Abstract
The clinical implications of HER-2/neu (HER2) expression in ductal carcinoma in situ (DCIS) lesions have yet to be clearly elucidated; this despite the more frequent expression of HER2 in high-grade DCIS lesions compared with invasive cancers. We hypothesized that HER2 overexpression in DCIS is associated with more rapid progression to invasive disease. Immunohistochemical staining for estrogen receptor, progesterone receptor, and HER2 was done on DCIS specimens. Univariate analysis and a multivariate logistic regression were done to determine whether estrogen receptor, progesterone receptor, or HER2 status, comedo necrosis, nuclear grade, lesion size, or patient age predicted the presence of associated invasive disease in patients with DCIS. Invasive foci were found in association with HER2 overexpressing DCIS at a higher frequency than with DCIS that did not overexpress HER2. Although high nuclear grade, large lesion size, and HER2 overexpression were all associated with the presence of invasive disease on univariate analysis, HER2 was the only significant predictor for the presence of invasive disease after multivariate adjustment (odds ratio, 6.4; P = 0.01). These data indicate that HER2 overexpression in DCIS lesions predicts the presence of invasive foci in patients with DCIS and suggest that targeting of HER2 in an early disease setting may forestall or prevent disease progression.
Introduction
Ductal carcinoma in situ (DCIS) now accounts for > 20% of all newly diagnosed breast cancers in the United States (1). If untreated, many of these lesions will progress to invasive cancer; however, the frequent heterogeneity of DCIS (both with regards to nuclear grade and expression of surface receptors) obfuscates efforts to distinguish truly premalignant from more dormant in situ disease (2). Despite its prognostic value in invasive cancer, the clinical implications of HER-2/neu (HER2) expression in DCIS lesions have yet to be clearly elucidated; this despite the more frequent expression of HER2 in high-grade DCIS lesions compared with invasive cancers (3).
We hypothesized that HER2 overexpression in DCIS lesions predicts more rapid progression to invasive disease. To compare the behavior of DCIS lesions with different phenotypes, we studied patients who were diagnosed with DCIS, either by core needle or needle localization surgical biopsy. Biopsy specimens were subjected to immunohistochemical staining for HER2, estrogen receptor (ER), and progesterone receptor (PR) expression. The frequencies at which invasive foci were subsequently found on final pathology in association with DCIS with different HER2, ER, and PR expression patterns were compared. Univariate analysis and a multivariate logistic regression were done to determine whether ER, PR, or HER2 status, comedo necrosis, nuclear grade, lesion size, or patient age predicted the presence of associated invasive disease.
Patients and Methods
One hundred and six patients diagnosed with DCIS by either core needle or excisional biopsies were studied between March 2003 and July 2007. All patients underwent magnetic resonance imaging and those with findings suggesting the presence of an infiltrating carcinoma >1 cm in diameter (and therefore, more advanced disease) were excluded. Immunohistochemical staining of biopsy specimens for HER2, ER, and PR expression was prospectively done in conjunction with an internal review board–approved clinical trial of an HER2-targeted vaccine at our institution (4). The size of DCIS lesions was estimated by measuring the area of calcifications on mammogram, or the area of enhancement on magnetic resonance imaging for mammographically occult lesions.
Specimens were characterized as high nuclear grade if they contained areas of high-grade disease. Specimens that did not contain high-grade disease were characterized as low or intermediate grade DCIS. Specimens were also assessed for the presence of comedo necrosis. All biopsy materials were immunostained with ER, PR test kits, and HercepTest (DAKO) and evaluated under the microscope by a single pathologist (PJZ). An Allred score of ER and PR nuclear immunoreactivity less than or equal to three was considered a positive result. Membrane 3+ HER2 staining was considered positive, as was membrane 2+ HER2 staining in >10% of tumor cells with fluorescence in situ hybridization evidence of HER2 gene amplification. Lesions were characterized as luminal A (ER+ or PR+, HER2−), luminal B (ER+ or PR+, HER2+), HER2 positive (ER− and PR−, HER2+), or basal like (ER−, PR−, and HER2−) following the classification system proposed by Perou (5).
Definitive resection specimens were evaluated for the presence of infiltrating disease. Univariate associations between the presence of invasive disease and ER, PR, and HER2 expression, patient age, tumor size (<3 cm versus ≥3 cm), presence of comedo necrosis, and grade (high versus intermediate or low) were assessed using χ2, Fisher’s exact and t tests as appropriate. A multivariate logistic regression was done to evaluate the association between HER2 overexpression and invasion, adjusting for the previously described characteristics. Rates of invasive disease associated with DCIS lesions with different phenotypes were also compared using Fisher’s exact tests.
Results
The mean patient age was 53.4 years. Sixty-six patients (62%) had high-grade disease; the remainder had low-or intermediate-grade DCIS. Comedo necrosis was present in 75 cases (71%). HER2 overexpression was seen in 39 cases (37%) and 32 of the 66 high-grade lesions (48%). ER and PR expression were seen in 77 cases (73%) and 67 cases (63%), respectively. Thirty-one lesions (29.2%) were >3 cm in largest dimension (Table 1). Seventy-three patients were diagnosed with DCIS by core needle biopsy. The remaining 33 patients were diagnosed by needle localization biopsy. The mean size of DCIS lesions diagnosed by core needle and needle localization biopsy were 2.6 cm and 1.7 cm, respectively.
Table 1.
Univariate analysis
| Variable | Total n (%) | DCIS only n (%) | DCIS with invasion n (%) | Odds ratio | P |
|---|---|---|---|---|---|
| Age (y) | 0.09 | ||||
| Mean (95% CI) | 53.4 (50.9, 55.8) | 54.5 (51.6, 57.3) | 49.5 (46.0, 53.1) | 0.96 | |
| Grade | 0.04 | ||||
| Low/intermediate | 40 (37.7) | 36 (90.0) | 4 (10.0) | 1 | |
| High | 66 (62.3) | 48 (72.7) | 18 (27.3) | 3.38 | |
| Comedo necrosis | 0.45 | ||||
| Absent | 31 (29.3) | 26 (83.9) | 5 (16.1) | 1 | |
| Present | 75 (70.7) | 58 (77.3) | 17 (22.7) | 1.52 | |
| HER-2 | 0.001 | ||||
| Negative | 67 (63.2) | 60 (89.6) | 7 (10.4) | 1 | |
| Positive | 39 (36.8) | 24 (61.5) | 15 (38.5) | 5.35 | |
| ER | 0.11 | ||||
| Negative | 29 (27.4) | 20 (69.0) | 9 (31.0) | 1 | |
| Positive | 77 (72.6) | 64 (83.1) | 13 (16.9) | 0.45 | |
| PR | 0.21 | ||||
| Negative | 37 (34.9) | 26 (70.3) | 11 (29.7) | 1 | |
| Positive | 67 (63.2) | 56 (83.6) | 11 (16.4) | 0.46 | |
| Unknown | 2 (1.9) | 2 (100.0) | 0 (0.00) | ||
| Size | 0.01 | ||||
| 3 cm | 66 (62.3) | 57 (86.4) | 9 (13.6) | 1 | |
| ≥3 cm | 31 (29.2) | 19 (61.3) | 12 (38.1) | 4.0 | |
| Unknown | 9 (8.5) | 8 (88.9) | 1 (11.1) |
Abbreviation: 95% CI, 95% confidence interval.
Twenty two patients (21%) were found to have invasive disease on final pathology. Invasive lesions ranged in size from 0.05 to 1.5 cm in greatest dimension. The average invasive lesion size was 0.6 cm. In three cases, multiple foci of microinvasion were noted. Twenty-four of the studied patients received a HER2 targeted vaccine after diagnosis of DCIS and before definitive surgery. Invasive disease was found in association with DCIS in a similar percentage of vaccinated (32%) and unvaccinated HER2+ patients (39%).
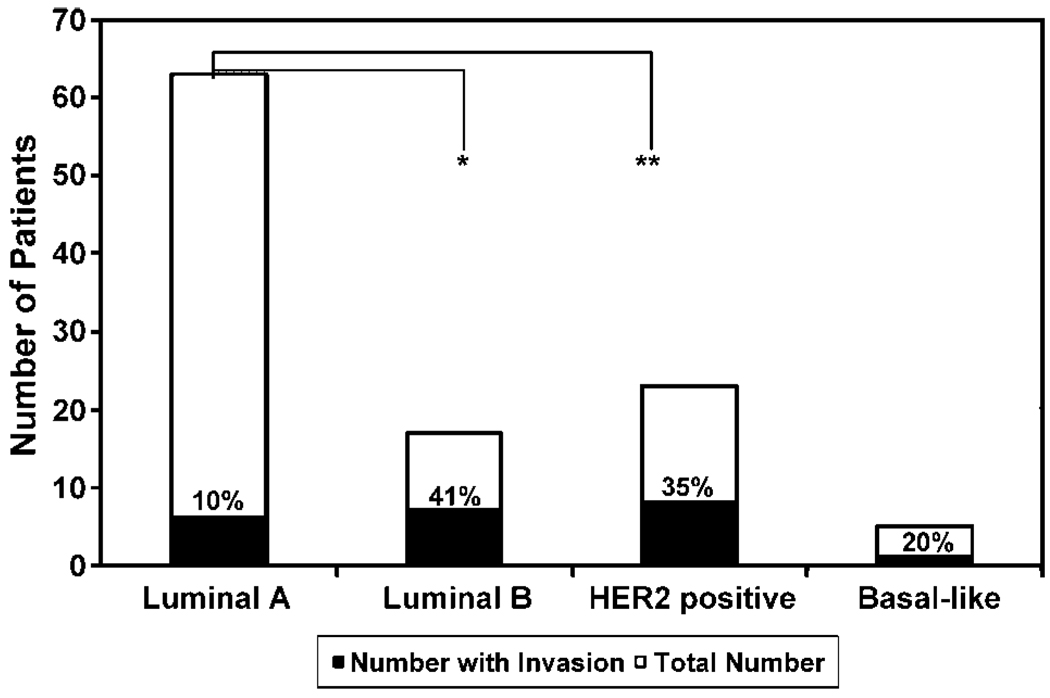
Univariate analysis revealed significant associations between invasion and high nuclear grade, HER2 overexpression, and tumor size of >3 cm (Table 1). Of these associations, only that with HER2 overexpression remained significant after multivariate adjustment (Table 2); specifically, DCIS lesions that overexpressed HER2 were over 6 times as likely to be associated with invasive disease than were DCIS lesions in which HER2 overexpression was not present (odds ratio, 6.4; P = 0.01). Luminal A was the most common tumor phenotype and accounted for 59% of lesions. Associated invasive disease was identified in 6 patients with such lesions (10%). The HER2-positive phenotypes, HER2 positive and luminal B, accounted for 21% and 17% of lesions, respectively. Invasive disease was seen in association with HER2 positive DCIS in 8 of 23 cases (35%) and with luminal B DCIS in 7 of 17 cases (41%). As noted in other studies, basal-like lesions were uncommon; only four patients with such lesions were identified (6). Rates of invasion differed significantly between groups (Fig. 1).
Table 2.
Multivariate logistic regression
| Variable | Odds ratio | P |
|---|---|---|
| HER-2 | ||
| Negative | 1 | Reference |
| Positive | 6.44 | 0.01 |
| ER+ | ||
| Negative | 1 | Reference |
| Positive | 0.84 | 0.84 |
| PR+ | ||
| Negative | 1 | Reference |
| Positive | 2.61 | 0.32 |
| Grade | ||
| Low/Intermediate | 1 | Reference |
| High | 2.98 | 0.147 |
| Comedo Necrosis | ||
| No | 1 | Reference |
| Yes | 0.59 | 0.455 |
| Size | ||
| <3 cm | 1 | Reference |
| ≥3 cm | 2.80 | 0.084 |
| Age | 0.95 | 0.129 |
Figure 1.
Rates of synchronous invasion in patient with different HER2/ER/PR expression patterns. The number of patients with invasive disease is shown (black bars) as a fraction of the total number of patients (white bars) with a given receptor expression pattern. Groups are defined as follows: luminal A (ER+or PR+, HER2−), luminal B (ER+ or PR+, HER2+), HER2 positive (ER− and PR−, HER2+), or basal-like (ER−, PR− and HER2−). A significant difference was noted between groups; *, 0.002; **, 0.004.
Discussion
A number of studies have aimed to identify factors that predict the presence of invasive disease in patients diagnosed with DCIS (7, 8), but few have included HER2 in their analyses. Although DCIS phenotypes similar to those seen in invasive disease have been previously identified (9), the clinical implications of these phenotypes in early disease have, likewise, infrequently been considered. Interestingly, in our series, multivariate logistic regression suggested that HER2 overexpression is a more powerful predictor for the presence of invasion than are size or high nuclear grade; factors associated with invasion in other series (7, 8). Moreover, tumors with HER2-overexpressing phenotypes were more likely to harbor invasive foci than were luminal A tumors.
Preclinical work associating HER2 signaling with tumor cell migration (10) and the expression of proangiogenic factors (11) and cyclooxygenase-2 (12) suggests a potentially significant role in inducing invasion or the elaboration of a stroma that supports tumor growth. Despite these findings, it has been previously suggested that the increased rate of HER2 overexpression in DCIS compared with invasive breast cancer reflects less aggressive biology of HER2 overexpressing DCIS (13, 14). Moreover, the low frequency of HER2 expression in advanced disease has been cited as evidence that HER2 down-regulation is an epiphenomenon of disease progression (14). An alternative explanation for the more frequent expression of HER2 in early disease, however, emerges from this study. HER2 expression may be characteristic of tumors at a discreet stage of pathogenesis and may represent a transient phenomenon. Specifically, HER2 may be up-regulated as in situ tumors progress to invasive disease and down-regulated again in more advanced tumors. The close association between HER2-overexpressing phenotypes and early invasive lesions seen in this study supports this latter explanation as does the occasional discordance in HER2 expression between in situ and associated invasive foci that has been observed in other studies (15).
If validated through additional study, the implications of this study are broad and highlight the power of diagnostic studies that identify pathogenic molecular mechanisms. The clustering of invasive foci in patients with HER2-overexpressing tumors suggests a disease pattern hinted at in other studies; specifically, HER2 expression may reflect an important pathway through which DCIS lesions may progress toward invasion. Although efforts to target early carcinomas that over-express HER2 with novel therapies are in an early phase of development, such strategies may ultimately allow for the prevention of many aggressive malignancies. Conversely, investigators must be cognizant that a substantial percentage of patients with HER2 overexpressing DCIS harbor invasive disease and must carefully weigh the potential benefits of a novel treatment approach against the risk of delaying conventional therapy in this group. As the molecular mechanisms underlying disease pathogenesis are more clearly delineated, novel molecular markers with prognostic value will play a more prominent role in guiding therapeutic approaches.
Acknowledgments
Grant support: NIH (R01-CA096997-02).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 2.Allred DC, Wu Y, Mao S, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–378. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- 3.Allred DC, Clark GM, Molina R, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23:974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- 4.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–621. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- 7.Huo L, Sneige N, Hunt KK, Albarracin CT, Lopez A, Resetkova E. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer. 2006;107:1760–1768. doi: 10.1002/cncr.22216. [DOI] [PubMed] [Google Scholar]
- 8.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–526. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf-Yadlin A, Kumar N, Zhang Y, et al. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol Syst Biol. 2006;2:54. doi: 10.1038/msb4100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen XF, Yang G, Mao W, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 12.Subbaramaiah K, Howe LR, Port ER, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 13.Mylonas I, Makovitzky J, Jeschke U, Briese V, Friese K, Gerber B. Expression of Her2/neu, steroid receptors (ER and PR), Ki67 and p53 in invasive mammary ductal carcinoma associated with ductal carcinoma In Situ (DCIS) Versus invasive breast cancer alone. Anticancer Res. 2005;25:1719–1723. [PubMed] [Google Scholar]
- 14.Hoque A, Sneige N, Sahin AA, et al. Her-2/neu gene amplification in ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2002;11:587–590. [PubMed] [Google Scholar]
- 15.Layfield LJ, Lewis C. In situ and invasive components of mammary adenocarcinoma: comparison of Her-2/neu status. Anal Quant Cytol Histol. 2007;29:239–243. [PubMed] [Google Scholar]