Abstract
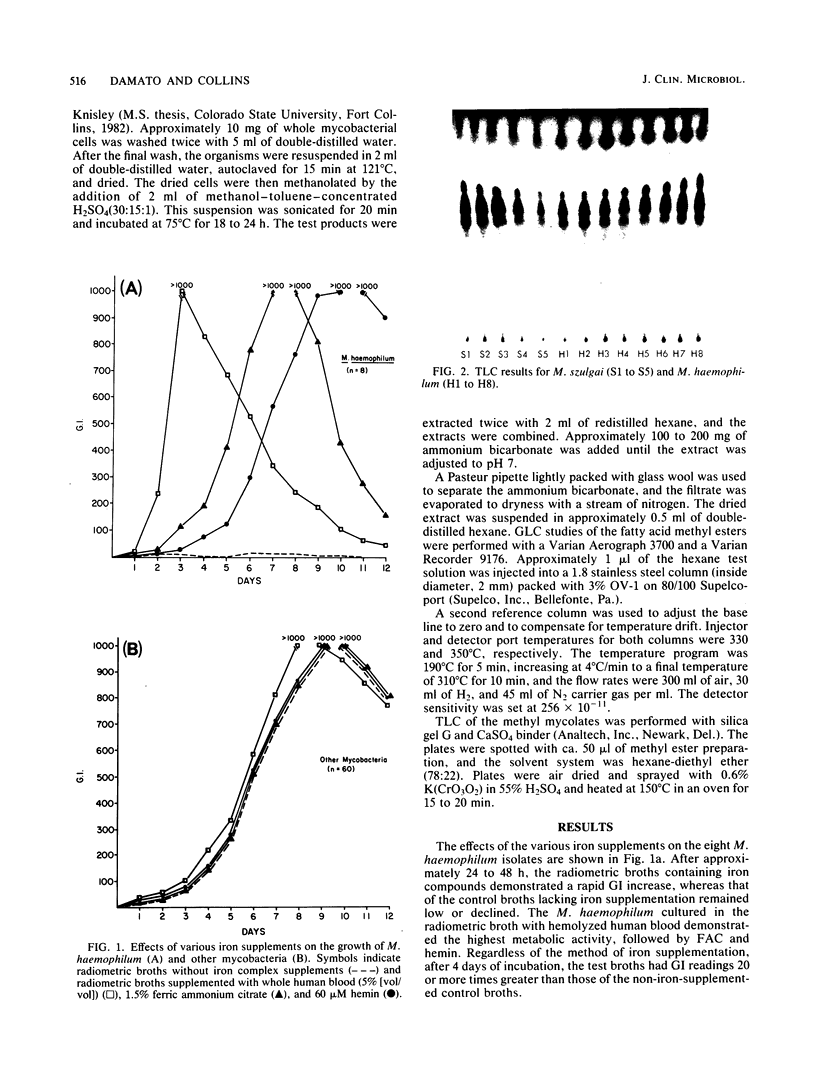
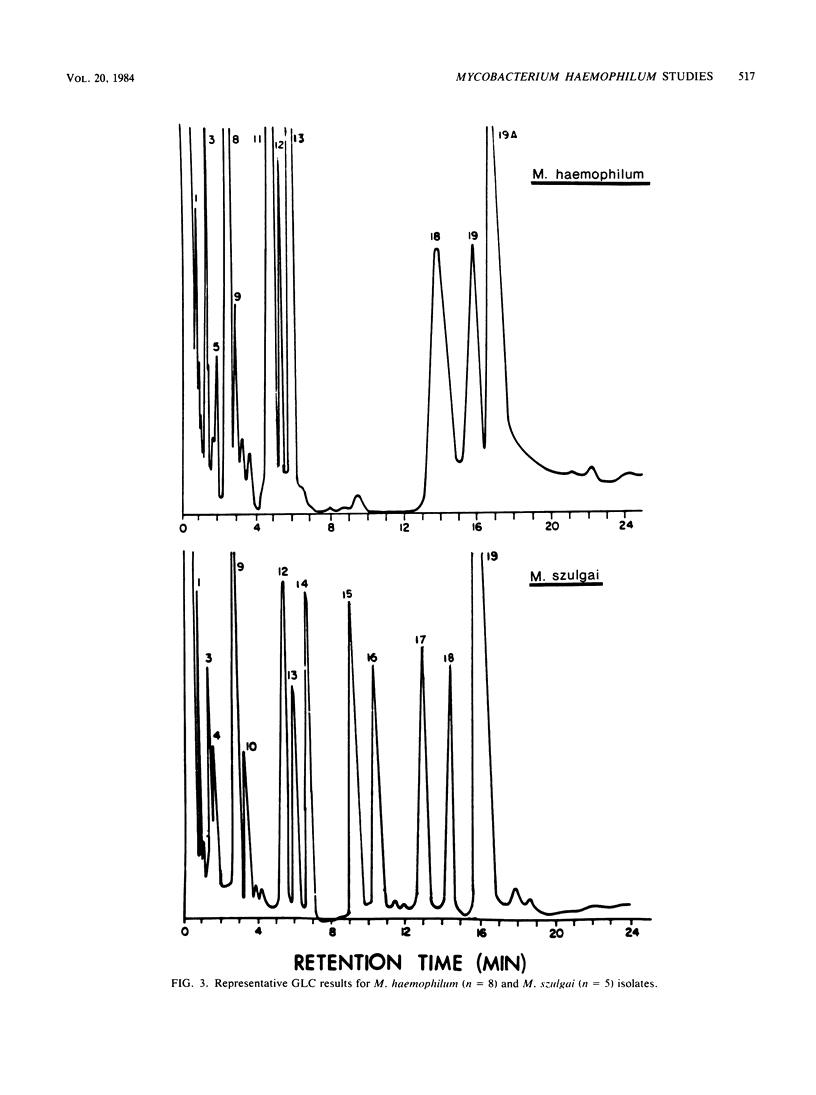
Eight isolates of Mycobacterium haemophilum were evaluated by radiometric methods to determine whether this test system could support the growth of these organisms as well as demonstrate their growth requirements for iron complexes such as hemin, ferric ammonium citrate, and blood. In addition, gas-liquid and thin-layer chromatography were evaluated to determine whether these procedures could further differentiate M. haemophilum from other mycobacteria. During the initial 24 to 48 h, there was no significant difference between the radiometric test broths containing iron complexes and control broths without iron supplementation. After 48 h, the test growth index readings rapidly increased, and control broth readings leveled off and declined. The mean growth index reading of the test broths after 6 days of incubation was 100 times that of the controls. The mean incubation time with supplemented 7H10 agar was 17 days. The use of radiometric media resulted in the demonstration of hemin dependence by M. haemophilum significantly earlier than with 7H10 agar. Of the three supplements studied, whole blood provided the greatest growth rate, followed by ferric ammonium citrate and hemin. When 12 species of mycobacteria other than M. haemophilum were radiometrically evaluated, no isolate demonstrated an iron complex requirement. Gas-liquid and thin-layer chromatography procedures were able to rapidly differentiate M. haemophilum from the other 12 Mycobacterium species.
Full text
PDF

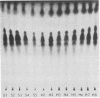
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damato J. J., Collins M. T., Rothlauf M. V., McClatchy J. K. Detection of mycobacteria by radiometric and standard plate procedures. J Clin Microbiol. 1983 Jun;17(6):1066–1073. doi: 10.1128/jcm.17.6.1066-1073.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. J., Blacklock Z. M., Kane D. W. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med J Aust. 1981 Sep 19;2(6):289–290. doi: 10.5694/j.1326-5377.1981.tb128321.x. [DOI] [PubMed] [Google Scholar]
- Dawson D. J., Jennis F. Mycobacteria with a growth requirement for ferric ammonium citrate, identified as Mycobacterium haemophilum. J Clin Microbiol. 1980 Feb;11(2):190–192. doi: 10.1128/jcm.11.2.190-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertcher J. A., Chen M. F., Charache P., Hwangbo C. C., Camargo E. E., McIntyre P. A., Wagner H. N., Jr Rapid radiometric susceptibility testing of Mycobacterium tuberculosis. Am Rev Respir Dis. 1978 Apr;117(4):631–637. doi: 10.1164/arrd.1978.117.4.631. [DOI] [PubMed] [Google Scholar]
- Middlebrook G., Reggiardo Z., Tigertt W. D. Automatable radiometric detection of growth of Mycobacterium tuberculosis in selective media. Am Rev Respir Dis. 1977 Jun;115(6):1066–1069. doi: 10.1164/arrd.1977.115.6.1066. [DOI] [PubMed] [Google Scholar]
- Moulsdale M., Harper J. M., Thatcher G. N. Unusual mycobacterium infection in a renal transplant recipient. Med J Aust. 1980 Oct 18;2(8):450–451. doi: 10.5694/j.1326-5377.1980.tb131920.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Lagziel A., Rosenberg I. Further studies of a new pathogenic mycobacterium (M. haemophilum sp. nov.). Can J Microbiol. 1979 Feb;25(2):217–226. doi: 10.1139/m79-033. [DOI] [PubMed] [Google Scholar]
- Walder B. K., Jeremy D., Charlesworth J. A., Macdonald G. J., Pussell B. A., Robertson M. R. The skin and immunosuppression. Australas J Dermatol. 1976 Dec;17(3):94–97. doi: 10.1111/j.1440-0960.1976.tb00798.x. [DOI] [PubMed] [Google Scholar]