Abstract
Light adaptation in vertebrate photoreceptors is mediated by multiple mechanisms, one of which could involve nuclear feedback and changes in gene expression. Therefore, we have investigated light adaptation-associated changes in gene expression using microarrays and real-time PCR in isolated photoreceptors, in cultured isolated retinas and in acutely isolated retinas. In all three preparations after 2 h of an exposure to a bright light, we observed an up-regulation of almost 100% of three genes, Sag, Guca1a and Guca1b, coding for proteins known to play a major role in phototransduction: arrestin, GCAP1 and GCAP2. No detectable up-regulation occurred for light exposures of less than 1 h. Functional in vivo electroretinographic tests show that a partial recovery of the dark current occurred 1–2 h after prolonged illumination with a steady light that initially caused a substantial suppression of the photoresponse. These observations demonstrate that prolonged illumination results in the up-regulation of genes coding for proteins involved in the phototransduction signalling cascade, possibly underlying a novel component of light adaptation occurring 1–2 h after the onset of a steady bright light.
Phototransduction is the process by which the absorption of a photon leads to a decrease of the photocurrent circulating across the photoreceptor membrane. Light initiates phototransduction by activating rhodopsin, which amplifies the signal by activating transducin, a member of the family of G proteins (Burns & Baylor, 2001; Arshavsky et al. 2002). This process is terminated by multiple phosphorylations of the C-terminus of rhodopsin (Wilden et al. 1986; Chen et al. 1999a; Mendez et al. 2000) and by the subsequent binding of arrestin. Therefore, arrestin is a potent effector for terminating phototransduction.
A striking property of photoreceptors is their ability to operate over a wide range of light intensities covering approximately 10 log units. This is made possible by a process referred to as light adaptation. Light adaptation has been extensively studied and a number of biochemical mechanisms for this process have been identified, including several dependent on intracellular calcium (Torre et al. 1986; Koutalos & Yau, 1996; Pugh, Jr. et al. 1999; Burns & Baylor, 2001; Fain et al. 2001; Burns & Arshavsky, 2005). One of these mechanisms leads to the activation of the enzyme guanylate cyclase and to the formation of new cGMP, which is hydrolysed by phosphodiesterase, an enzyme activated by active transducin (Arshavsky & Bownds, 1992). Another possible mechanism contributing to light adaptation is the light-driven redistribution of transducin and arrestin between the outer and inner segment: in the presence of a bright light, transducin migrates from the outer to the inner segment, while arrestin follows the opposite path (Philp et al. 1987; Whelan & McGinnis, 1988; Sokolov et al. 2002; Elias et al. 2004; Nair et al. 2005; Strissel et al. 2006; Lobanova et al. 2007; Frechter et al. 2007). The trafficking of these proteins is thought to lead to a reduction in photoreceptor sensitivity and thus to light adaptation.
During prolonged changes of light, not only photoreceptors modify their electrical responsiveness, but also other biological mechanisms are involved in adapting to the background illumination and the circadian rhythm. Indeed, the rate of disk shedding (LaVail, 1976; Basinger et al. 1976), the rhodopsin level (Penn & Williams, 1986) and the expression level of several genes, such as c-fos, c-jun and jun B (Yoshida et al. 1993; Imaki et al. 1995), vary as the ambient light changes and during the day and night cycle. All these homeostatic mechanisms help photoreceptors to adapt properly to the external conditions.
In the present investigation we have asked whether the light adaptation that occurs after exposure to a continuous bright light of several hours is also mediated by changes in gene expression, which so far have been thought to play only a marginal role in phototransduction. We have investigated light adaptation-associated changes in gene expression by microarray and real-time PCR in three different preparations from the mouse retina: isolated photoreceptors, cultured isolated retinas and acutely harvested retinas from live animals. In all of these preparations we observed a consistent up-regulation of almost twofold of three genes involved in phototransduction: Sag (coding for arrestin), Guca1a (coding for guanylate cyclase activating protein (GCAP) 1) and Guca1b (coding for GCAP2) (Ngo et al. 1990; Palczewski et al. 1994b; Dizhoor et al. 1995).
Methods
Microarray analysis of gene expression in isolated photoreceptors
Harvesting of isolated photoreceptors from mouse retinas
Dark-adapted C57/Bl6 mice were killed by cervical dislocation under an infrared light source and photoreceptors were isolated enzymatically and mechanically using a buffer containing papain 0.1%, DNAse 400 U, NaCl 150 mm, KCl 3.5 mm, CaCl2 1 mm, MgCl2 2.4 mm, Hepes 5 mm, d-glucose 10 mm, incubating for 3 min at 37°C. After dissociation, samples were plated in two different dishes and positioned on two distinct set-ups: one always in the dark under infrared light and the other under 10 lux light. Small aggregates of isolated photoreceptors were harvested every 5 min with suction pipettes. The harvested cell aggregates were expelled into 50 μl of Trizol (Invitrogen) on ice and stored at −80°C.
Global polyadenylation PCR amplification (GA)
Total RNA from each sample was isolated from Trizol (Invitrogen) following the addition of 100 ng of polyinositol. RNA recovery by precipitation in isopropanol was optimized by using linear polyacrylamide (Ambion) as a carrier. All of the harvested RNA was resuspended in 4.5 μl of ice-cold cell reverse transcription mixture containing: 47 μl lysis buffer (100 μl 10× PCR buffer (Roche), 60 μl 25 mm MgCl2 (Roche), 5 μl of NP-40 (American Bioanalytical, Natick, MA, USA), 50 μl of 0.1 DTT (Gibco/Invitrogen), 725 μl of Diethylpyrocarbonate (DEPC)-treated water), 1 μl RNAse inhibitors mix (1 : 1 prime RNA inhibitor (Eppendorf) and RNAguard ribonuclease inhibitor (Porcine)), 1 μl anchor T primer (final concentration 200 ng ml−1), 1 μl 2.5 mm dNTP (Takara Bio, Shiga, Japan). Total RNA was reverse transcribed to cDNA, tailed with poly-A and amplified for 30 cycles of PCR as described (Subkhankulova & Livesey, 2006). PCR products were purified with the CyScribe GFX Purification kit (Amersham Biosciences) and directly labelled for microarray hybridization with dCTP-Cy3/Cy5 (Amersham) with the BioPrime DNA labelling system (Invitrogen).
Microarray hybridization and data analysis
Expression microarrays containing 23 232 65-mer oligonucleotides (Sigma-Genosys) were printed on Codelink slides (Amersham). The majority of known photoreceptor-specific genes were represented on the array, including Rcvrn, Pdc, Gnat1, Gnat2, Guca1a, Guca2a, Guca2b, Pde6b, Pde6d, Pde6g, Sag, Crx, Nrl, Rom1, Prph, Prph2, UNC119, Rs1, Pias3, Tulp1, Rgs9, Gnb5 and Cnga1. Hybridized arrays were scanned in an Axon microarray scanner at a resolution of 10 μm at maximum laser power and photomultiplier tube voltage of 60–80%. Image and feature analysis were performed with GenePix Pro 4.0 (Axon Instruments, Inc.). Statistical analysis of microarray data was conducted in the R environment using the R package ‘Statistics for Microarray Analysis’ (http://www.stat.berkeley.edu/users/terry/zarray/Software/smacode.html). Data normalization was performed using scaled loess normalization (Limma package (Smyth et al. 2005)). Differentially expressed genes were selected and clustered using the Maanova package (http://research.jax.org/faculty/churchill), according to their temporal expression pattern.
Real-time PCR
Animals
All mouse and rat experiments were carried out according to the Italian and European guidelines for animal care (d.l.116/92; 86/609/C.E.). C57/Bl6 mice and Long–Evans rats were bred and maintained under a 12 h light–dark cycle (07.00 h–19.00 h). For lighting environment changes, two groups of overnight dark-adapted animals were maintained in either a darkened or a lighted cage. A 60 W bulb was used as an adjustable light source. For each time point at least six animals were killed by cervical dislocation, the eyes enucleated, the lenses removed and the retinas collected in Trizol (Invitrogen).
Acute and cultured retinas
The retina culture system was established according to the experimental procedures previously published by Reidel et al. (2006). In brief, intact eyes of adult Long–Evans rat were immediately removed from animals that had been killed and incubated with 1.2 mg ml−1 Proteinase K (Sigma-Aldrich) for 30 min at 37°C. Proteinase K activity was stopped by transferring the eyes to the culture medium containing 10% fetal calf serum for 5 min. After rinsing the eyes four times in serum-free culture medium for Proteinase K removal, retinas were dissected in basal culture medium after removal of the sclera, ocular tissue, and the hyaloid vessel, preserving the pigmented epithelium. All these procedures were performed under dim red light conditions. Retinas were spread with the retinal pigmented epithelial cells facing down on ME 25/31 culture membranes (Schleicher and Schuell, Dassel, Germany). Acute retinas were immediately exposed to light or dark, whereas cultured retinas were kept in Dulbecco's modified Eagle's medium with F12 supplement (DMEM–F12) and 10% fetal calf serum, penicillin and streptomycin (Sigma-Aldrich) and maintained at 37°C with 5% CO2 for 1 or 2 days before exposure. After light or dark treatment, acute or cultured retinas were collected in Trizol (Invitrogen).
Real-time PCR protocol
Total RNA from retinas was extracted in Trizol (Invitrogen) according to the manufacturer's instructions. In brief, retinas were homogenized in 1 ml of Trizol by passing several times through a glass pipette. After adding 0.2 ml of chloroform, samples were centrifuged at 12 000 g at 4°C for 20 min. After centrifugation, the RNA-containing aqueous phase was collected in a fresh tube. Subsequently, RNA was precipitated by adding 0.5 ml of isopropyl alcohol overnight at −20°C. RNA was then pelleted by centrifuging at 12 000 g at 4°C and washed with 70% ethanol in DEPC-treated water (12 000 g at 4°C for 20 min). RNA pellets were resuspended in 10 μl RNAse-free water (Qiagen, Hilden, Germany). RNA was further purified using an RNeasy column (Qiagen) and quantified using an ND-1000 Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Total RNA (∼500 ng) was treated with DNAse I (Invitrogen) to remove any genomic DNA contamination and converted to cDNA using Superscript II reverse transcriptase (Invitrogen). Twenty microlitres of PCR reaction mixtures contained cDNA, SYBR green master mix (Bio-Rad), H2O, and custom primers designed for each gene of interest. The PCR reactions were performed in an iQ5 thermocycler (Bio-Rad). Each reaction was performed at least in duplicate, and threshold cycles (CT) were calculated using the second derivative of the reaction. The CT of each gene was normalized against that of the control reference transcript Gapdh. Fold changes were determined using the –ΔΔCT method, using the average of dark control set to zero (Livak & Schmittgen, 2001; Pfaffl, 2001; Yuan et al. 2006). RNA controls were performed to ensure that amplification of products did not come from genomic DNA contamination.
Primers used for Real-Time PCR were as follows:
Gapdh mouse for: GCTGCCCAGAACATCATCCC
Gapdh mouse rev: ATGCCTGCTTCACCACCTTC
Sag mouse for: TTACAAGCCTTCCAACCTCTGAC
Sag mouse rev: ACCAGCACAACACCATCTACAG
Pde6b mouse for: TGCTGACTGTGAGGAGGATGAG
Pde6b mouse rev: GGGAATCTGGAACTTTCGGACTAC
Guca1a mouse for: CCCTCAGCCAGCCAGTATGTG
Guca1a mouse rev: ACTTCTGTTCCACTTTGCCCTTG
Gapdh rat for: CAAGTTCAACGGCACAGTCAAGG
Gapdh rat rev: ACATACTCAGCACCAGCATCACC
Sag rat for: GTGTCATACCATATCAAAGTGAAGC
Sag rat rev: GGAACGGCACCTCAGTAGC
Guca1a rat for: CAACGGGGATGGGGAACTG
Guca1a rat rev: GGTCAAGTCCAGGCTTCGG
Guca1b rat for: GCTTCTTCAAGGTCACTGGTAATG
Guca1b rat rev: GTAGATTGCCTCCACGATGTCC
Sag intron rat for: CCCTTGCCCTGTGAGGTTATCTG
Sag intron rat rev: ACCTTGTAATTTGTCACCGAAGTCAG
Guca1a intron rat for: CCCTCAGCCAGCCAGTATGTG
Guca1a intron rat rev: CTTCCCATCCCTCCCGTCCTC
Guca1b intron rat for: TTCTTCAAGGTCACTGGTAATG
Guca1b intron rat rev: GATGGAAAGGTCACTCAATGG
Immunohistochemistry
Arrestin translocation detection
Enucleated eyes were prefixed in paraformaldehyde (PFA) 4% in PBS for 30 min. Successively, eyecups obtained after lens and sclera removals were fixed in PFA 4% overnight. The lens removal allows fixative to penetrate the tissue diffusely. After fixation, samples were cryoprotected with scalar dilution of sucrose (10%, 20% and 30%), embedded in OCT (Sakura Tissue-Tek OCT Compound, Zoeterwoude, The Netherlands) and cryosectioned at 16 μm at −20°C. Immunolabeling was performed by standard protocols using anti-arrestin PA1–731 (Affinity BioReagents, Golden, CO, USA) as primary antibody and DAPI (Boehringer Mannheim GmbH, Ingelheim, Germany) for nuclear staining.
TUNEL assay
Retinal cryosections, processed as described in the previous section, were rinsed in phosphate buffered saline (PBS) 1× and permeabilized with 0.1% Triton X-100 and 0.1% Sodium Citrate for 2 min at 4°C. Subsequently, 100 μl of TUNEL reaction mixture (Chemicon) was added to each slide and kept for 60 min at 37°C in a humid chamber. After washing in PBS, 100 μl of 1:1000 DAPI (Boehringer Mannheim) were applied for 5 min at room temperature for nuclear staining. Slides were mounted with Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) for microscopy analysis. The positive control was treated, after permeabilization, with DNase I solution (100 μl of 200 μg ml−1) for 10 min at room temperature. In the negative control reaction mixture, terminal transferase enzyme was omitted. As shown in Fig. 1A very few apoptotic cells were detected in retinas after 2 days of culture.
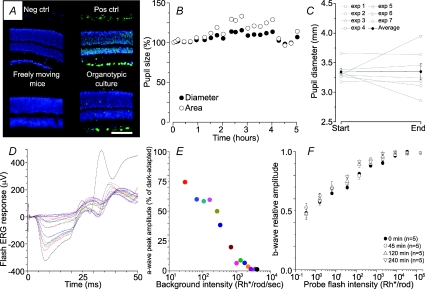
Figure 1. Effect of the light intensity on apoptosis, pupil constriction, a-wave amplitude and ERG sensitivity.
A, TUNEL assay on retinas harvested from freely moving mice and on cultured retinas. Upper left, negative control; upper right, DNase-treated positive control; lower left, apoptotic cells in retinas harvested from freely moving mice after 2 h of exposure to a steady light equivalent to 1200 lux illumination; lower right, apoptotic cells in cultured retinas after 2 days of culturing and exposed for 4 h to a light intensity equivalent to 105 Rh* rod−1 s−1. Nuclei stained in blue, apoptotic cells in green; scale bar: 40 μm. B, time course of pupil diameter (filled circle) and area (open circle) during a representative experiment lasting 5 h. Pupil was dilated with 1% tropicamide at the beginning of the experiment. C, changes of pupils diameter in 7 experiments from start to end (4–5 h later) of the ERG recording sessions. Averaged pupil diameter (filled circles) in mm ±s.e.m.: initial: 3.34 ± 0.06; final: 3.35 ± 0.14 (n= 7; paired t-test: P= 0.97, not significant). D and E, reduction of the amplitude of the a-wave as a function of the intensity of the steady light in a calibration experiment. After recording the amplitude of the a-wave evoked by a flash of light equivalent to 1.5 × 105 Rh* per rod in dark-adapted conditions (black trace), the flash of light was superimposed to backgrounds of steady light of increasing intensity. Each background was held for 10 min before delivering the flash of light. Recorded ERG responses are shown in different colours and have been superimposed. F, normalized amplitude of ERG responses to flashes of light of increasing intensity delivered at different times in dark adapted conditions. No significant change of sensitivity and responsiveness was observed during 4 h after the initiation of the experiment (mean ±s.e.m.; n= 5).
Western blotting
Retinas, dissected from light- or dark-exposed mice, were homogenized in lysis buffer on ice (50 mm Tris pH 7.5; 150 mm NaCl; 1% Triton X-100; 10 mm MgCl2). The total amount of protein was determined using the BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The homogenate was diluted with sample buffer, subjected to scalar dilutions, run on SDS-PAGE and Western blotted using the following antibodies: rabbit anti-visual arrestin (PA1-731, Affinity Bioreagents, Golden, CO, USA), mouse anti-GCAP1 (MA1-724, Affinity BioReagents), rat anti-HSC70 (SPA-815, StressGen, Ann Arbor, MI, USA) and rabbit anti beta-tubulin III (Sigma-Aldrich, Milano, Italy). HSC70, heat-shock cognate protein 70, and β-tubulin III are constitutively expressed proteins and were used as control of protein loading. Signals were detected analysing the optical density of the spots.
Electroretinogram
Animal preparation for ERG recording
Anaesthesia was induced by intraperitoneal injection of 120 mg per 100 g of urethane (ethyl carbamate) producing a long lasting and stable anaesthesia with minimal physiological changes (Berardi et al. 1990; Vaegan & Millar, 1994; Gargini et al. 2004). This was sufficient to maintain the animal deeply anaesthetised throughout the entire experimental session, as verified by the absence of corneal reflexes. The general conditions were continuously monitored by recording the electrocardiogram (ECG). Body temperature was constantly monitored and maintained throughout the whole experimental session at 37°C with an electric blanket and with any change greater than 0.1°C readily compensated by a feedback circuit. In all cases the observed temperature changes were less than 0.2°C with negligible effect on the ERG (Kong & Gouras, 2003). The general conditions were continuously monitored by recording the electrocardiogram (ECG). Pupils were dilated with 1% tropicamide (Sigma-Aldrich). A thin layer of methylcellulose solution (Lacrinorm, Farmigea Pisa, Italy) protected the cornea. We verified possible changes of sensitivity caused by the prolonged anaesthesia and we measured the amplitude of the a- and b-wave. Flashes of light intensity from 0.3 to 105 Rh* per rod were used and the normalized amplitude of the b-wave was computed at different times from the onset of the experiment, while the rat was kept in dark adapted conditions. As shown in Fig. 1F, no change of sensitivity was observed during 4 h of anaesthesia.
Optical stimulation
Full field illumination of the eyes was obtained via a Ganzfeld sphere (30 cm diameter), allowing a precise determination of the amount of light impinging the retina (Gargini et al. 2004; Lobanova et al. 2007). An electronic flash unit (SUNPAK B3600 DX) generated a stimulus and its energy decayed in time (τ= 1.7 ms). Saturating a-wave responses were obtained by delivering flashes of white light, the scotopic efficacy of which was evaluated according to Lyubarsky & Pugh (1996). The estimated retinal illuminance of the flash was 1.4 × 105 Rh* per rod. A steady background was obtained by illuminating the Ganzfeld sphere with four green light emitting diodes (LEDs) with a λ= 520 nm (Opto Diode Corp., Newbury Park, CA, USA). Fluctuations of the background light intensity were less than 13% during the entire length of the experiments.
ERG recording
ERGs were recorded in a completely darkened room via coiled gold electrodes making electrical contact with the moist cornea. A small gold plate placed in the mouth was used as reference. Responses were amplified differentially, band-pass filtered at 0.3–500 Hz, digitized at 0.078 ms intervals by a PC interface (National instruments LabVIEW 6.1, Italy). Responses to flashes were averaged with an interstimulus interval of 60 s and measured at fixed intervals after background exposure up to 240 min. Since a typical experimental session spanned a relative long period of time (4 to 5 h), special care was paid to perform recordings at the same time of the day (starting at midday), in order to minimize the influence of circadian rhythms on ERG measurements (Barattini et al. 1981; Barnard et al. 2006; Storch et al. 2007).
Measurements of the pupil size
The pupil size was estimated by measuring its radius at the beginning and at the end of the ERG recording session. A digital camera was used in the red eye removal mode and the pupil radius measured by image analysis software (GIMP 2.4.2; GNU Image Manipulation Program, http://www.gimp.org). In control experiments, we simultaneously measured the amplitude of a-wave and the area and diameter of the pupil (as shown in Fig. 1B). In these experiments, changes of pupil area and diameter were within 25% of their initial value, measured at the beginning of the experiment. Collected data (n= 7) show that the pupil diameter never changed by more than 20% from the start to end of the experimental session lasting at least 4 h (as shown in Fig. 2C) and that on average (black dots in Fig. 1C) no appreciable change of the pupil diameter was observed.
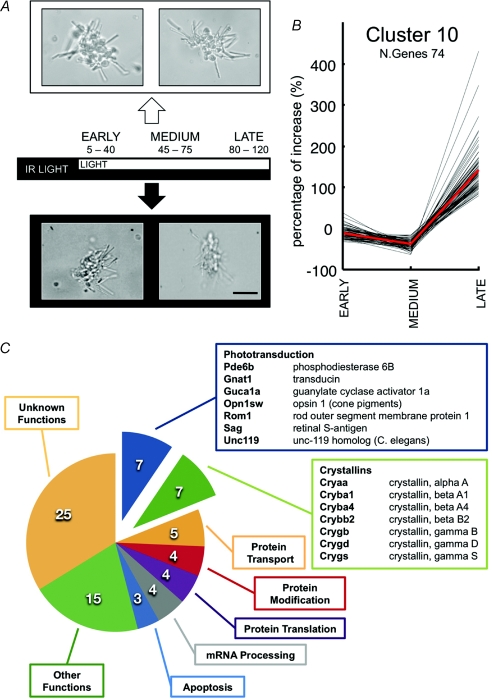
Figure 2. Microarray analysis in isolated photoreceptors.
A, dark-adapted retinas were dissociated under infrared illumination. Small bunches of rods were harvested simultaneously every 5 min in dark and light conditions. Rods were grouped in three different categories for early (from 0 to 40 min; n= 14), medium (from 40 to 80 min; n= 22) and late (from 80 min up to 2 h; n= 16) changes of gene expression. The light intensity impinging on the set-up used for harvesting light adapted rods was approximately 10 lux. Scale bar: 20 μm. B, time course of changes in gene expression in one cluster (Cluster 10) after a short, intermediate and long light exposure; cluster centroid indicated in red. C, gene onthology analysis of genes in Cluster 10.
Light measurement
In experiments with freely moving animals, the light intensities were measured using an Illuminometer (mod. 5200, Kyoritsu Electrical Instruments Works, Ltd, Tokyo, Japan). As animals were free to move and their pupils were not dilated by any pharmacological treatment, we report only the cage illumination in lux (stated as ambient light). In ERG and acute retinas experiments, the light intensity was measured using a Radiometric probe (Model 818-ST, Newport Corp., Irvine, CA, USA) connected to an Optical Power Meter (Model 1815-C, Newport). The number of photoisomerizations in a single rod per second, Φ/Δt, was estimated using the following equations (Lyubarsky et al. 2004):
| (1) |
| (2) |
where F(λ) is the photon density (photons m−2 s−1), I is the measured irradiance (W m−2), h is Planck's constant (6.626 × 10−34 J s−1), c is the speed of light in a vacuum (2.99792 × 108 m s−1), λ is the light wavelength (m), τ(λ) is the transmission of the pre-photoreceptor ocular media (1 for experiments with isolated retinas and 0.7 in ERG recordings with intact animals), ac(λ) is the ‘end-on collecting area’ of the photoreceptor (m2) assumed to be equal to 1.3 μm2 (Pennesi et al. 1998) and Spupil/Sretina is the ratio of the pupil and retina area, assumed to be 1 in isolated retinas and equal to 7.1/55 (Pennesi et al. 1998) in the intact rat eye during ERG recordings.
Determination of the relation between steady light intensity and fraction suppressed photocurrent
The relation between steady light intensity measured in Rh* rod−1 s−1 and fractional suppressed photocurrent in rat rods was obtained from ERG recordings in which the amplitude of the a-wave evoked by a bright flash of light (equivalent to 1.4 × 105 Rh* rod−1) was measured in the presence of steady lights of increasing intensity (Fig. 1D). From these experiments we derived the relation between fractional suppressed rod photocurrent and steady light intensity (Fig. 1E). This relation was used to determine the range of light intensity which to study light adaptation in rods, comprised between 20 and 2000 Rh* rod−1 s−1.
Results
The aim of the present investigation is to explore the relation between light adaptation in murine rods and possible concomitant changes of gene expression. This analysis requires the determination of changes of gene expression and protein levels and long lasting electrical recordings from rod photoreceptors. This combined analysis is not straightforward at the level of a single photoreceptor and a biochemical/genomic analysis is more practical in mouse rods, due to the availability of a larger variety of probes and antibodies. Long lasting and stable electrical recordings with ERG are more practical in rats, because of their larger size and strength. Therefore we have repeated similar experiments in mice and rats to confirm our observations in at least two species, but using primarily mice for the determination of protein levels with Western blot analysis and rats for ERG recordings.
We have investigated possible changes in gene expression during phototransduction in three different preparations: small aggregates of isolated photoreceptors, as those shown in Fig. 2A, in cultured intact retinas and in retinas acutely isolated from freely moving mice and rats. The mRNA extracted from bunches of isolated photoreceptors predominantly comes from rods. However, rods dissociated from mice and rat retina are extremely fragile and often degenerate after 2 h or so of intense light exposure. Retinas extracted from intact animals are in more physiological conditions, but the extracted mRNA comes from all retinal neurons, although the rodent retina is composed of approximately 70% rod photoreceptors (Morrow et al. 1998).
Microarray analysis in isolated photoreceptors
The two eyes of a dark-adapted mouse were isolated and dissociated under infrared light. Small bunches of isolated photoreceptors were positioned on the perfusion chamber of two distinct set-ups: one set-up was kept in complete darkness and mechanical manipulations were performed in infrared light; the other set-up was kept in a constant light of 10 lux.
Small bunches of rods were harvested from both set-ups at a range of times up to 2 h and grouped in three categories: those exposed to a short period of light (from 0 to 40 min, n= 14 bunches of rods), those exposed to an intermediate period of light (from 40 to 80 min, n= 22) and those exposed for a long period of time (from 80 min up to 2 h, n= 16). Single rod photoreceptors isolated from rodent retinas are fragile and often after 2 h of light exposure show clear signs of loss of morphological integrity. Therefore, harvesting was restricted to bunches of photoreceptors and not to isolated rods and was not prolonged beyond 120 min. After harvesting, mRNA from groups of rods was amplified by the global polyadenylation PCR amplification technique (Subkhankulova & Livesey, 2006) and expression profiled using custom-made two-channel oligonucleotide arrays representing over 22 000 genes. Several hundred of genes exhibited significant changes of gene expression and were grouped in 10 different clusters, according to their temporal expression pattern (with the Maanova Package).
One cluster (termed cluster 10) had 74 genes up-regulated by more than 100% specifically for the long light exposure period (Fig. 2B and C) and was noteworthy for containing several genes coding for proteins involved in phototransduction. In this cluster of genes, there are seven genes known to be part of the phototransduction machinery: arrestin (Sag), the β subunit of phosphodiesterase (Pde6b), the guanylate cyclase activator 1a (Guca1a), the guanine nucleotide binding protein, α transducing 1 (Gnat1), opsin-1 cone pigment (Op1sw), Unc-119 homologue C. elegans (Unc119) and the rod outer segment membrane protein 1 (Rom1). This cluster also contained seven genes coding for crystallins (γ S, γ B, β A4, β B2, α A, γ D and β A1). As shown in Fig. 2C, this cluster also contained genes coding for protein transport (5), protein modification (4), protein translation (4), mRNA processing (4), apoptosis (3) and other functions (15).
Real-time PCR analysis for selected genes in retinas from intact mice
Three of the up-regulated genes reported in Fig. 2C are particularly relevant for phototransduction: Sag, Guca1a and Pde6b. Up-regulation of genes activating guanylate cyclase, such as Guca1a and Guca1b, would be expected to elevate the cGMP level, while up-regulation of Sag would be expected to reduce the ability of photo-isomerized rhodopsin to close cGMP-gated channel. As a consequence, up-regulation of Guca1a, Guca1b and Sag is likely to reactivate the photocurrent and thus to contribute to light adaptation.
Therefore, we decided to confirm the changes in expression of Sag, Guca1a, Guca1b, Pde6b and Gnat1 with real-time PCR in retinas extracted from freely moving mice kept in complete darkness and mice exposed to a continuous light. As these genes are known to be expressed only or primarily in rod photoreceptors (Gorczyca et al. 1994; Palczewski et al. 2004), changes in their expression detected in whole retinas are due to changes occurring in rod photoreceptors. Under these conditions, long light exposures can be used without retinal deterioration, as observed in dissociated rod photoreceptors. However, under these conditions, the amount of light directly impinging on photoreceptors cannot be precisely calculated and only the ambient light was precisely measured.
Changes in gene expression at each time point were obtained using at least six pairs of retinas from mice kept in 1000 lux ambient light (test condition) and six pairs of retinas from mice kept in darkness (control condition). The ratio of observed changes is shown in Fig. 3A. After 2 h of light exposure, the level of mRNA of Sag, Guca1a and Guca1b increased by approximately 75% from the dark level. Sag, Guca1a and Guca1b transcripts remained elevated at longer times, up to 6–12 h. Changes in Pde6b transcript levels were statistically significant (P < 0.05) but were smaller in magnitude, whereas the changes in Gnat1 expression were not statistically significant (P > 0.1).
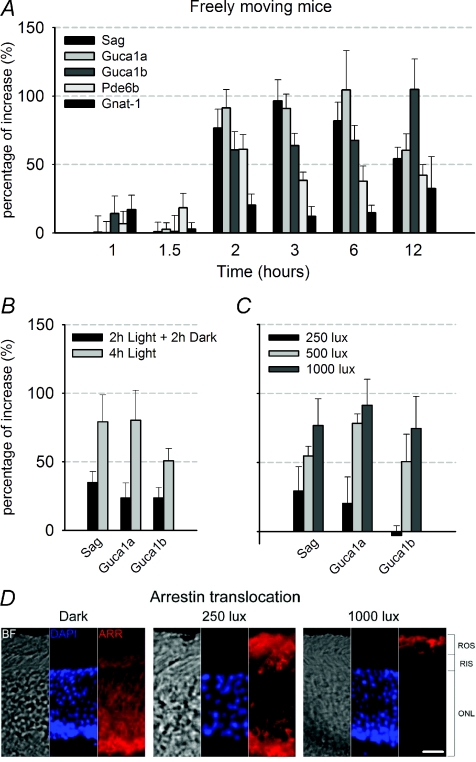
Figure 3. Real time PCR analysis of the selected genes in freely moving mice.
A, time course of changes in expression (relative to the control level in dark conditions) induced by light of Sag, Guca1a, Guca1b, Pde6b and Gnat1 genes in retinas harvested from freely moving mice exposed to an ambient light of 1000 lux. Light exposures varied from 1 to 12 h (mean ±s.e.m.; n≥ 6). B, changes in gene expression observed in retinas extracted from freely moving mice exposed to 1000 lux ambient light for 2 h and for 2 h in darkness compared to retinas from mice kept for 4 h in the same light. As a control, a group of mice were kept for 4 h in darkness (mean ±s.e.m.; n= 3). C, relation between changes in gene expression and ambient light intensity in freely moving mice exposed at 250, 500 and 1000 lux after 2 h of illumination (mean ±s.e.m.; n= 6). D, Immunofluorescence images of light-dependent arrestin translocation, following 2 h of illumination in retinas extracted from freely moving mice. Arrestin migration occurs from the outer nuclear layer (ONL) and rod inner segments (RIS) (in dark), to rod outer segments (ROS) when mice are kept in a bright ambient light of 1000 lux. A partial migration is observed when the ambient light was decreased to 250 lux. Columns from left to right show bright field images (BF), nuclear localization with DAPI staining in blue and arrestin labelling in red (ARR). Scale bar: 20 μm.
Having established that light elevated the total amount of mRNA of genes coding for Sag, Guca1a and Guca1b, we asked whether the elevated mRNA levels were maintained upon return to darkness and, if so, for how long. To do so, we exposed mice to a steady light for 2 h followed by complete darkness for 2 h. After this treatment, transcripts of Sag, Guca1a and Guca1b were still elevated but to a lesser extent (Fig. 3B). These results indicate that the light-dependent up-regulation of Sag, Guca1a and Guca1b transcripts was transient, returning to lower levels when placed in darkness, with a time constant of approximately 2 h.
We also analysed the dependence of up-regulation of Sag, Guca1a and Guca1b expression on light intensity in freely moving mice. As shown in Fig. 3C, the ambient light producing half of maximal gene up-regulation after 2 h of exposure was between 250 lux and 500 lux for the three studied genes.
As arrestin is known to migrate from the cell body and inner segment towards the outer segment, we correlated the level of up-regulation of expression with arrestin migration towards rods outer segments. Six freely moving mice were exposed to different ambient light levels for 2 h and six were kept in darkness. After dissection, half of these retinas were used to quantify gene expression by real-time PCR and the remaining eyes used to determine arrestin migration by immunofluorescence imaging. As shown in immunofluorescence images (Fig. 3D), arrestin completely migrates towards the outer segment in the brightest light for which maximal gene up-regulation was observed. At a lower light (250 lux), a partial migration of arrestin towards the outer segments was observed and transcripts coding for Sag, Guca1a and Guca1b are up-regulated to a sub-maximal level. Therefore, light-dependent gene up-regulation and arrestin translocation (Strissel et al. 2006) occur in the same range of light intensity.
Light induced changes of expression of Sag, Guca1a and Guca1b were typically increased by almost 100% and were reliably detected when retinas from at least two or three animals were pooled. We attempted to determine changes in expression between retinas from the same animal, where the eyelid of one eye was sealed and the other eye was exposed to ambient light. In these experiments, mice and rats attempted to free the closed eyelid and appeared to be stressed. To calm the animals, mild anaesthesia was used and in those experiments we could detect a light-induced up-regulation of Sag, Guca1a and Guca1b, comparing the mRNA level extracted from retinas from the eye with the closed and open eyelid of the same animal. After 3 h of illumination with a 1000 lux steady light, the differential expression, calculated as the ratio between the open and closed eye, was increased by 34.7 ± 32%, 16.5 ± 12% and 38.5 ± 34% for Sag, Guca1a and Guca1b, respectively. The high variability of the obtained results is attributed to different degrees of light reaching the retina of the eye with the presumed closed lid.
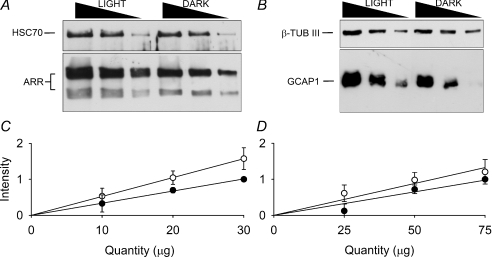
In order to verify whether up-regulation of Sag, Guca1a and Guca1b genes resulted in an increased level of related protein, the expression levels of arrestin and GCAP1 were determined by semi-quantitative Western blotting (Fig. 4) from retinas of freely moving mice. We were not able to quantify expression level of GCAP2 due to lack of a reliable commercial antibody against that specific mouse protein. For the semi-quantitative Western blot analysis, different lines with scaled concentrations of retinal homogenates were selected (Fig. 4A and B). Retinas from mice kept in dark adapted conditions and exposed to a steady bright light equivalent to 1000 lux for 3 h were used. Western blot analysis showed that the concentration of both forms of arrestin, the full-length with a molecular weight of 48 kDa and its splice variant with a molecular weight of 44 kDa (Palczewski et al. 1994a; Pulvermuller et al. 1997), increased by approximately 57% (Fig. 4C) and GCAP1 by 36% (Fig. 4D) after 3 h of light exposure. Therefore the observed gene up-regulation leads to an increase in protein synthesis.
Figure 4. Western blot analysis of arrestin and GCAP1.
A, representative Western blot quantifying the expression of the two isoforms of arrestin protein (48 kDa and 44 kDa) in retinal homogenates of light (3 h of exposure at 1000 lux) or dark-exposed mice retinas. For each line, 30, 20 and 10 μg of total protein was loaded. HSC70 was used as housekeeping control. B, same conditions as described above, representative Western blot quantifying the expression of GCAP1 in retinal homogenates. For each line, 75, 50 and 25 μg of the total protein was loaded. β-Tubulin III was used as housekeeping gene control. C, the relative increase of arrestin in light-exposed mice, calculated as the ratio of the slopes of light and dark-exposed (open and filled symbols respectively) mice, was 1.57-fold (mean ±s.e.m.; n= 3). D, the relative increase of GCAP1 in light-exposed mice was 1.36-fold (mean ±s.e.m.; n= 3).
Real-time PCR analysis for selected genes in retinas from intact rats
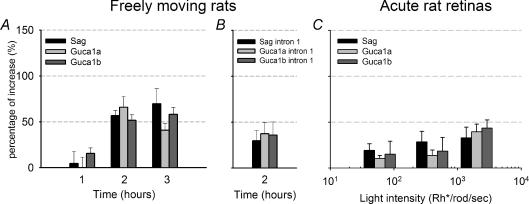
In order to extend the observation to another species we repeated similar experiments in rats, where ERG electrical recordings are easier to be performed over long periods of time (see next section). As in mice, up-regulation of Sag, Guca1a and Guca1b expression in the rat retina was observed at 2 h after onset of light exposure (P < 0.05; Fig. 5A). The increased levels of mRNAs described in Figs 3 and 5 could be due to an increased rate of transcription or to a decrease of the degradation of those mRNAs. To distinguish between these two possibilities, we quantified the levels of unspliced transcripts of Sag, Guca1a and Guca1b as one estimate of new RNA synthesis. As shown in Fig. 5B, the levels of these transcripts containing intron 1 of each gene increased by approximately 35% after 2 h of light exposure, indicating that the observed increases in mRNA are caused by higher rates of transcription.
Figure 5. Real time PCR analysis of Sag, Guca1a and Guca1b in rat retinas.
A, time course of changes in expression (relative to the control level in dark conditions) induced by light of Sag, Guca1a and Guca1b genes in retinas from freely moving rats exposed to an ambient light of 1000 lux (mean ±s.e.m.; n= 6). B, changes of unspliced Sag, Guca1a and Guca1b transcripts in rats after a 2 h light exposure, detected by primers matching the intron 1 region (mean ±s.e.m.; n= 5). C, up-regulation of Sag, Guca1a and Guca1b after 4 h of exposure to a steady light equivalent to 60, 410 and 2000 Rh* rod−1 s−1 in acute rat retinas (mean ±s.e.m.; n= 5).
Rat rod photoreceptors are saturated and the photocurrent completely abolished for steady lights brighter than 2000 Rh* rod−1 s−1 (Fig. 1E). Therefore, an up-regulation of Sag, Guca1a and Guca1b transcript levels could play a physiological role during light adaptation, if these genes are up-regulated for steady lights comprising between 20 and 2000 Rh* rod−1 s−1. Therefore we analysed changes of gene expression in acute rat retinas for light intensities in this range. As shown in Fig. 5C, a small but statistically significant up-regulation of these genes is observed following an exposure for 4 h of a steady light equivalent to 60, 410 and 2000 Rh* rod−1 s−1 (P < 0.05).
Analysis of late light adaptation in intact rats by ERG
The results presented so far show that exposure to a steady light equivalent to 60 Rh* rod−1 s−1 or brighter (Fig. 5C) causes a small but significant up-regulation of expression of genes coding for arrestin and the two activators of guanylate cyclase, both in vivo and in explanted retinas. The up-regulation of these genes could reactivate the photocurrent mediating a late phase of light adaptation. Therefore, we investigated the possible reactivation of the photocurrent in vivo by ERG recordings, in experiments where eyes are exposed to steady light with intensities varying from 60 to 12 000 Rh* rod−1 s−1 for several hours.
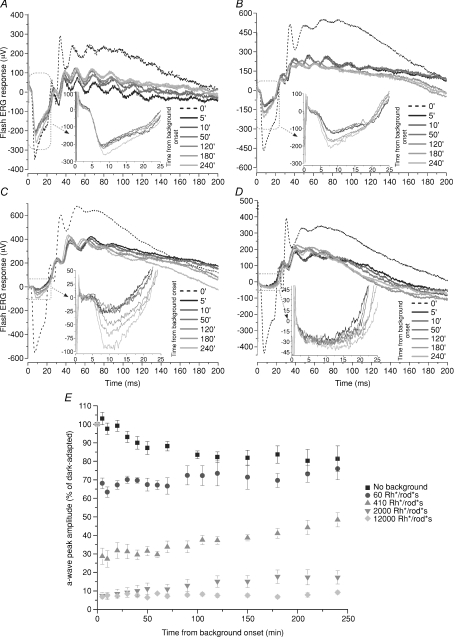
We used steady green lights equivalent to approximately 60, 410, 2000 and 12 000 Rh* rod−1 s−1 suppressing approximately 35% (Fig. 6A), 70% (Fig. 6B), 93% (Fig. 6C) and 96% (Fig. 6D) of the circulating photocurrent. For steady lights between 60 and 2000 Rh* rod−1 s−1 a significant recovery of the photocurrent was observed over the next 1–4 h and the largest fractional increase of the amplitude of the a-wave occurred with the steady light equivalent to 2000 Rh* rod−1 s−1 (Fig. 6C). The brightest steady light equivalent to 12 000 Rh* rod−1 s−1 completely suppressed the rod circulating photocurrent, (Fig. 6D) and no appreciable recovery of the amplitude of the a-wave was observed for light exposures up to 4 h. In the presence of this steady light, rods are expected to be saturated but not cones. Therefore the lack of the recovery of the amplitude of the a-wave observed with a steady light equivalent to 12 000 Rh* rod−1 s−1 and its recovery with the dimmer steady lights equivalent to 60, 410 and 2000 Rh* rod−1 s−1 strongly suggests that the recovery of the a-wave is produced by the rod and not the cone system.
Figure 6. Effect of light backgrounds on the recovery of the a-wave.
A–D, ERG recordings in dark adapted conditions in response to a bright flash of light delivering 1.4 × 105 Rh* rod−1 (dotted curve) and at different times after the onset of a steady light corresponding to 60 (A), 410 (B), 2000 (C) and 12 000 (D) Rh* rod−1 s−1. The steady light was switched on at time 0 and the first response was obtained 5 min after the initiation of the steady light (black curve). Responses at later times are shown as shaded grey curves. The inset reproduces the ERG responses in the dotted box. E, normalized amplitude of the a-wave peak as a function of time in the absence of a steady light (squares; n= 6) and with backgrounds of light corresponding to 60 (circles; n= 5), 410 (triangles; n= 6), 2000 (inverted triangles; n= 6) and 12 000 Rh* rod−1 s−1 (diamonds; n= 5). The amplitude of the a-wave was normalized to the amplitude of the a-wave measured at the beginning of the experiment in dark adapted conditions (mean ±s.e.m.).
These ERG recordings lasted for several hours. Therefore, it is crucial to analyse the stability of the components of the recording system and possible changes in the optical properties of the eye associated to a prolonged anaesthesia. Fluctuations of the light intensity emitted by the diodes illuminating the rat eyes during the entire experiment were less than 13% (see Methods). We also verified that modifications of the measured circulating photocurrent were not caused by changes in the electrical contacts of the recording electrodes. Indeed, the amplitude of the a-wave in dark adapted conditions (n= 5) slightly decreased during experimental sessions lasting longer than 4 h by about 20%, as shown by the filled squares in Fig. 6E. As shown in Fig. 1B and C the pupil size did not change appreciably during the experimental sessions. Another possible source of variability could be generated by changes in optical transmission of the crystalline during prolonged light exposures: indeed in the presence of steady lights brighter than 2 × 104 Rh* rod−1 s−1, the crystalline of mice became opaque in some animals after 3–4 h of light exposure. In ERG recordings from rats the crystalline did not become opaque in the presence of steady lights lower than 2 × 104 Rh* rod−1 s−1.
Collected data showed that steady lights equivalent to 60, 410, 2000 and 12 000 Rh* rod−1 s−1 immediately suppressed the circulating photocurrent by 35 ± 1.4% (circles, n= 5), 71 ± 2.4% (triangles, n= 6) and 89 ± 1.4% (inverted triangles, n= 6) of the circulating photocurrent, respectively (Fig. 6E). After 1 h of steady illumination the amplitude of the a-wave started to recover and a clear increase of the circulating current was observed 2 h after switching on the steady light. The amplitude of the a-wave after 240 min recovered by 12 ± 7.8%, 69 ± 10.7% and 157 ± 48.6% for the steady lights equivalent to 60, 410 and 2000 Rh* rod−1 s−1. We did not observe any recovery of the amplitude of the a-wave in the presence of a steady light equivalent to 12 000 Rh* rod−1 s−1, which is likely to completely saturate rods but not cones. During two experiments, we simultaneously measured the amplitude of a-wave and the size of the pupil (see Methods). In these experiments, the pupil size did not change by more than 14% over 4 h. Therefore, the recovery of the amplitude of the a-wave cannot be ascribed to changes in the background light intensity falling onto rod photoreceptors. Therefore, following a steady light that initially decreased the circulating photocurrent between 30 and 90%, a partial recovery in the photocurrent was observed after 1–2 h.
Discussion
The present paper provides experimental evidence for an up-regulation of genes coding for arrestin and the two activators of guanylate cyclase initiated by light exposures lasting longer than 2–3 h. These findings were obtained in three different rodent preparations: in isolated photoreceptors, in cultured isolated retinas and in acutely isolated retinas (Figs 2, 3 and 5). Gene up-regulation led to an increased level of concentration of the related protein (Fig. 4).
Observed changes of gene expression could be one aspect of the homeostatic mechanisms by which photoreceptors absorb the ‘optimal’ number of photons and adapt properly to the light environment, a phenomenon known as photostasis (Penn & Williams, 1986). Indeed, the up-regulation of the gene coding for arrestin could help the cell to avoid light-induced apoptosis, occurring in rods of transgenic mice lacking arrestin in continuous dim light (Chen et al. 1999b).
The up-regulation of Sag, Guca1a and Guca1b was observed following 2–3 h of light intensities ranging between 60 and 2000 Rh* rod−1 s−1. Under similar experimental conditions a partial recovery of the photocurrent was also observed in ERG recordings (Fig. 6), establishing a correlation between these two phenomena. In what follows we will consider and discuss this possibility. The establishment of a causal relationship between these events requires additional experiments, possibly using RNA interference of these genes.
Reactivation of photocurrent after prolonged light exposures
The in vivo ERG recordings from rats show that the circulating photocurrent, initially suppressed by a bright steady light, reactivates significantly after 1–2 h (Fig. 6). The possibility that the observed reactivation of the photocurrent may be due to a progressive closure of the animal pupil, leading to a decrease of the amount of light impinging on photoreceptors, was ruled out by pupil size measurements that found no significant changes over the entire experimental session. Similarly we have verified the stability of the light source, possible changes of sensitivity of rod photoreceptors caused by a prolonged anaesthesia (Fig. 1F) and changes of the transmission properties of the crystalline. Therefore, we conclude that the observed reactivation of the photocurrent is a genuine effect caused by events occurring inside rod photoreceptors.
The reactivation of the photocurrent here described is different from the ‘light rise of the photopic ERG’ (Bui & Fortune, 2006). In this phenomenon, the amplitude of the ERG doubles its amplitude over a period of 15 min in the presence of a rod-saturating background (Fig. 9 of Bui & Fortune, 2006), and this is considerably faster than in the reactivation observed here (see Fig. 6). In addition, this phenomenon is observed with steady lights equivalent to 105 Rh* rod−1 s−1 or brighter: under these conditions rods are completely saturated and the delayed increase of the amplitude of the a-wave is produced by cones and not rods. In the presence of steady lights equivalent to less than 2000 Rh* rod−1 s−1, as those used here (Fig. 6), the late increase of the a-wave originates from rods.
Roles of arrestin and GCAP1 and GCAP2
Arrestin is a cytosolic protein, known to block the interaction between photoactivated rhodospin and transducin (Kuhn, 1978; Xu et al. 1997; Vishnivetskiy et al. 2000) and an elevation of its concentration is expected to decrease the efficiency of photons in shutting off the photocurrent. In experiments with mice mutant for arrestin (Xu et al. 1997), the kinetics of the photoresponse is almost unchanged in +/− heterozygous mice, suggesting that a decrease of the concentration of arrestin by 1/2 does not affect the kinetics of photoresponses. In fact, the kinetics of photoresponses to brief light flashes is primarily controlled by regulators of G protein signalling (Krispel et al. 2006) and not by arrestin. In wild-type mice, the ratio between rhodopsin Rh and arrestin molecules is nearly 1 to 1 (Strissel et al. 2006). When more than 1% of the total rhodopsin Rh is bleached, a higher level of arrestin protein is expected to lead to a more effective shutting-off of activated Rh* and therefore to a partial reactivation of the photocurrent.
It is well known that GCAPs inhibit guanyl cyclase (GC) when Ca2+ levels are high and that GCAPs activate GC when Ca2+ levels are low, by binding to the GC intracellular domain in a Ca2+-dependent manner (Palczewski et al. 2000; Mendez et al. 2001). In vitro studies show that GC activity depends on the GCAP1 concentration (Wilkie et al. 2000) and it has been calculated that EC50 for GCAP1 is around 1 μm and for GCAP2 is around 6 μm (Pugh et al. 1997) while the GC density in mammalian rod membrane is 50 μm−2, corresponding to 2 × 105 GC molecules per rod (Pugh & Lamb, 2000). Similarly, the estimated number of GCAP1 and GCAP2 molecules per rod is in the range of 2 × 104 and 1 × 105, respectively. Therefore GC molecules are not completely covered by GCAPs and consequently an increase in GCAP concentration is expected to lead to GC activation. Moreover, GCAPs +/− mice that express approximately half the normal levels of GCAP1 and GCAP2 show delayed kinetics of photoresponses, i.e. an intermediate state between knockout and wild-type rods (Mendez et al. 2001). According to these considerations, an increase of the cytoplasmic concentration of the two GCAPs is expected to lead to a higher GC activity and consequently to a partial reactivation of the circulating photocurrent.
Western blotting analysis (Fig. 4) has shown that in the presence of intense steady lights the level of arrestin and of GCAP1 proteins increased by about 57 and 36%, respectively. The combined increase of the concentration of these two proteins is consistent with a reactivation of the photocurrent of about 100% observed in the presence of a steady light equivalent to 2000 Rh* rod−1 s−1, which is likely to bleach between 3 and 15% of the total rhodopsin.
Comparison of gene activation and recovery of photocurrent
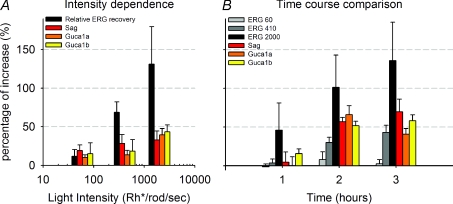
In order to establish a more compelling link between up-regulation of Sag, Guca1a and Guca1b and the a-wave recovery it is useful to compare the light dependence and time course of these two phenomena. As shown in Fig. 7A, the fractional recovery of the a-wave after 4 h of prolonged steady light and the up-regulation of the three genes under investigation (Sag, Guca1a and Guca1b) have a similar dependency on light intensity. In the presence of a steady light equivalent to 2000 Rh* rod−1 s−1, the fractional increase of the rod photocurrent and the sum of the up-regulation of the three genes is also quantitatively very similar consisting of about 100% of the increase.
Figure 7. Comparison between gene up-regulation and a-wave recovery.
A, comparison of fractional reactivation of the photocurrent and gene up-regulation measured with real time-PCR as a function of light intensity. Fractional reactivation of the photocurrent was calculated as the ratio between final and initial a-wave peak from the ERG data shown in Fig. 6E. Gene up-regulation from data shown in Fig. 5C. B, comparison of fractional reactivation of the photocurrent and time course of gene up-regulation measured with real time-PCR (data from Figs 6E and 5A).
A very similar behaviour is observed when the time courses of the fractional recovery of the rod photocurrent and gene up-regulation are compared (Fig. 7B). After 1 h of steady light, the fractional recovery of the rod photocurrent and gene up-regulation is about half of what is observed after 4 h. These results suggest – but do not prove – the existence of a causal relation between the observed gene up-regulation and recovery of the rod photocurrent. Our results, however, indicate the existence of a novel biochemical pathway initiated by rhodopsin activation, leading to a new late component of light adaptation.
Gene activation during intense light exposures causing degeneration
The three genes under investigation in the present study, Sag, Guca1a and Guca1b, are up-regulated by light intensities corresponding to 60–2000 Rh* rod−1 s−1 which do not cause any degeneration in retinal neurons. When brighter light intensities and longer exposures are used, degeneration of retinal neurons can be triggered (Noell & Albrecht, 1971). Under these conditions, intense light illumination affects the transcription in the mouse retina by activating the transcription factor AP-1 (Hao et al. 2002). AP-1 is a heterodimer composed of members of the Fos (Fos, FosB, Fra-1 and Fra-2) and the Jun (c-Jun, JunB and JunD) families. In albino or pupil-dilated pigmented mice, members of these families were up-regulated by steady illuminations brighter than 10 000 lux and the activation of these genes induces retinal apoptosis and the transcription of antioxidant genes (ceruloplasmin, heme oxygenase), apoptosis-related genes (caspase-1, bag3) and factors involved in metal homeostasis (metallothionein I and II) (Grimm et al. 2000; Roca et al. 2004; Chen et al. 2004; Krishnan et al. 2008).
Conclusion
The signalling pathway by which light regulates transcription of these genes in rod photoreceptors is currently unknown. The decrease of intracellular Ca2+ occurring during light adaptation (Torre et al. 1986; Koutalos & Yau, 1996; Pugh, Jr. et al. 1999; Burns & Baylor, 2001; Fain et al. 2001; Burns & Arshavsky, 2005) is a potential part of this signalling pathway, but the combination of different experimental techniques will be required to unravel this new pathway of phototransduction. Our experimental results show that the effect of light in visual photoreceptors is not limited to events occurring in the cytoplasm, but acts also in the nucleus by regulating transcription. As such, transcriptional control of visual transduction in rods and cones is a novel finding and describes a heretofore unappreciated level of control of this process. Similar changes of gene expression may play a similar role in other sensory systems, such as in chemoreception and possibly also in mechano-transduction and thermo-reception.
Acknowledgments
We thank Prof. U. Wolfrum and T. Goldmann for the useful guidance on retina culture protocols; M. Pocecco for technical assistance on the real-time PCR experiments; S. Pifferi for his support on simultaneous harvesting in double set-up experiments. We are indebted to Prof. T. D. Lamb for very useful criticisms and for suggesting to us several necessary control experiments. This work was supported by EU Grant NFG LSHG-CT-2003-503221, EU Grant NEURO NEST-012788, Italian Grant FIRB RBLA03AF28_007, Italian Grant PRIN 2006 and GRAND funding from Friuli Venezia Giulia.
Glossary
Abbreviations
- GC
guanyl cyclase
- GCAP
guanylate cyclase activating protein
- ERG
electroretinogram
- SAG
retinal S-antigen
Author contributions
P.C., C.G., F.J.L., L.C. and V.T. designed research; P.C. performed microarray screening and real-time PCR experiments; D.E.B. performed immunofluorescence assays; L.D.S. performed ERG experiments; P.C., L.D.S. and T.S. analyzed data; P.C. and V.T. wrote the paper.
References
- Arshavsky VY, Bownds MD. Regulation of deactivation of photoreceptor G protein by its target enzyme and cGMP. Nature. 1992;357:416–417. doi: 10.1038/357416a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Barattini S, Battisti B, Cervetto L, Marroni P. Diurnal changes in the pigeon electroretinogram. Rev Can Biol. 1981;40:133–137. [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194:1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- Berardi N, Domenici L, Gravina A, Maffei L. Pattern ERG in rats following section of the optic nerve. Exp Brain Res. 1990;79:539–546. doi: 10.1007/BF00229323. [DOI] [PubMed] [Google Scholar]
- Bui BV, Fortune B. Origin of electroretinogram amplitude growth during light adaptation in pigmented rats. Vis Neurosci. 2006;23:155–167. doi: 10.1017/S0952523806232024. [DOI] [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, Hurley JB, Baylor DA, Simon MI. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci U S A. 1999a;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness) Invest Ophthalmol Vis Sci. 1999b;40:2978–2982. [PubMed] [Google Scholar]
- Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79:239–247. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and α-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Frechter S, Elia N, Tzarfaty V, Selinger Z, Minke B. Translocation of Gq alpha mediates long-term adaptation in Drosophila photoreceptors. J Neurosci. 2007;27:5571–5583. doi: 10.1523/JNEUROSCI.0310-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargini C, Bisti S, Demontis GC, Valter K, Stone J, Cervetto L. Electroretinogram changes associated with retinal upregulation of trophic factors: observations following optic nerve section. Neuroscience. 2004;126:775–783. doi: 10.1016/j.neuroscience.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci U S A. 1994;91:4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Hafezi F, Reme CE. Gene expression in the mouse retina: the effect of damaging light. Mol Vis. 2000;6:252–260. [PubMed] [Google Scholar]
- Hao W, Wenzel A, Obin MS, Chen CK, Brill E, Krasnoperova NV, Eversole-Cire P, Kleyner Y, Taylor A, Simon MI, Grimm C, Reme CE, Lem J. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- Imaki J, Yamashita K, Yamakawa A, Yoshida K. Expression of jun family genes in rat retinal cells: regulation by light/dark cycle. Brain Res Mol Brain Res. 1995;30:48–52. doi: 10.1016/0169-328x(94)00270-o. [DOI] [PubMed] [Google Scholar]
- Kong J, Gouras P. The effect of body temperature on the murine electroretinogram. Doc Ophthalmol. 2003;106:239–242. doi: 10.1023/a:1022988332578. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Chen J, Shin KJ, Hwang JI, Han SU, Lee G, Choi S. Gene expression profiling of light-induced retinal degeneration in phototransduction gene knockout mice. Exp Mol Med. 2008;40:495–504. doi: 10.3858/emm.2008.40.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Kuhn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978;17:4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–DDCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Song H, Tsang SH, Chen CK, Sokolov M, Skiba NP, Arshavsky VY. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27:1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Pugh EN., Jr Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci. 1996;16:563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Roca A, Lem J, Wu LW, Simon MI, Baylor DA, Chen J. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28:153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98:9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Belliveau MJ, Cepko CL. Two phases of rod photoreceptor differentiation during rat retinal development. J Neurosci. 1998;18:3738–3748. doi: 10.1523/JNEUROSCI.18-10-03738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo JT, Klisak I, Sparkes RS, Mohandas T, Yamaki K, Shinohara T, Bateman JB. Assignment of the S-antigen gene (SAG) to human chromosome 2q24-q37. Genomics. 1990;7:84–87. doi: 10.1016/0888-7543(90)90521-u. [DOI] [PubMed] [Google Scholar]
- Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971;172:76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Buczylko J, Ohguro H, Annan RS, Carr SA, Crabb JW, Kaplan MW, Johnson RS, Walsh KA. Characterization of a truncated form of arrestin isolated from bovine rod outer segments. Protein Sci. 1994a;3:314–324. doi: 10.1002/pro.5560030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Polans AS, Baehr W, Ames JB. Ca2+-binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Sokal I, Baehr W. Guanylate cyclaseactivating proteins: structure, function, and diversity. Biochem Biophys Res Commun. 2004;322:1123–1130. doi: 10.1016/j.bbrc.2004.07.122. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994b;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Penn JS, Williams TP. Photostasis: regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp Eye Res. 1986;43:915–928. doi: 10.1016/0014-4835(86)90070-9. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Lyubarsky AL, Pugh EN., Jr Extreme responsiveness of the pupil of the dark-adapted mouse to steady retinal illumination. Invest Ophthalmol Vis Sci. 1998;39:2148–2156. [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–132. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Duda T, Sitaramayya A, Sharma RK. Photoreceptor guanylate cyclases: a review. Biosci Rep. 1997;17:429–473. doi: 10.1023/a:1027365520442. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Phototransduction in Vertebrate Rods and Cones: Molecular Mechanisms of Amplification, Recovery and Light Adaptation. Elsevier Science; 2000. [Google Scholar]
- Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opin Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- Pulvermuller A, Maretzki D, Rudnicka-Nawrot M, Smith WC, Palczewski K, Hofmann KP. Functional differences in the interaction of arrestin and its splice variant, p44, with rhodopsin. Biochemistry. 1997;36:9253–9260. doi: 10.1021/bi970772g. [DOI] [PubMed] [Google Scholar]
- Reidel B, Orisme W, Goldmann T, Smith WC, Wolfrum U. Photoreceptor vitality in organotypic cultures of mature vertebrate retinas validated by light-dependent molecular movements. Vision Res. 2006;46:4464–4471. doi: 10.1016/j.visres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Roca A, Shin KJ, Liu X, Simon MI, Chen J. Comparative analysis of transcriptional profiles between two apoptotic pathways of light-induced retinal degeneration. Neuroscience. 2004;129:779–790. doi: 10.1016/j.neuroscience.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Smyth GK, Thorne NP, Wettenhall JHO. Limma: Linear Models for Microarray Data User's Guide. 2005. http://bioinf.wehi.edu.au/limma. [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subkhankulova T, Livesey FJ. Comparative evaluation of linear and exponential amplification techniques for expression profiling at the single-cell level. Genome Biol. 2006;7:R18. doi: 10.1186/gb-2006-7-3-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V, Matthews HR, Lamb TD. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986;83:7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaegan, Millar TJ. Effect of kainic acid and NMDA on the pattern electroretinogram, the scotopic threshold response, the oscillatory potentials and the electroretinogram in the urethane anaesthetized cat. Vision Res. 1994;34:1111–1125. doi: 10.1016/0042-6989(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez MG, Gurevich VV. An additional phosphatebinding element in arrestin molecule. Implications for the mechanism of arrestin activation. J Biol Chem. 2000;275:41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- Wilden U, Hall SW, Kuhn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, Hurley JB, Bhattacharya SS, Warren MJ, Hunt DM. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–3073. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–1054. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]