907-914. [DOI] [PubMed] [Google Scholar]
- Chang Y and Weiss DS (2002) Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat Neurosci 5 1163-1168. [DOI] [PubMed] [Google Scholar]
- Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125 924-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov GN and White MM (1995) The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol 48 379-384. [PubMed] [Google Scholar]
- Galzi JL, Bertrand S, Corringer PJ, Changeux JP, and Bertrand D (1996) Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J 15 5824-5832. [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Giniatullin R, Skorinkin A, and Yakel JL (2008) Aromatic residues at position 55 of rat α7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. J Physiol 586 1105-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA and Yakel JL (2007) Gating of nicotinic ACh receptors; new insights into structural transitions triggered by agonist binding that induce channel opening. J Physiol 584 727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C and Clementi F (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74 363-396. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, and Malysz J (2007) Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72 715-724. [DOI] [PubMed] [Google Scholar]
- Günther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, and Lang Y. (1995) Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 92 7749-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, and Bourne Y (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24 3635-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25 4396-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB (1989) Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A 86 2199-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3 102-114. [DOI] [PubMed] [Google Scholar]
- Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, and Millar NS (2005) RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol 68 1431-1438. [DOI] [PubMed] [Google Scholar]
- Lyford LK, Sproul AD, Eddins D, McLaughlin JT, and Rosenberg RL (2003) Agonist-induced conformational changes in the extracellular domain of alpha 7 nicotinic acetylcholine receptors. Mol Pharmacol 64 650-658. [DOI] [PubMed] [Google Scholar]
- McLaughlin JT, Barron SC, See JA, and Rosenberg RL (2009) Conformational changes in alpha 7 acetylcholine receptors underlying allosteric modulation by divalent cations. BMC Pharmacol 9 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JT, Fu J, and Rosenberg RL (2007) Agonist-driven conformational changes in the inner β-sheet of α7 nicotinic receptors. Mol Pharmacol 71 1312-1318. [DOI] [PubMed] [Google Scholar]
- McLaughlin JT, Fu J, Sproul AD, and Rosenberg RL (2006) Role of the outer β-sheet in divalent cation modulation of α7 nicotinic receptors. Mol Pharmacol 70 16-22. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, and Luetje CW (2006) Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol 70 801-805. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, and Beaudet AL (1997) Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17 9165-9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual JM and Karlin A (1998) State-dependent accessibility and electrostatic potential in the channel of the acetylcholine receptor. Inferences from rates of reaction of thiosulfonates with substituted cysteines in the M2 segment of the α subunit. J Gen Physiol 111 717-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless SA, Dibas MI, Lester HA, and Lynch JW (2007) Conformational variability of the glycine receptor M2 domain in response to activation by different agonists. J Biol Chem 282 36057-36067. [DOI] [PubMed] [Google Scholar]
- Purohit P and Auerbach A (2009) Unliganded gating of acetylcholine receptor channels. Proc Natl Acad Sci U S A 106 115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, and Changeux JP. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353 846-849, 1991. [DOI] [PubMed] [Google Scholar]
- Sharkey LM and Czajkowski C (2008) Individually monitoring ligand-induced changes in the structure of the GABAA receptor at benzodiazepine binding site and non-binding-site interfaces. Mol Pharmacol 74 203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, Ahring PK, Peters D, Holst D, Chrsitensen JK, et al. (2007) An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther 323 294-307. [DOI] [PubMed] [Google Scholar]
- Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346 967-989. [DOI] [PubMed] [Google Scholar]
- Venkatachalan SP and Czajkowski C (2008) A conserved salt bridge critical for GABAA receptor function and loop C dynamics. Proc Natl Acad Sci U S A 105 13604-13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Hanek AP, Wang J, Lester HA, and Dougherty DA (2005) A unified view of the role of electrostatic interactions in modulating the gating of Cys loop receptors. J Biol Chem 280 41655-41666. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, and Millar NS (2008) Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 105 14686-14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue F, and Chang Y (2009) Agonist- and antagonist-induced conformational changes of loop F and their contributions to the rho1 GABA receptor function. J Physiol 587 139-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, and Dougherty DA (1998) From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor. Proc Natl Acad Sci U S A 95 12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.