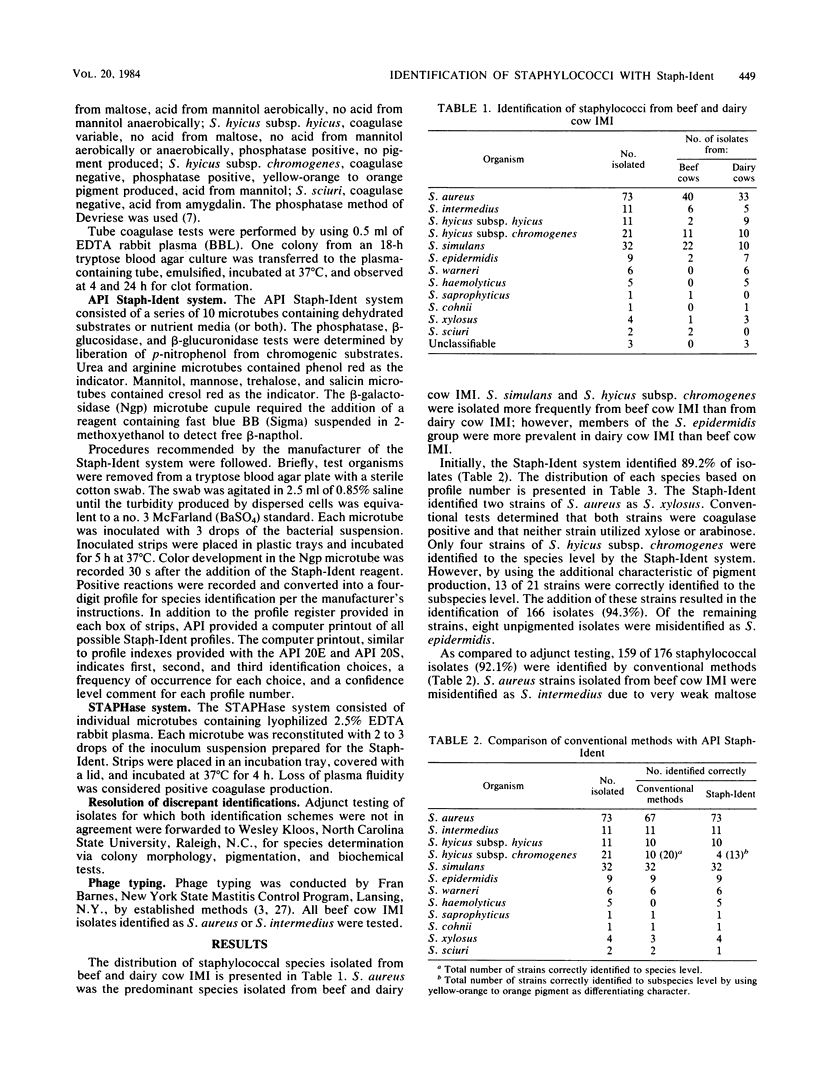
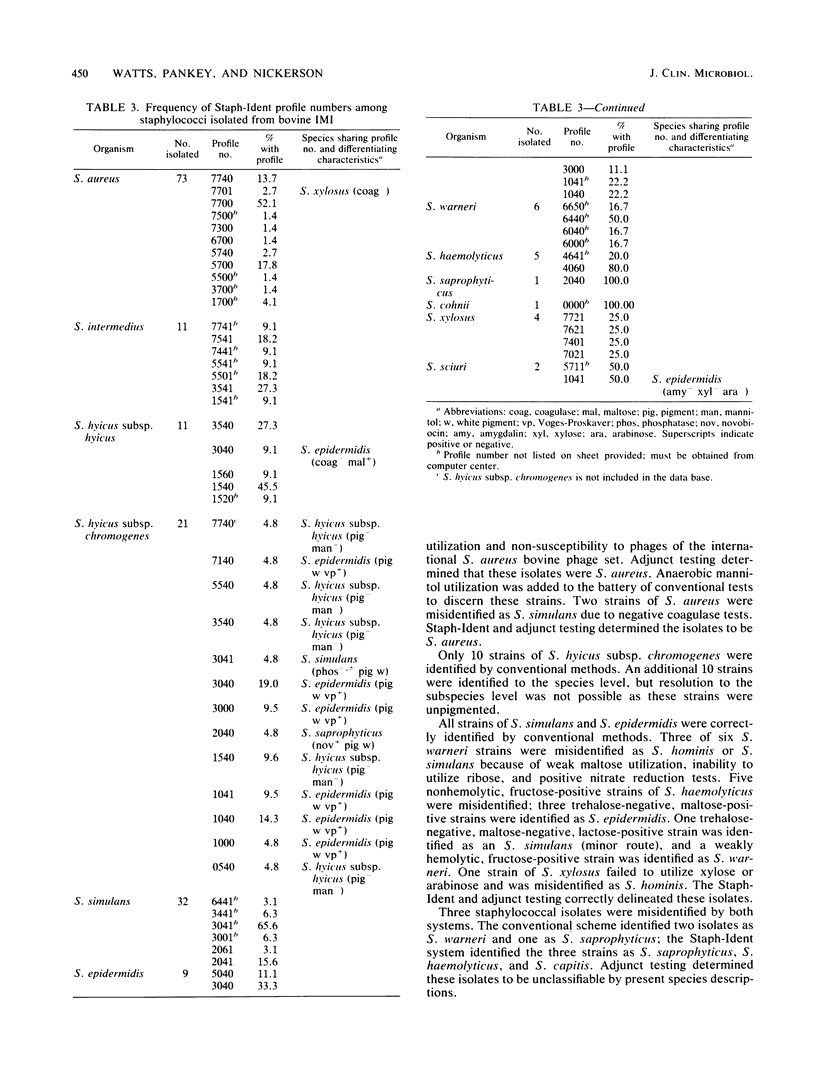
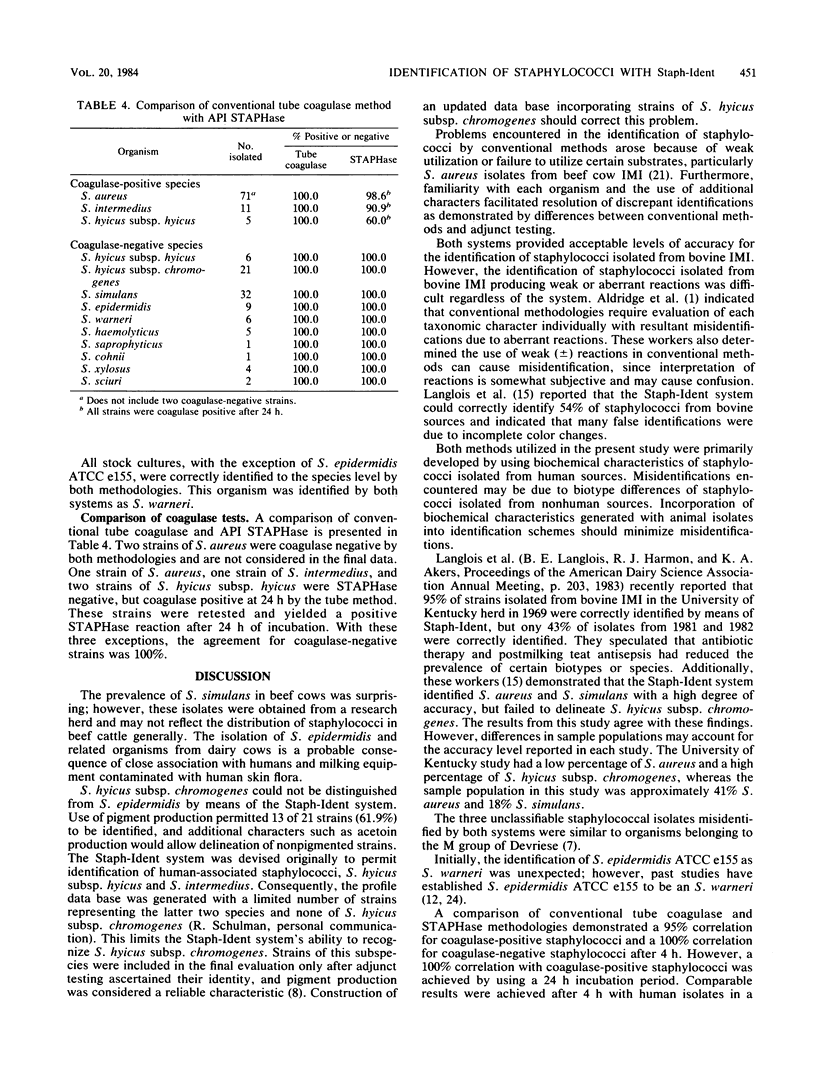
Abstract
The Staph-Ident and STAPHase systems (Analytab Products, Plainview, N.Y.) were compared with conventional methods for identification of staphylococci isolated from bovine intramammary infections. Adjunct testing by colony morphology, pigmentation, and biochemical tests was conducted to resolve discrepant identifications. The initial accuracies of the conventional scheme and Staph-Ident were 92.1 and 89.2%, respectively. Staphylococcus hyicus subsp. chromogenes could not be identified by means of the Staph-Ident test, but the addition of pigment production as a key character permitted identification of most strains. The final accuracy of the Staph-Ident was 94.3%. The STAPHase system was as accurate as the conventional tube coagulase method. The Staph-Ident and STAPHase systems are acceptable alternatives to conventional methods for identification of staphylococcal species isolated from bovine intramammary infections.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Stratton C. W., Patterson L. S., Evans M. E., Hodges R. L. Comparison of the Staph-Ident system with a conventional method for species identification of urine and blood isolates of coagulase-negative staphylococci. J Clin Microbiol. 1983 Mar;17(3):516–520. doi: 10.1128/jcm.17.3.516-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley A. J. The effect of subclinical Staphylococcus epidermidis infection of the lactating bovine udder on its susceptibility to infection with Streptococcus agalactiae or Escherichia coli. Br Vet J. 1978 Mar-Apr;134(2):146–151. doi: 10.1016/s0007-1935(17)33538-8. [DOI] [PubMed] [Google Scholar]
- Devriese L. A. Identification of clumping-factor-negative staphylococci isolated from cows' udders. Res Vet Sci. 1979 Nov;27(3):313–320. [PubMed] [Google Scholar]
- Goldstein J., Roberts J. W. Microtube coagulase test for detection of coagulase-positive staphylococci. J Clin Microbiol. 1982 May;15(5):848–851. doi: 10.1128/jcm.15.5.848-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N. C. Common mammary pathogens and factors in infection and mastitis. J Dairy Sci. 1979 Jan;62(1):128–134. doi: 10.3168/jds.S0022-0302(79)83214-2. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois B. E., Harmon R. J., Akers K. Identification of Staphylococcus species of bovine origin with the API Staph-Ident system. J Clin Microbiol. 1983 Nov;18(5):1212–1219. doi: 10.1128/jcm.18.5.1212-1219.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse G. E. Studies of bovine mastitis: a bacteriologically correct method of culturing milk for the detection of udder infection. Proc Annu Meet U S Anim Health Assoc. 1967;71:596–614. [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Intramammary infections in primigravid heifers near parturition. J Dairy Sci. 1983 May;66(5):1180–1183. doi: 10.3168/jds.S0022-0302(83)81916-X. [DOI] [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Susceptibility of bovine mammary gland to infections during the dry period. J Dairy Sci. 1983 May;66(5):1162–1166. doi: 10.3168/jds.S0022-0302(83)81913-4. [DOI] [PubMed] [Google Scholar]
- Phillips W. E., Jr, Kloos W. E. Identification of coagulase-positive Staphylococcus intermedius and Staphylococcus hyicus subsp. hyicus isolates from veterinary clinical specimens. J Clin Microbiol. 1981 Dec;14(6):671–673. doi: 10.1128/jcm.14.6.671-673.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosypal S., Rosypalová A., Horejs J. The classification of micrococci and staphylococci based on their DNA base composition and adansonian analysis. J Gen Microbiol. 1966 Aug;44(2):281–292. doi: 10.1099/00221287-44-2-281. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kocur M. Classification of staphylococci based on chemical and biochemical properties. Arch Mikrobiol. 1973 Oct 4;93(1):65–85. doi: 10.1007/BF00666081. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Schumacher-Perdreau F., Götz F., Popp B. Chemical and biochemical studies for the differentiation of coagulase-positive staphylococci. Arch Microbiol. 1976 Nov 2;110(23):263–270. doi: 10.1007/BF00690237. [DOI] [PubMed] [Google Scholar]
- Symposium: bovine mastitis. J Dairy Sci. 1979 Jan;62(1):117–176. [PubMed] [Google Scholar]
- Thörne H., Hallander H. O. Phage typing of Staphylococcus aureus from bovine mastitis. A comparison of phages according to Davidson to the conventional phage set. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):425–428. [PubMed] [Google Scholar]
- Ward G. E., Madl J. E., Lyon R. H. Mannitol agar for microbiologic diagnosis of bovine mastitis. J Am Vet Med Assoc. 1981 May 15;178(10):1061–1064. [PubMed] [Google Scholar]


