Abstract
Background and Methods
Abnormal circulating endothelial cell (CEC) and circulating progenitor cell (CPC) numbers are present in cancer, but their relationship with angiogenesis, apoptosis, vascular biology, and prognosis is unclear. We prospectively studied 160 patients with breast cancer and 63 age-matched controls free of breast cancer, measuring CECs (CD45-/CD146+/CD34+) and CPCs (CD45-/CD133+/CD34+) by flow cytometry and plasma markers of endothelial damage/dysfunction (von Willebrand factor), apoptosis (Fas/Fas-L) and angiogenesis (vascular endothelial growth factor [VEGF], angiogenin) by ELISA. These were compared with clinicopathophysiologic features and the Nottingham Prognostic Index (NPI). An additional blood sample was taken 6 to 8 weeks after surgery from 15 women to test the effect of tumor removal.
Results
CECs were significantly higher in the NPI poor prognostic group compared with moderate and good prognostic groups, and the cancer-free controls, whereas CPCs were lower in the poor prognosis group (both P < .05). Levels of von Willebrand factor, VEGF, angiogenin, and Fas-L (but not soluble Fas) were abnormal in breast cancer compared with controls (P < .05), with no relationship to prognosis groups. VEGF (P = .04) and angiogenin (P = .001) were markedly different after surgery. In multivariate analysis, vascular invasion (P < .05) and tumor size (P < .001) were independently associated with CECs. CPCs did not significantly associate with NPI in a linear regression model; age (P < .05) was a negative predictor, whereas Her-2 status (P < .05) positively predicted CPCs. After adjustment, no variable independently predicted CPC levels.
Conclusions
CECs and CPCs demonstrate a strong relationship with NPI groups, but only CECs positively predict higher NPI scores and correlate with tumor invasiveness and size, possibly reflecting total tumor vascular volume.
Introduction
Recent advances in the vascular biology of various diseases have identified a number of nonleukocyte endotheliod-like cells in the blood. One such population, circulating endothelial cells (CECs), defined by membrane component CD146, are reputedly a marker of vascular damage/dysfunction [1]. Believed to be cells shed from the intima into the lumen in the presence of vascular insult, their presence in peripheral blood has been associated with worse outcome in cardiovascular disease and prognostic for adverse events after an acute myocardial infarction. In cancer, CECs are present in abnormally high numbers, but current evidence would suggest a different pathophysiology from that of cardiovascular disease [2]. A second endotheliod population, circulating progenitor cells (CPCs), is derived from the bone marrow and is recognized by other membrane components such as CD34 and/or AC133. CPCs, present in cardiovascular and neoplastic disease, are believed to have regenerative/restorative properties [2–4].
There is a developing hypothesis that CECs and/or CPCs may have a more direct role in tumor biology and angiogenesis [4–6]. In a typical solid tumor such as breast cancer, the tumor increases in size and vasculature and metastasizes, possibly under the direction of angiogenic growth factors such as vascular endothelial growth factor (VEGF). Tumor-associated angiogenesis is very much evident, often with ingrowing of surrounding existing vessels as well as de novo vessel formation, to meet the metabolic requirements of the growing tumor and to be an avenue for tumor cell dissemination. However, increased apoptosis of tumor cells and/or their feeding vasculature may be one route to limiting tumor growth [7]. CEC levels in patients with cancer might well reflect the abnormally high turnover rate of tumor endothelium, as well as the disordered nature of tumor angiogenesis, and relate to tumor vascular volume [2]. For example, tumors are known to encourage CPC mobilization from the bone marrow, which affects, either directly or indirectly, tumor angiogenesis, and animal models suggest that this may be important in tumor vascularity [4–6]. However, a lack of consensus about the definition of these endotheliod cells (of whatever origin), using different cell surface glycoproteins provides difficulty in the interpretation of these and other data [8–12].
We tested the hypotheses that, in breast cancer, both CEC and CPC levels (defined by flow cytometry) are related 1) to clinicopathologic indices of tumor load, 2) to the validated clinical prognostic systems of the Nottingham Prognostic Index (NPI) [13] (widely used to select patients anticipated to benefit from adjuvant therapy after surgery), and 3) to plasma markers of angiogenesis (VEGF, angiogenin) [14,15], endothelium damage/dysfunction (von Willebrand factor [vWf]) [16,17], and apoptosis (soluble Fas [sFas] and soluble Fas-ligand [sFas-L]) [7,18,19]. We also tested the hypothesis that tumor debulking would normalize numbers of the CECs, CPCs, and levels of the plasma markers.
Patients and Methods
Patients with breast cancer, confirmed by core biopsy, were recruited from breast preadmission and oncology clinics. Patients were recruited before surgery, radiotherapy, and chemotherapy. Fifteen women were seen again 6 to 8 weeks after surgery for removal of their tumor; all had confirmed histologic clearance of disease, after undergoing either wide local excision or mastectomy with axillary clearance. Twelve of the 15 patients were in early stages of the disease (i.e., stage I, II, or ductal carcinoma in situ), and their conditions were diagnosed with invasive ductal carcinoma. The remaining patients had stage III disease.
Subjects with benign breast disease but free of breast cancer were recruited from screening clinics, with a benign pathologic lesion confirmed by core biopsy. All participants were fully informed and gave written consent according to approved local ethics and research protocol and were required to undergo a full medical assessment including blood pressure measurements and routine blood tests. Exclusion criteria included any history of previous cancer or inflammatory/infectious, cardiovascular, endocrine, or autoimmune disease. Routine histopathologic data were acquired after the final surgical intervention. All NPI scores in the study were calculated according to the established formulae [13] (based on invasive size, lymph node involvement, and histopathologic grade), and patients were grouped according to their prognostic group: good prognostic group (GPG), moderate prognostic group (MPG), and poor prognostic group (PPG).
Enumeration of CECs and CPCs by Flow Cytometry
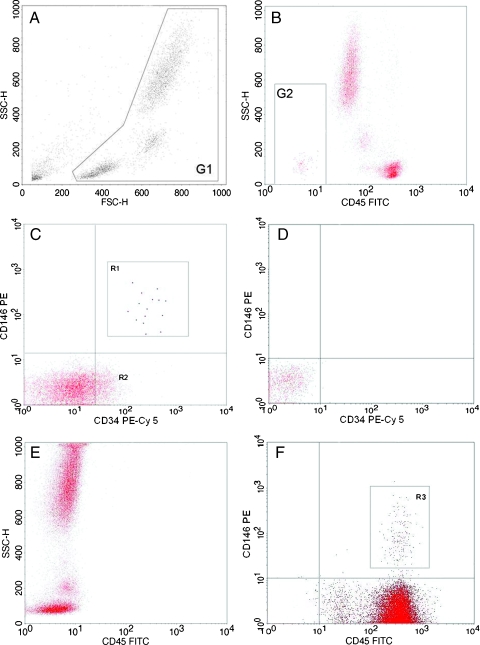
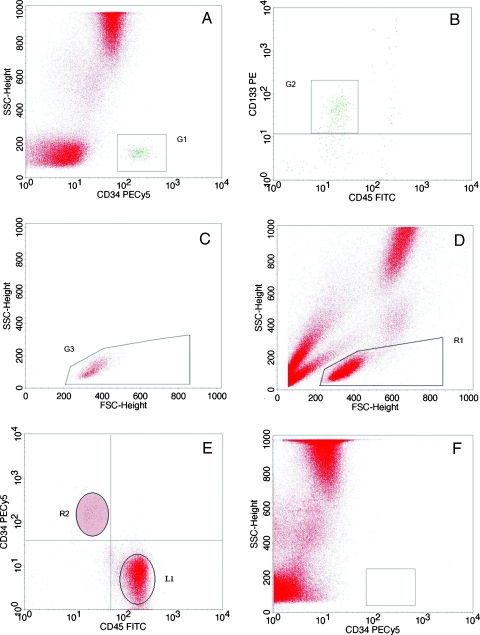
The method for measuring CECs has been previously described [20,21]. Briefly, 1 ml of venous blood (anticoagulant: EDTA) is prepared by lysing red cells with 10 ml of FACS lysing solution (10x, diluted 1:10) for 10 minutes. The white cells are then blocked with 20 µl of specific Fc-receptor antibodies (Octagam; Octapharma, Coventry, United Kingdom) and 200 µl of mouse serum (Sigma, Gillingham, United Kingdom) for a minimum of 20 minutes at room temperature. Next, the cells are incubated with fluorochrome-labeled monoclonal anti-human mouse antibodies, namely, FITC-CD45, PE-CD146, and PE-Cy 5-CD34 (Becton Dickinson, Oxford, United Kingdom) for 20 minutes at room temperature, washed with cell buffer solution (PBS + 1% bovine serum albumin + 0.05% sodium azide), and centrifuged at 500g to repellet the cells. The cells are then fixed with 200 µl of 2% paraformaldehyde for 20 minutes at 4°C and made up to a final volume of 1 ml with cell buffer solution ready for analysis. Blood for CPC analysis was prepared in a similar fashion, and antibodies used were FITC-CD45, PE-Cy 5-CD34 (Becton Dickinson), and PE-CD133 (Miltenyi Biotec, Bisley, United Kingdom). Lysing as described previously was first determined not to affect the antigen staining process by validation work using non-fixative-containing lyse solutions (HYL-250; Caltag Laboratories, Bucks, United Kingdom). All samples were analyzed using a three-color FACScan flow cytometer (Becton Dickinson). CECs were defined as CD45-/CD146+/CD34+ cells, whereas CPCs were, in turn, defined as CD45dim-to-intermediate/CD133+/CD34+. Our interassay and intra-assay coefficients of variation were both less than 10%. Full strategy and representative flow cytometer plots are presented in Figures 1 and 2.
Figure 1.
Flow cytometry strategy for CECs. CECs are defined as CD45-/CD34+/CD146+ using CD45-FITC, CD34-PE Cy5, CD146-PE conjugated antibodies. Sequential gating strategy for CECs: (A) Forward (FSC) and side scatter (SSC) plot of white blood cells and gating region G1 to include all mononuclear and polymorphonuclear cell events while excluding platelets, dead cells, and microparticles. (B) Dump channel to exclude CD45+ cells and very high side scatter events. (C) Cellular events from gated region G2, with CECs in R1 (CD34+/146+). R1 cells are backscattered to ensure that they meet the minimal FSC requirements, that is, size equal to or greater than that of lymphocytes (Figure 2: CPC strategy CD where R2 indicates progenitor cells (CD34+)). CECs have been highlighted in bold for clarity. (D and E) Negative controls using fluorochrome-matched isotype control antibodies. (F) R3 illustrates small population of CD146+ leukocytes that are likely to be lymphocytes.
Figure 2.
Flow cytometer strategy for CPCs. CPCs are defined as CD45dim/CD34+/CD133+ using CD45-FITC, CD34-PE Cy5, CD133-PE conjugated antibodies. Gating strategy for peripheral blood CPC, a modification of the International Society for Hematotherapy Graft Engineering stem cell strategy. (A) All CD45+ events are displayed on a side scatter (SSC) versus CD34 plot. G1 events include the CD34+ cluster with low SSC. (B) G1 events are displayed by CD45 versus CD133 scatter plot. Cells demonstrating positive CD133 fluorescence and dim-to-intermediate CD45 expression (G2) are gated onto a third plot. (C) G3 displays events from G1 + G2, with characteristic forward scatter (FSC) and SSC. The lower limit of FSC of G3 is determined by backscattering lymphocytes (R1 + L1), so that G3 only includes events no smaller than lymphocytes. CPCs are defined as CD45dim/CD34+/CD133+ cells with characteristic FSC/SSC. (D) Ungated population demonstrates FSC/SSC of mononuclear cells of low SSC (majority probably lymphocytes) R1 population. (E) CD34 versus CD45 plot of R1 demonstrating region L1 (CD34-ve leukocytes), the clear CD34+ events (R2), and the establishment of the lower limit of CD45 expression by CD34+ events. (F) SSC versus CD34 plot of CD45+/IgG1-PE Cy 5 events (isotype control).
Plasma Markers
Venous blood was taken into sodium citrate, and plasma was obtained by centrifugation at 3000 rpm (1000g) for 20 minutes at 4°C. All aliquots were stored at -70°C to allow batch analysis. Plasma vWf, VEGF, angiogenin, soluble Fas (sFas) and soluble Fas-ligand (sFas-L) were measured by ELISA using commercial reagents (e.g., R&D Systems, Abingdon, Oxfordshire, United Kingdom; DakoCytomation, Ely, Cambs, United Kingdom). The interassay and intra-assay coefficients of variation for all ELISA assays were less than 5% and less than 10%, respectively.
Power Calculation
On the basis of published data on CECs in subjects with breast cancer [20–24], we defined a minimum sample of at least 100 patients and 50 healthy control subjects to detect a difference of at least 6% in mean CEC levels, achieving a 1 - β statistical power of 0.8 and an α power of less than 0.05.
Statistical Analyses
After applying the Shapiro-Wilks test to determine a normal distribution for data, noncategorical data distributed normally are expressed as mean (SD) and data distributed nonnormally are expressed as median (interquartile range [IQR]). Correlations were sought by the Spearman rank method. Categorical data were analyzed by the χ2 test. Differences between groups (NPI vs noncancer controls) were analyzed with Kruskal-Wallis (nonparametric), post hoc test (Dunn), or one-way analysis of variance (parametric), post hoc test (Tukey), as appropriate. Analysis was by linear regression, with a hierarchical approach applied in multivariate analyses. A multiple linear regression model was tested to determine independent variables predicting change in the NPI (excluding tumor size, nodal status, and grade, which make up NPI). Also, a model was tested in which significant variables (univariate) were entered at step 1 followed by the NPI stage to determine the factors accounting for variance in CEC or CPC levels. To test the predictive accuracy of CECs for the diagnosis of breast cancer, as well as for PPG (NPI), area under the ROC curve (AUC) was calculated by comparing the healthy control cohort to the group with cancer. From these ROC curves, the optimum cutoff CEC value (95% confidence interval [CI]) for diagnosis and prognostication was calculated, together with positive and negative predictive values. For the null hypothesis, AUC = 0.50. Analyses and power calculations were done using SPSS Version 14.0 (SPSS, Inc, Chicago, IL). P < 0.05 was considered statistically significant.
Results
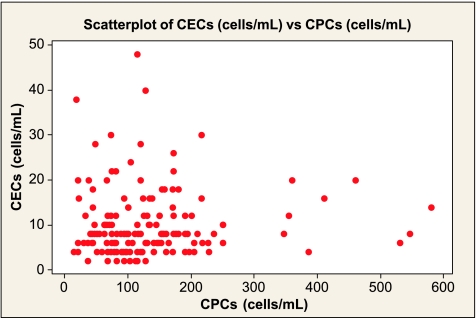
The two groups were matched for all recorded indices (Table 1). Patients with breast cancer had higher plasma vWf, VEGF, and angiogenin (as expected) but had lower sFas-L than the controls (all P < .05), but there was no difference in levels of sFas (Table 2). The CEC count was higher (median, 9.4 cells/ml; IQR, 5.0–12.7) and the CPC count was lower in patients with cancer (median, 121 cells/ml; IQR, 81–186; both P < .05). Within the group with breast cancer, numbers of CECs and CPCs failed to correlate (Spearman r = 0.075, P = .369; Figure 3).
Table 1.
Clinical and Demographics Details of Patients with Breast Cancer and Controls.
| Demographic | All Cancer Patients | Controls Free of Breast Cancer | P | ||
| n | % | n | % | ||
| n | 160 | 100 | 63 | 100 | — |
| Sex | |||||
| Female | 159 | 99.3 | 62 | 98.4 | .49 |
| Male | 1 | 0.7 | 1 | 0.6 | |
| Age (years) | |||||
| Mean (SD) | 62 (12) | — | 60 (7) | — | .37 |
| BMI (kg/m2) | |||||
| Mean (SD) | 28.0 (6.2) | — | 27.8 (5.7) | — | .91 |
| Systolic BP (mm Hg) | |||||
| Mean (SD) | 142 (19.9) | — | 139 (17.3) | — | .52 |
| Smoker | 26 | 16 | 7 | 11 | .20 |
| Ethnicity/race | |||||
| White European | 150 | 94 | 60 | 95 | .60 |
| Afro-Caribbean | 2 | 1.2 | 1 | 1.6 | |
| South Asian | 8 | 4.9 | 2 | 3 | |
All group comparisons used χ2 test, t test, or one-way analysis of variance.
BMI indicates body mass index; BP, blood pressure.
Table 2.
Clinical Details of Patients with Breast Cancer.
| Factor | All Cancer Patients | |
| n | % | |
| n | 160 | 100 |
| Route of diagnosis | ||
| Screening | 73 | 46 |
| Symptom | 97 | 54 |
| Family history | 50 | 31 |
| Multifocal disease | 18 | 11 |
| Tumor type | ||
| DCIS/LCIS | 6 | 4 |
| IDC | 118 | 74 |
| ILC | 19 | 12 |
| Special type | 17 | 10 |
| Tumor size | ||
| T1 (<2 cm) | 83 | 52 |
| T2 (>2 cm, <5 cm) | 57 | 35 |
| T3/T4 (>5 cm, skin, chest) | 20 | 13 |
| Vascular invasion | ||
| +ve | 37 | 23 |
| -ve | 98 | 61 |
| Unknown | 25 | 16 |
| Histologic grade | ||
| Low | 37 | 23 |
| Moderate | 76 | 48 |
| High | 47 | 29 |
| ER/PR status | ||
| ER+ | 127 | 79 |
| ER/PR- | 24 | 15 |
| Unknown | 9 | 6 |
| Nodal status | ||
| -ve | 86 | 54 |
| 1–3 nodes + ve | 39 | 24 |
| >4 nodes + ve | 22 | 14 |
| Unknown | 13 | 8 |
| Her-2 | ||
| +ve | 16 | 10 |
| -ve | 31 | 19 |
| Unknown | 113 | 71 |
| Metastatic | 9 | 6 |
DC indicates invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ER, estrogen receptor; ILC, invasive lobular carcinoma; LCIS, lobular carcinoma in situ; PR, progesterone receptor.
Figure 3.
Correlation scatterplot of the number of CECs and CPCs per milliliter of whole blood in the patients with breast cancer (Spearman r = 0.075, P = .369).
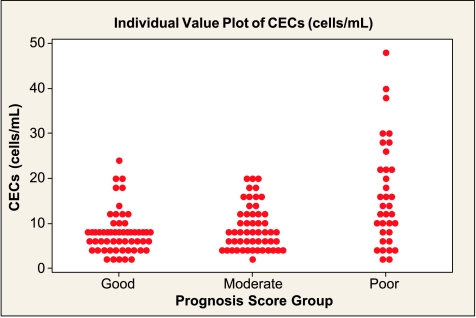
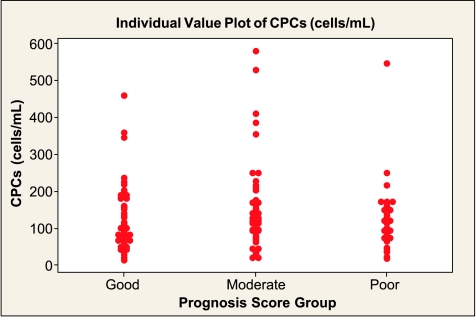
On the basis of the NPI score, CEC levels in the GPG and MPG were comparable to controls but were significantly higher in the PPG (Table 3 and Figure 4). CPC numbers were also significantly different in the PPG compared with controls, but unlike CECs, the values were lower, the worse the prognosis (Table 3 and Figure 5). There were no statistically significant trends in levels of vWf, VEGF, angiogenin, sFas, or sFas-L levels across the three NPI groups (Table 3).
Table 3.
Serum Levels of vWf, VEGF, Angiogenin, Fas-L, and sFas and Numbers of CECs and CPCs in Different Prognostic Groups According to the NPI.
| Variable | Healthy Controls (n = 63) | Breast Cancer NPI Groups (n = 160) | P* | ||
| GPG (n = 58) | MPG (n = 59) | PPG (n = 35) | |||
| vWf (IU/dl) | 116 (108–130) | 130 (120–145) | 123 (107–136) | 140 (124–157) | <.0001† |
| VEGF (pg/ml) | 20 (3–1600) | 135 (10–2175) | 250 (10–2900) | 30 (2–800) | .26 |
| Angiogenin (pg/ml) | 243 (163–326) | 338 (280–420) | 320 (270–390) | 345 (278–475) | <.0001‡ |
| sFas-L (pg/ml) | 182 (114–1128) | 153 (100–433) | 146 (80–338) | 154 (84–435) | .26 |
| sFas (pg/ml) | 1635 (1023–2643) | 1600 (1260–2500) | 1410 (1120–2170) | 1600 (960–2250) | .70 |
| CECs (cells/ml) | 7.7 (6–10) | 8.0 (4–8) | 8.0 (4–12) | 14.0 (8–22) | <.0001§ |
| CPCs (cells/ml) | 169 (106–241) | 113 (73–190) | 132 (96–204) | 120 (72–150) | .004¶ |
Data are median with IQRs in parentheses.
Kruskal-Wallis P value over the four groups. Subgroup analysis was by Dunn test.
HC versus GPG, P < .001; PPG, P < .001; MPG versus PPG, P < .01.
HC versus GPG, MPG, and PPG, all P < .001.
HC versus PPG, P < .001; GPG versus PPG, P < .001; MPG versus PPG, P < .05.
HC versus GPG, P < .05; HC versus PPG, P < .01.
Figure 4.
Number of CECs according to NPI GPG (median [IQR], 8 [4–8] cells/ml), MPG (8 [4–12] cells/ml), and PPG (14 [8–22] cells/ml). The Kruskal-Wallis test reports a significant difference at P < .001 with higher levels in the PPG compared with the other two groups (Tukey post hoc test, P < .05).
Figure 5.
Number of CPCs according to NPI GPG (median [IQR], 113 [73–190] cells/ml), MPG (132 [96-204] cells/ml), and PPG (120 [72–150] cells/ml). The Kruskal-Wallis test reports no significant difference between these three groups (P = .320).
In the univariate analyses of the clinicopathologic factors associated with CECs, age, vascular invasion, tumor grade, lymph node involvement, Her-2+ status (all P < .05), tumor size, and metastasis (both P < .001) were all positively linked, with diagnosis by screening (P < .05) having a negative association. In the multivariate analysis, only vascular invasion (P < .05) and tumor size (P < .001) were associated with CEC count. CPCs did not significantly associate with NPI in a linear regression model, and of the clinicopathologic factors, only age (negative; P < .05) and Her-2 status (positive; P < .05) were associated with CPCs; however, these lost significance after multivariate adjustment.
Table 4 shows the 6- to 8-week change after surgery in 15 women in those indices that were abnormal on baseline assessment. There was no change in the number of CECs, but although there was an increase of 16% in the number of CPCs in the direction of the non-cancer controls, this was not significant. Similarly, the fall in vWf of 8% was not significant.
Table 4.
Levels of Cellular and Plasma Markers Before and After Surgery.
| Variable | Before Surgery | After Surgery | P* |
| CEC (cells/ml) | 10 (8–18) | 10 (8–16) | .93 |
| CPC (cells/ml) | 128 (62–226) | 148 (70–236) | .56 |
| vWf (IU/dl) | 133 (120–141) | 123 (106–137) | .72 |
| VEGF (pg/ml) | 230 (5–2500) | 70 (1–420) | .04 |
| Angiogenin (pg/ml) | 335 (302–400) | 200 (125–310) | .001 |
Data are median with IQRs in parentheses. P values in boldface emphasis are significant.
Wilcoxon signed rank test.
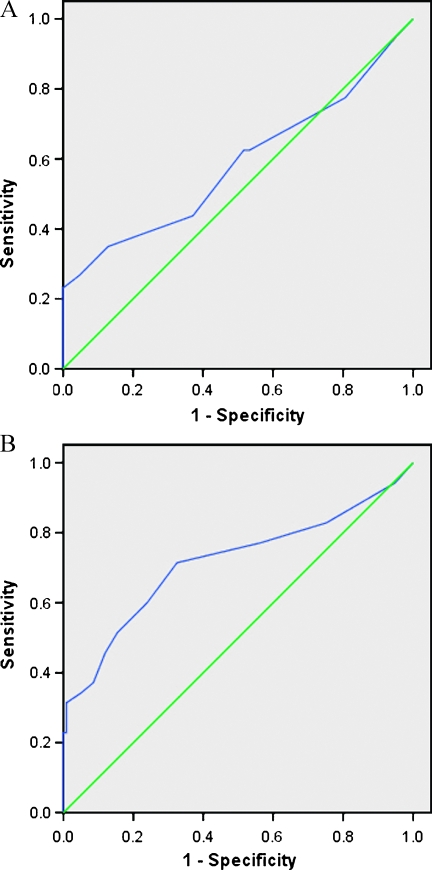
By comparing CECs in the cancer and healthy cohorts (Figure 6A), the AUC was 0.58 (95% CI, 0.51–0.66; P = .05). When CECs were used to predict PPG patients from GPG and MPG (Figure 6B), the AUC was 0.74 (95% CI, 0.63–0.85; P < .0001). A CEC cutoff value of 15/ml or higher provided a positive predictive value of 80% and a negative predictive value of 61% for predicting an NPI higher than 5.4 (i.e., PPG) in an appropriate clinical setting (no confounding factors present).
Figure 6.
Receiver-operator characteristic curves. (A) Predictive value of CECs for the diagnosis of breast cancer. (B) Predictive value of CECs for diagnosing PPG patients (NPI > 5.4).
Discussion
CECs and CPCs are thought to represent novel surrogate markers of vascular disruption, repair, and angiogenesis in health and disease [1,2,4–6]. In cancer, high levels of CECs have been reported [20–24], but as in many cases that levels fail to associate with prognosis or stage, their significance is still undetermined. Goodale et al. [25] reported higher CD146+/CD45- CECs in patients with localized breast cancer compared with metastatic breast cancer and healthy controls. Similarly, there are reports of raised [26] and normal numbers [27] of endothelial progenitor cells (EPCs) in cancer, whereas another [28] reported raised CD34+/FLK-1+ EPCs as a proportion of peripheral blood mononuclear cells in breast cancer that related to tumor size and where levels fell after tumor excision. Naik et al. [29] reported raised CD133+/KDR+ EPCs as a proportion of peripheral blood mononuclear cells in 25 patients with breast cancer that were higher in the most adverse disease stage. Our finding of lower CD34+/CD133+ EPCs in breast cancer counters these reports, but we also report altered CECs in breast cancer with some relationships with some tumor indices, but no relationship with plasma markers of vascular integrity [17], apoptosis [7,18,19], and angiogenic growth factors [14,30]. However, although CECs had the strongest relationship with the NPI, numbers did not fall 6 to 8 weeks after tumor excision, although levels of angiogenic growth factors did improve.
A continuing problem in drawing common themes is use of different CD molecules in cell definition [9–12,19–29]. Although CD146 is found nonspecifically on endothelial cells (including those within tumors [31]), some cancer cells (e.g., melanoma, where it is known as MUC18, and other cancers [32–34]), circulating EPCs and some leukocytes, in analysis many workers use CD45 to exclude the latter [1,9,35]. We colabeled with CD34 because it is reported to be present on endothelial progenitors as well as on hemopoietic progenitors [9,36,37], and CD34+ cells alone can repopulate bone marrow in vivo [38]. CD133 is a more immature hemopoietic stem cell marker, possibly at the level of the hemangioblast, defining cells which can differentiate to endothelial cells in vitro [8,36], whereas VEGFR2 (also known as KDR) is an endothelial marker [36,39]. Thus, CD34+/CD133+ cells more likely reflect immature progenitor cells [8,40], which we are taking to be CPCs. Clearly, multiple phenotypes exist, and different cells can coexpress alternative CD molecules according to their differentiation status [35–41].
The NPI [13] finds the combination of lymph node stage, tumor size, and pathologic grade to be superior to lymph node stage alone in predicting survival, although the latter is still important [42]. Our data suggest a relationship between these CECs and overall tumor vascular bulk. As tumors get progressively larger, this is mirrored by an increase in total vascular volume, and we hypothesize that this provides an opportunity for more CECs to enter the circulation. This is interesting because our study demonstrates that the chief independent tissue predictor for CEC levels is tumor size and vascular invasion, with nodal stage and grade losing significance after adjustment. Of note, other predictors of prognosis (e.g., estrogen receptor positivity) had no effect on either CEC or CPC levels, whereas patients whose conditions were diagnosed through screening clinics have lower CECs and possibly reflect a biologic difference between symptomatic versus screen-detected cancer implied previously [43]. Smoking has a negative association with NPI score in this study even after correction; large studies have shown no additional breast cancer risk with smoking [44], and it is notable that smoking may influence EPC biology [40,45].
Tumors are reliant on, and/or may also secrete, angiogenic factors such as VEGF and angiogenin, and raised plasma levels and tissue expression of these molecules are present in certain stages of cancer [14,15,46–49]. Furthermore, VEGF may stimulate the release of CECs and/or EPCs [50]. We were therefore surprised to find that levels of VEGF neither related the prognosis grouping nor correlated with either cell type [23]. This is certainly not due to lack of statistical power, although it may reflect relatively moderate disease or other pathophysiological processes. Similarly, generalized plasma endothelial cell marker vWf [17] failed to relate to prognosis group; hence, raised levels may simply reflect nonspecific pathophysiological changes. Also against expectation was the failure to find changes in CECs and CPCs after surgery, although we did find the expected improvements in VEGF and angiogenin [50,51]. However, others have found that CECs were a good marker of response to chemotherapy [52]. Measurement of sFas and sFas-L (reflecting apoptosis) [53] was, with the exception of low sFas-L in the group with cancer, uninformative.
In this study, the diagnostic accuracy of CECs for distinguishing patients with primary breast cancer from healthy subjects was “poor,” which probably reflects the fact that most of the patients were in the early stages of the disease, as well as the relatively nonspecific nature of CECs. However, CECs had a “moderate” diagnostic accuracy when used to diagnose the conditions of patients with an NPI higher than 5.4 (PPG), when a cutoff point of CEC = 15/ml was chosen. The use of CECs as a diagnostic or predictive marker has been reported previously in cardiovascular disease to predict positive myocardial infarction [54]. Their use in predicting PPG patients with a degree of accuracy is significant for a number of reasons: it may herald a new and easier way to identify such high-risk patients earlier because NPI scoring can only be achieved after the final surgical histologic finding, whereas CEC measurements can be obtained potentially on the day of diagnosis. This might lead to earlier decision making for such patients requiring adjuvant treatment such as chemotherapy.
There are several limitations to this study. Despite efforts to minimize confounding factors by careful screening of patients and controls, it is possible that other factors remain and have not been adequately controlled for. The definitions of CPCs and CECs are evolving [55], and a consensus is yet to be formalized not merely because of the intrinsic difficulties of flow cytometry. Therefore, our descriptions of these cells may not be universally applicable and makes comparisons with other published work difficult [56]. Indeed, Mancuso et al. [57] have offered a protocol that, if widely accepted, will reduce interlaboratory variability in the definition of CPCs and CECs and perhaps initiate a multicenter study using common CD molecules as standard. Multicenter clinical outcome based on adequate follow-up of these patients is still pending and will be considered when taking into account prognostication and validation work. However, data suggesting that CECs may be useful as a marker of clinical response to chemotherapy are becoming available [58,59]. Accordingly, we believe that the value of CECs is perhaps more useful as a biologic marker of tumor vascular status, and possible of response to treatment, rather than a long-term clinical prognostic marker, and may therefore have potential as a marker of response to antiangiogenic treatment.
Acknowledgments
The authors thank the Research and Development Committees of the Sandwell and West Birmingham NHS Trust and Dudley Group of Hospitals NHS Trust for their support of the Haemostasis, Thrombosis and Vascular Biology Unit.
References
- 1.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 2.Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wojakowski W, Kucia M, Kaźmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 4.Ergün S, Hohn HP, Kilic N, Singer BB, Tilki D. Endothelial and hematopoietic progenitor cells (EPCs and HPCs): hand in hand fate determining partners for cancer cells. Stem Cell Rev. 2008;4:169–177. doi: 10.1007/s12015-008-9028-y. [DOI] [PubMed] [Google Scholar]
- 5.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 6.Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, Lang G, Dome P, Bogos K, Tovari J. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: from biology to therapy. Crit Rev Oncol Hematol. 2009;69:108–124. doi: 10.1016/j.critrevonc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Sun T, Xue L, Han X, Zhang B, Lu N, Shi Y, Tan W, Zhou Y, Zhao D, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–1073. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 8.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 9.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 11.Fan CL, Li Y, Gao PJ, Liu JJ, Zhang XJ, Zhu DL. Differentiation of endothelial progenitor cells from human umbilical cord blood CD34+ cells in vitro. Acta Pharmacol Sin. 2003;24:212–218. [PubMed] [Google Scholar]
- 12.Aoki M, Yasutake M, Murohara T. Derivation of functional endothelial progenitor cells from human umbilical cord blood mononuclear cells isolated by a novel cell filtration device. Stem Cells. 2004;22:994–1002. doi: 10.1634/stemcells.22-6-994. [DOI] [PubMed] [Google Scholar]
- 13.Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, Nicholson RI, Griffiths K. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GYH. Plasma angiopoietin-1, angiopoietin-2 and tie-2 in breast and prostate cancer: a comparison with VEGF and flt-1. Eur J Clin Invest. 2003;33:883–890. doi: 10.1046/j.1365-2362.2003.01243.x. [DOI] [PubMed] [Google Scholar]
- 15.Duranyildiz D, Camlica H, Soydinc HO, Derin D, Yasasever V. Serum levels of angiogenic factors in early breast cancer remain close to normal. Breast. 2009;18:26–29. doi: 10.1016/j.breast.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–290. [PubMed] [Google Scholar]
- 17.Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49–55. [PubMed] [Google Scholar]
- 18.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 19.Boos CJ, Balakrishnan B, Blann AD, Lip GY. The relationship of circulating endothelial cells to plasma indices of endothelial damage/dysfunction and apoptosis in acute coronary syndromes: implications for prognosis. J Thromb Haemost. 2008;6:1841–1850. doi: 10.1111/j.1538-7836.2008.03148.x. [DOI] [PubMed] [Google Scholar]
- 20.Goon PKY, Boos CJ, Stonelake PS, Blann AD, Lip GYH. Detection and quantification of mature circulating endothelial cells using flow cytometry and immunomagnetic beads: a methodological comparison. Thromb Haemost. 2006;96:45–52. doi: 10.1160/TH06-04-0185. [DOI] [PubMed] [Google Scholar]
- 21.Goon PKY, Watson T, Shantsila E, Boos CJ, Lip GYH. Standardization of circulating endothelial cell enumeration by the use of human umbilical vein endothelial cells. J Thromb Haemost. 2007;5:870–872. doi: 10.1111/j.1538-7836.2007.02411.x. [DOI] [PubMed] [Google Scholar]
- 22.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable of circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 24.Fürstenberger G, von Moos R, Lucas R, Thürlimann B, Senn HJ, Hamacher J, Boneberg EM. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodale D, Phay C, Brown W, Gray-Statchuk L, Furlong P, Lock M, Chin-Yee I, Keeney M, Allan AL. Flow cytometric assessment of monocyte activation markers and circulating endothelial cells in patients with localized or metastatic breast cancer. Cytometry B Clin Cytom. 2009;76:107–117. doi: 10.1002/cyto.b.20449. [DOI] [PubMed] [Google Scholar]
- 26.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 27.Kim HK, Song KS, Kim HO, Chung JH, Lee KR, Lee YJ, Lee DH, Lee ES, Kim HK, Ryu KW, et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198:83–88. doi: 10.1016/s0304-3835(03)00268-4. [DOI] [PubMed] [Google Scholar]
- 28.Richter-Ehrenstein C, Rentzsch J, Runkel S, Schneider A, Schönfelder G. Endothelial progenitor cells in breast cancer patients. Breast Cancer Res Treat. 2007;106:343–349. doi: 10.1007/s10549-007-9505-z. [DOI] [PubMed] [Google Scholar]
- 29.Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL, Christos PJ, de Dalmas T, Donovan D, Rafii S, et al. Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat. 2008;107:133–138. doi: 10.1007/s10549-007-9519-6. [DOI] [PubMed] [Google Scholar]
- 30.Burstein HJ, Chen YH, Parker LM, Savoie J, Younger J, Kuter I, Ryan PD, Garber JE, Chen H, Campos SM, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 2008;14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 31.Duda DG, Cohen KS, di Tomaso E, Au P, Klein RJ, Scadden DT, Willett CG, Jain RK. Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol. 2006;24:1449–1453. doi: 10.1200/JCO.2005.04.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GJ, Peng Q, Fu P, Wang SW, Chiang CF, Dillehay DL, Wu MW. Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene. 2004;327:201–213. doi: 10.1016/j.gene.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Kristiansen G, Yu Y, Schlüns K, Sers C, Dietel M, Petersen I. Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal Cell Pathol. 2003;25:77–81. doi: 10.1155/2003/574829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia S, Dalès JP, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, Andonian C, Lavaut MN, Allasia C, Bonnier P, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Jacques N, Vimond N, Conforti R, Griscelli F, Lecluse Y, Laplanche A, Malka D, Vielh P, Farace F. Quantification of circulating mature endothelial cells using a whole blood four-color flow cytometric assay. J Immunol Methods. 2008;337:132–143. doi: 10.1016/j.jim.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 37.Delorme B, Basire A, Gentile C, Sabatier F, Monsonis F, Desouches C, Blot-Chabaud M, Uzan G, Sampol J, Dignat-George F. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–1279. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 38.Brugger W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Reconstitution of hematopoiesis after high-dose chemotherapy by autologous progenitor cells generated ex vivo. N Engl J Med. 1995;333:283–287. doi: 10.1056/NEJM199508033330503. [DOI] [PubMed] [Google Scholar]
- 39.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 40.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vroling L, Yuana Y, Schuurhuis GJ, van Hinsbergh VW, Gundy C, de Haas R, van Cruijsen H, Boven E, Hoekman K, Broxterman HJ. VEGFR2 expressing circulating (progenitor) cell populations in volunteers and cancer patients. Thromb Haemost. 2007;98:440–450. [PubMed] [Google Scholar]
- 42.Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Mazzarol G, Pruneri G, Luini A, Intra M, Veronesi P, Galimberti V, et al. Size of breast cancer metastases in axillary lymph nodes: clinical relevance of minimal lymph node involvement. J Clin Oncol. 2005;23:1379–1389. doi: 10.1200/JCO.2005.07.094. [DOI] [PubMed] [Google Scholar]
- 43.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screendetected cancers. J Natl Cancer Inst. 1999;91:2020–2028. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 44.London SJ, Colditz GA, Stampfer MJ, Willett WC, Rosner BA, Speizer FE. Prospective study of smoking and the risk of breast cancer. J Natl Cancer Inst. 1989;81:1625–1631. doi: 10.1093/jnci/81.21.1625. [DOI] [PubMed] [Google Scholar]
- 45.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 46.Capillo M, Mancuso P, Gobbi A, Monestiroli S, Pruneri G, Dell'Agnola C, Martinelli G, Shultz L, Bertolini F. Continuous infusion of endostatin inhibits differentiation, mobilization and clonogenic potential of endothelial cell progenitors. Clin Cancer Res. 2003;9:377–382. [PubMed] [Google Scholar]
- 47.Eppenberger U, Kueng W, Schlaeppi JM, Roesel JL, Benz C, Mueller H, Matter A, Zuber M, Luescher K, Litschgi M, et al. Markers of tumor angiogenesis and proteolysis independently define high- and low-risk subsets of node-negative breast cancer patients. J Clin Oncol. 1998;16:3129–3136. doi: 10.1200/JCO.1998.16.9.3129. [DOI] [PubMed] [Google Scholar]
- 48.Caine GJ, Lip G, Zanetto U, Maheshwari M, Stonelake PS, Blann AD. A comparison of plasma versus histologic indices of angiogenic markers in breast cancer. Appl Immunohistochem Mol Morphol. 2007;15:382–388. doi: 10.1097/01.pai.0000213137.01536.ca. [DOI] [PubMed] [Google Scholar]
- 49.Sheen-Chen SM, Eng HL, Chen WJ, Chou FF, Chen HS. Serum level of angiogenin in breast cancer. Anticancer Res. 2000;20:4769–4771. [PubMed] [Google Scholar]
- 50.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caine GJ, Stonelake PS, Lip GY, Blann AD. Changes in plasma vascular endothelial growth factor, angiopoietins, and their receptors following surgery for breast cancer. Cancer Lett. 2007;248:131–136. doi: 10.1016/j.canlet.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 53.Mollinedo F, Gajate C. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist Updat. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 55.Pearson JD. Endothelial progenitor cells—hype or hope? J Thromb Haemost. 2009;7:255–262. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 56.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancuso P, Antoniotti P, Quarna J, Calleri A, Rabascio C, Tacchetti C, Braidotti P, Wu HK, Zurita AJ, Saronni L, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 58.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torrisi R, Bagnardi V, Cardillo A, Bertolini F, Scarano E, Orlando L, Mancuso P, Luini A, Calleri A, Viale G, et al. Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER- and/or PgR-positive breast cancer: clinical and biological activity. Br J Cancer. 2008;99:1564–1571. doi: 10.1038/sj.bjc.6604741. [DOI] [PMC free article] [PubMed] [Google Scholar]