Chronic severe pain is a significant global health problem [1]. In the US alone, one third of Americans suffer some form of chronic pain, and in these individuals over 30% of reported pain is resistant to analgesic therapy [1]. The economic impact of pain is equally large at approximately $100 billion annually [1]. While selective cyclooxygenase-2 (COX-2) inhibitors are effective for several forms of chronic pain, their occasional side-effects including increased risks of heart attack and stroke [2] prompted the precipitous withdrawal of some of them (i.e. Vioxx) from the market in 2004.
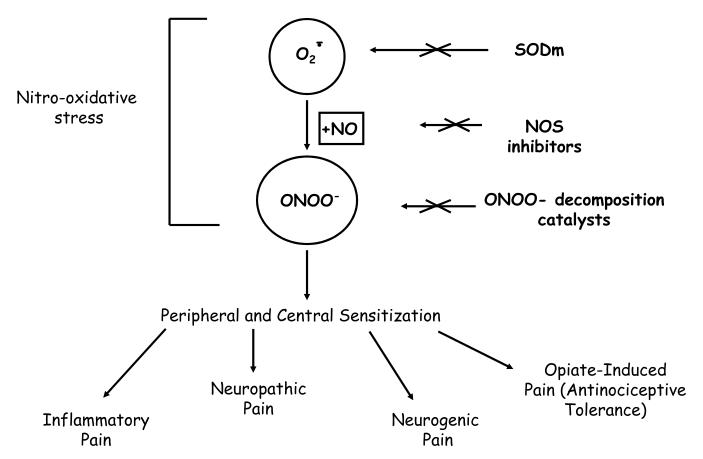
Morphine sulfate and other opiate/narcotic analgesics are the most effective treatments for acute and chronic severe pain. However, their clinical utility is often hampered by the development of analgesic tolerance as well as by de novo painful hypersensitivity to innocuous and noxious stimuli with such phenomena observed in both animal and human studies [3; 4; 5]. For morphine in particular, development of tolerance necessitates escalating doses to achieve equivalent pain relief [6], even as the onset of morphine-induced hypersensitivity subverts the therapeutic impact of such dose increases [3; 4; 5]. This complex pathophysiological cycle contributes significantly to decreased quality of life in the growing population of subjects with chronic pain due to oversedation, reduced physical activity, respiratory depression, constipation, potential for addiction, and other side-effects [6]. Accordingly, there is growing interest in new approaches that would maintain opiate efficacy during repetitive dosing without engendering tolerance or unacceptable side-effects. Considerable evidence implicates nitroxidative stress in the development of pain of several etiologies and importantly in opiate antinociceptive tolerance, caused by the presence of superoxide, O2·-, nitric oxide, ·NO and more recently peroxynitrite (ONOO- or its protonated counterpart ONOOH) that is the product of their interaction (Figure 1). In addition to the 3 routes of reducing ONOO- toxicity depicted in Figure 1, there is a fourth: scavenging of the radicals from ONOOH (urate, methionine and tyrosine peptides are examples in this category) [7].
Figure 1.
Peroxynitrite (ONOO-) Mediated Nitro-Oxidative Stress in Pain
The objectives of this first mini-review written on peroxynitrite and morphine antinociceptive tolerance are to discuss the importance of nitroxidative stress in this process and argue that peroxynitrite is a rational target for therapeutic intervention in pain management. These concepts provide a pharmacological basis for developing inhibitors of peroxynitrite biosynthesis as novel non-narcotic analgesics, thus addressing a large and currently unmet medical need with major socioeconomic consequences.
Morphine-induced antinociceptive tolerance: Is there a role for peroxynitrite?
Prolonged use of opiates results in antinociceptive tolerance, such that higher doses are required to achieve equivalent analgesia [6] or antinociception [5; 8; 9]. Adaptative modifications in cellular responsiveness, particularly desensitization and downregulation of opioid receptors, underlie this phenomenon [10]. By contrast, a competing hypothesis is that stimulation of opioid receptors over time triggers activation of anti-opioid systems that reduce sensory thresholds, thus causing hypersensitivity to tactile stimulation (allodynia) and noxious thermal stimulation (hyperalgesia) [8; 11; 12]. As a corollary, such opioid-induced hypersensitivity paradoxically diminishes the net analgesic effect of the opioid agonist [8; 11; 12]. In vivo support for this alternative hypothesis has been found in animals [3; 13; 14] and in humans [4; 15; 16]. Thus, analgesic tolerance likely arises when pain facilitatory systems become sensitized or hyperactive after repeated opioid use.
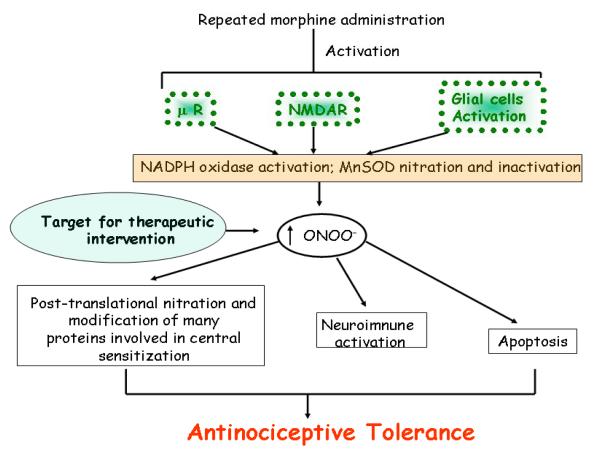
The mechanisms by which prolonged opiate exposure induce tolerance and hypersensitivity remain unclear, although a role for peroxynitrite-mediated nitroxidative stress has been identified [17]. Peroxynitrite is a potent pro-inflammatory and pro-apoptotic reactive species [18; 19; 20] and a potent inducer of hyperalgesia (defined as augmented pain intensity in response to painful stimuli) [21]. Besides its role in the development of morphine-induced antinociceptive tolerance that will be reviewed herein, peroxynitrite is also implicated in the development of hyperalgesia associated with acute and chronic inflammation and in response to spinal activation of the N-methyl-D-aspartate (NMDA) receptor [22; 23; 24] (Figure 1). We reasoned that since inhibiting formation of peroxynitrite precursors (O2·- or ·NO) blocks the development of morphine antinociceptive tolerance, then peroxynitrite is most likely the common and final signaling mediator of nitroxidative stress accompanying antinociceptive tolerance [17]. In support, it has been repeatedly shown that non-selective inhibitors, as well as those selective for iNOS and nNOS, prevent development of morphine-induced antinociceptive tolerance [25; 26; 27; 28; 29; 30; 31; 32; 33; 34]. These beneficial effects of NOS inhibition were associated with attenuation of spinal neuroimmune activation and reduced release of pro-inflammatory and pro-nociceptive cytokines, achieved at least in part by blocking redox-sensitive transcription factors such as p38 MAPK [17; 35; 36; 37; 38]. While links among morphine hypersensitivity, tolerance and ·NO production clearly exist, the contributions of different isoforms by pharmacological, antisense and genetic approaches remain controversial. In general nNOS is considered the primary source, although evidence also implicates iNOS [32; 39]. A defined role of eNOS must await development of selective inhibitors of this isoform; one study using eNOS knockout mice indicated that these animals develop tolerance in a manner similar to wild types [32]. Inhibition of O2·- formation with superoxide dismutase mimetics blocks tolerance events and is associated with suppressed spinal formation of TNF-α, IL-1βand IL-6, and reduced apoptosis [17]. Repeated administration of morphine in rodents promotes the nitration and thus the enzymatic inactivation of spinal manganese superoxide dismutase (MnSOD). Consequently, morphine may provide a critical source of spinal peroxynitrite that contributes to the development of morphine antinociceptive tolerance through three well-defined biochemical pathways within the dorsal horn of the spinal cord: (1) post-translational nitration of proteins involved in glutamate homeostasis (2) neuroimmune activation (release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα), interleukin (IL)-1β, and IL-6) and (3) neuronal apoptosis [17]. Thus, reducing ONOO- formation either indirectly (with nitric oxide synthase inhibitors or superoxide dismutase inhibitors) or directly (using pharmacological approaches to catalytically decompose ONOO-) inhibits these three events [17]. Collectively then, experimental evidence points to peroxynitrite as a canonical signaling molecule in morphine antinociceptive tolerance. The mechanisms leading to nitroxidative stress upon repeated administration of morphine during the development of antinociceptive tolerance are not known to date but are the subject of current investigation in my laboratories. However a link between morphine and oxidative stress has been documented. For example, morphine-induced O2·- production seems to occur as a result of activation of μreceptors, leading to the activation of the phospholipase D pathway, and an increase in Ca2+, leading to the activation of NADPH oxidase; generation of superoxide through this pathway evokes apoptosis in macrophages [40; 41]. Furthermore, morphine has been shown to exert oxidative stress in various cells including cells that we know play a critical role in antinociceptive tolerance namely neurons, microglial cells and astrocytes [42; 43]. Another potential source for peroxynitrite in response to repeated administration of morphine and the development of antinociceptive tolerance includes the activation of NMDA receptors and glial cells. Why? Substantial evidence has been gathered over the last decade to demonstrate that NMDA receptor activation as well as the activation of glial cells play a key role in the development of morphine tolerance since NMDA receptor antagonists, inhibitors of glial cell activation and anti-cytokine therapies block morphine antinociceptive tolerance [26; 35; 44; 45; 46; 47; 48; 49; 50; 51; 52]. NMDA receptor activation and glial cell activation release the precursors in the formation of ONOO- namely O2·- and ·NO [53; 54; 55; 56; 57; 58; 59; 60]. We therefore propose that repeated administration favors the formation of ONOO- as a result of at least in part μ receptor activation, NMDAR activation and glial cell activation (Figure 2). In this paradigm the enzymatic sources in ONOO- formation include NOS (already discussed), nitration and enzymatic inactivation of MnSOD (vide infra) and activation of the NADPH oxidase. The O2·- -generating enzyme, NADPH-oxidase, is dormant in resting cells and produces superoxide only upon activation. Unlike the regulation of NOS, the principal regulation of NADPH oxidase is post-translational and depends on assembly of several membrane-bound and cytosolic components to form an active enzyme complex. In resting cells, the enzyme consists of two membrane-bound components, gp91phox and p22phox, and several cytosolic components, including p47phox, p40phox, p67phox, and rac1-2 [61]. Gp91phox is a flavocytochrome and the catalytic core of the enzyme. Upon activation, the cytosolic components translocate to the membrane and associate with membrane components to form an assembled, activated, and superoxide-producing enzyme complex. Although this enzyme is best characterized in immune cells and leukocytes for its involvement in superoxide production, it is now known that various protein components of NADPH oxidase are expressed in neurons, astrocytes and microglia [61; 62; 63; 64]. These include the following NADPH oxidase subunits: gp91phox, p22phox, p40phox, p47phox, and p67phox [64; 65]. Furthermore, a recent study with hippocampal slices has demonstrated a link between NMDA and production of superoxide through NADPH oxidase [66]. Cytokines such as TNF-α and IL-1β activate this enzyme and activated glial cells generate ONOO- by iNOS and NADPH oxidase leading to neuronal death [58; 59; 67; 68]. Importantly, superoxide autoaugments superoxide formation by upregulating gp91phox creating a self-perpetuating cascade [67]. The role of this enzyme in superoxide formation during pathological settings is supported by the following observations. First, apocynin, a well-known inhibitor of the NADPH-oxidase prevents serine phosphorylation of p47phox, and blocks its association with gp91phox [69; 70]. This blunts NADPH oxidase activation leading to beneficial effects in animal models of oxidative stress including rheumatoid arthritis, diabetes, atherosclerosis, neurodegeneration, stroke and ischemia-reperfusion injuries [71; 72; 73; 74; 75; 76; 77; 78]. Second, these pharmacological observations are supported by genetic approaches demonstrating that mice lacking a functional NADPH oxidase subunit (gp91phox) show substantial decrease in O2·- and ONOO- formation and reduced oxidative stress in animal models [79]. Our preliminary results have shown that besides nitration and enzymatic inactivation MnSOD, the NADPH oxidase is also an important target source in the generation of ONOO- via O2·-. Thus, co-administration of morphine with apocynin, a well-characterized specific inhibitor of this enzyme blocked antinociceptive tolerance (Salvemini, manuscript in preparation). As discussed above NOS activation will provide ·NO, the second precursor in ONOO- formation.
Figure 2.
Peroxynitrite a Viable Target for Novel Therapeutic Intervention in Pain
Role of peroxynitrite in the development of morphine antinociceptive tolerance: Proposed molecular and biochemical pathways
A. Post-translational nitration and protein modification
Considerable evidence supports the notion that a key biologically relevant feature of peroxynitrite is post-translational tyrosine nitration and consequent modification of protein function [80; 81; 82]. The biological importance of post-translational nitration is thus underscored by compelling evidence linking this phenomenon to diseases driven by overt production of peroxynitrite including sepsis, ischemia/reperfusion injury, cancer, neurodegenerative disorders [22; 23; 83; 84; 85; 86; 87; 88], and more recently for pain and opiate antinociceptive tolerance [17; 21; 23; 24; 89].
A1: Protein nitration and superoxide/peroxynitrite homeostasis
Several proteins are nitrated, a modification associated with loss, gain or change of function [90; 91; 92]. A key example of lost enzyme activity due to nitration in vivo is mitochondrial MnSOD that normally keeps concentrations of superoxide under tight control [93]. The MnSOD protein is nitrated by peroxynitrite on Tyr-34 by a Mn-catalysed process which leads to enzyme inactivation [94]. Nitration of MnSOD, and its subsequent enzymatic inactivation, favor the accumulation of peroxynitrite which then nitrates and alters additional proteins and receptors, thereby perpetuating and extending the initial damage [80; 81; 82; 95]. To determine likely sources of sustained production of peroxynitrite during antinociceptive tolerance, we asked whether nitration/inactivation of MnSOD was a possibility. Our studies revealed that repeated administration of morphine leads to spinal nitration and enzymatic inactivation of MnSOD and that inhibition of peroxynitrite blocks nitration, restores the enzymatic activity of the enzyme and blocks tolerance suggesting the key role of nitrated MnSOD as a source of peroxynitrite in tolerance [17]. Interestingly, St. Clair and colleagues reported that when activated glial cells release cytokines such as TNF-α, iNOS is induced in neighbouring neurons; as a consequence formation of ·NO-derived peroxynitrite in such neurons nitrates MnSOD causing neuronal cell death [96]. Their results led us to postulate that nitration and inactivation of MnSOD contributes to the neuronal death often accompanying antinociceptive tolerance, and this hypothesis is being evaluated in our laboratory.
A2: Protein nitration and glutamate homeostasis
Dysfunction of the glutamatergic pathway is a key component of nociception [3; 35; 36; 46; 56]. Peroxynitrite alters glutamate homeostasis through post-translational nitration and modification of key proteins involved in maintaining a normal glutamate balance. Indeed research in diverse fields including amyotrophic lateral sclerosis and septic shock have demonstrated that peroxynitrite nitrates and inactivates 1) NMDA receptors [97; 98; 99], 2) the transport activity of sodium-dependent high-affinity glutamate transporters (GTs) [100; 101] and 3) glutamine synthase [102; 103; 104]. While these excitatory amino acid transporters also transport cysteine, for simplicity we shall refer to them as glutamate transporters GTs, and not excitatory amino acid transporters, EAATs.
We will next discuss why these observations are critically important in the context of morphine-induced antinociceptive tolerance and associated hyperalgesia
Glutamate neurotransmission, in particular that mediated via NMDA receptors under chronic pain conditions, is fundamentally involved in the development of opioid tolerance, especially tolerance arising from μ-opioid receptor stimulation [26; 44]. cDNA cloning has revealed that the NMDA receptor is formed by several NMDA receptor subunits. The coexpression of NR1 with various NR2 subunits is required for a fully functional ion channel receptor and the combined expression of NR1 with different NR2 subunits results in channel with distinct pharmacological and physiological properties that define NMDA receptor heterogeneity [105]. Peroxynitrite interacts with the NMDA receptor leading to nitration of the tyrosine residues present on the NMDA receptor subunits. This nitration is an irreversible reaction that leads to a constant potentiation of synaptic currents, calcium influx, and ultimately excitotoxicity [97; 98; 99].
Glutamate, as the primary endogenous ligand for the NMDA receptor, is not metabolized by extracellular enzymes but must be removed from the synaptic cleft by cellular uptake. Thus, homeostasis of extracellular glutamate is tightly regulated by GTs in the plasma membranes of both neurons and glia [106; 107; 108; 109]. There are five membrane GTs, termed GLAST (EAAT1), GLT-1 (EAAT2), EAAC1 (EAAT3), EAAT4, and EAAT5 [110]. Of these, GLAST and GLT-1 are localized primarily to astrocytes and EAAC1, EAAT4 and EAAT5 to neurons. EAAT4 and EAAT5 are restricted to cerebellar Purkinje cells and the retina, respectively, whereas EAAC1 is widely expressed in the CNS [111]. Astrocyte glutamate transporters are limited to glutaminergic synapses, whereas EAAC1 is detected diffusely over cell bodies and processes [112]. Three glutamate transport protein subtypes isolated in the spinal cord [GLAST and GLT-1 associated with glial cells, and EAAC1 associated with neurons [113; 114; 115; 116; 117; 118]], are considered essential to maintain low resting levels of glutamate (< 1μM), and to prevent overstimulation of GTs [108; 119; 120; 121; 122]. Knockdown expression of GLAST or GLT-1 in rats using antisense oligonucleotides increased the extracellular glutamate concentration [123]. Notably, these glutamate transport proteins are concentrated in the superficial dorsal horn of the spinal cord and are responsible for > 80% of total glutamate transport [110]. In elucidating potential mechanisms of morphine-induced antinociceptive tolerance and hypersensitivity, activation of NMDA receptors can lead to neurotoxicity under many circumstances [124; 125; 126; 127]. Thus, peripheral nerve injury has been shown to activate spinal cord NMDA receptors, causing intractable neuropathic pain and neuronal apoptosis [128; 129; 130; 131]. Furthermore, crosstalk between the pathways underlying opioid tolerance and neuropathic pain has been proposed, suggesting that a common cellular mechanism may be causal in both conditions [3; 132]. Extending this reasoning, it is possible that the cellular process leading to the development of opioid tolerance may also cause neurotoxic changes in response to prolonged opioid administration [133]. Thus, a number of studies indicate that functional glutamate transporters prevent glutamate neurotoxicity under both physiological and pathological conditions [101; 108; 109; 121; 134]. In brain tissue, decreases in GLT-1 mRNAs have been observed after naloxone-precipitated morphine withdrawal [135]. Of note, the activity of glutamate transporters decreases during morphine tolerance and is associated with spinal apoptosis [136]. Glutamate transporter inhibitors, or GT activators such as MS-135, increase and decrease respectively the development of spinal apoptosis, hyperalgesia and tolerance [136; 137]. In addition, agents such as dexamethasone or amitryptiline attenuate analgesic tolerance to morphine in part by preventing the downregulation of glutamate transporters, with consequent reduction in synaptic levels of glutamate [138; 139]. Not unexpectedly, nitration of GLT-1 by peroxynitrite inhibits its glutamate transport capacity and causes excitotoxicity [103].
Besides regulating synaptic levels of glutamate, these GTs play a crucial role in the uptake of cysteine, and thus contribute to the overall thiol redox state of cells that is regulated by intracellular levels of glutathione (GSH). GSH plays a critical role in protecting cells from oxidative stress as well as maintaining the thiol redox state. GSH depletion enhances oxidative stress leading to neuronal degeneration as shown in several studies [140; 141]. GSH is a tripeptide composed of glutamate, cysteine and glycine. In neurons, cysteine is the rate-limiting substrate for GSH synthesis [142] and in neurons approximately 90% of total cysteine uptake is mediated by EAATs [143; 144; 145]. Thus, EAAC1 transports cysteine at a rate comparable to that of glutamate, with an affinity 10- to 20-fold higher than that of GLAST or GLT-1 [146]. Recent studies have shown that peroxynitrite-mediated nitration of EAAC1 in neurons reduces the uptake capacity of cysteine leading to a depletion of intracellular GSH and neuronal cell death [100]. Integrating these findings, tolerance could develop due to excitotoxicity from increased synaptic concentrations of glutamate and a decrease in neuronal thiol redox state due to decreased intracellular levels of cysteine and thus GSH. We are currently evaluating such a concept.
In contradistinction to the central role of GTs in regulating the homeostasis of extracellular glutamate, glutamine synthase (GS) plays a pivotal role in glutamate’s intracellular metabolic fate. Once taken up into glial cells, glutamate is converted into nontoxic glutamine by endogenous GS [147]. In the brain, GS is located mainly in astrocytes; a primary roles of these cells is to protect neurons against excitotoxicity by taking up excess ammonia and glutamate, converting them into glutamine [detoxification of ammonia by GS will not be discussed here for simplicity]. Studies have shown that in glutamatergic brain areas, the distribution of both glial glutamate receptors and glial transporters parallels the location of GS suggesting a functional coupling between the two systems to prevent damage [148; 149; 150]. Furthermore, through feedback regulation, a decrease in GS activity can reduce the activity of GTs [148]. Thus, dysfunctional glutamate metabolism likely contributes to antinociceptive tolerance [133; 137; 138; 139]. These observations prompted us to show that post-translational tyrosine nitration of spinal glutamate transporters (GLT-1) and GS by peroxynitrite contributes to the development of antinociceptive tolerance to morphine [17]. Increased levels of glutamate can be decreased by reducing the production of cytokines such as TNF-αand IL-6 that have been shown to inhibit glutamate uptake [151]. Since peroxynitrite increases cytokine production (vide infra) it is likely that peroxynitrite modulates glutamate homeostasis via the cytokine signaling pathway.
B: Inflammation
Peroxynitrite is a potent pro-inflammatory nitroxidative species with an established role in “neuronal inflammation” (defined here as neuroimmune activation which includes activation of glial cells and release of proinflammatory cytokines) [35; 45; 46; 47; 48; 49; 50; 51; 52]. Chronic administration of morphine promotes activation of spinal cord glial cells, as well as production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 and spinal sensitization [35; 36; 52]. Thus, inhibitors of glial cell metabolism and/or anti-proinflammatory cytokine approaches block morphine-induced antinociceptive tolerance and hyperalgesia [35; 36; 52]. In addition, other anti-inflammatory agents including dexamethasone [138] [152], non-steroidal anti-inflammatory drugs [45; 51], IL-10 [48], NOS inhibitors [27; 28; 45], p38 kinase inhibitors [38] and superoxide dismutase mimetics [153]have been shown to inhibit morphine-induced antinociceptive tolerance and hyperalgesia. The possible mechanisms for chronic morphine-induced glial cell activation are not known with certainty. Although μ-opiate receptors are present on microglia and astrocytes [154], acute administration of morphine does not activate these cells [52]. On the other hand, morphine primes glial cells for enhanced production of pro-inflammatory cytokines [155]. In inflammation, peroxynitrite induces endothelial cell damage and increased microvascular permeability [156; 157], activates redox-sensitive transcription factors including NF-κ B and AP-1 that in turn regulate genes encoding various pro-inflammatory and pronociceptive cytokines genes such as interleukin-1β, tumor necrosis factor-α and interleukin-6 (IL-1β, TNF-α and IL-6 respectively [158; 159; 160; 161; 162; 163; 164]. Peroxynitrite also up-regulates adhesion molecules such as ICAM-1 and P-selectin to recruit neutrophils at sites of inflammation [163; 165], auto-catalyzes the destruction of neurotransmitters and hormones such as norepinephrine and epinephrine [166; 167], lipid peroxidation and oxidation [20]. In the development of morphine antinociceptive tolerance, inhibition of peroxynitrite formation with NOS inhibitors, superoxide dismutase mimetics or decomposition of peroxynitrite with peroxynitrite decomposition catalysts, block spinal formation of IL-1β, TNF-α and IL-6 [17]. The cyclooxygenase (COX) pathway has also been implicated in tolerance. In animals, a number of studies have confirmed that neuronal cyclooxygenase (COX) activity contributes to the expression of opioid tolerance and that certain COX inhibitors can be used for the prevention, and even the reversal of morphine tolerance [168; 169] As discussed previously in this review article, it has also been established that NOS inhibitors can effectively attenuate opioid tolerance [25; 26; 27; 28; 29; 30; 31; 32; 33; 34; 39] In this setting, another potential molecular pathway by which peroxynitrite may influence the development of antinociceptive tolerance is through the constitutive (COX-1) and inducible (COX-2) enzymes. A significant body of experimental evidence suggests a relationship between NO biosynthesis and PG generation [170; 171; 172]. As originally reported by our group [173] and subsequently extended by several other investigators [170; 172; 174; 175; 176; 177] the COX enzymes (constitutive COX-1 and inducible COX-2) are “receptor targets” for the multifaceted action of ·NO and as such are regulated in its presence. Although the mechanisms by which ·NO activates COX enzymes remain undefined, we now know that ONOO- is involved in this activation through the oxidative inactivation and/or modification of key amino acids residues in the COX polypetide backbone [178; 179]. Other possibilities in this complex reaction biochemistry have been raised and discussed in detail [172; 176; 180]. In addition to effects on COX-2 enzyme activity, ·NO and ONOO- increase the production of PGs from macrophages by acting post-transcriptionally or translationally to increase COX-2 protein levels or to increase its mRNA stability, at least in part through O2·- and the p38 MAPK pathway [174; 175; 181; 182; 183; 184]. Furthermore, iNOS binds COX-2, and iNOS-derived ·NO increases the catalytic activity of COX-2 through S-nitrosylation in a macrophage cell line [185]. Furthermore, and as discussed nitroxidative species activate transcription factors such as AP-1 and NF-kB as well as mitogen activated protein kinases (MAPK) such as p38 MAP kinase, which is known to induce COX-2 protein expression during inflammation [159; 161; 162; 186]. Substantial evidence supports the conclusion that the activation or induction of COX enzymes by nitro-oxidative stress augments the production of pro-inflammatory and pro-nociceptive prostaglandin PGE2 (PGE2) at sites of inflammation [170; 173]. It is therefore likely that the beneficial effects of peroxynitrite decomposition catalysts are due to suppressed production of local and spinal pro-inflammatory and pronociceptive cytokines and prostaglandins.
C: Apoptosis
Peroxynitrite is a potent pro-apototic and cytotoxic molecule and a role for spinal neuronal apoptosis in morphine antinociceptive tolerance is well established [136; 187; 188]. Peroxynitrite is considered the major oxidant responsible for DNA strand breakage which then activates the nuclear enzyme poly(ADP-ribose) polymerase (PARP). Rapid activation of PARP depletes the intracellular concentration of its substrate, nicotinamide adenine dinucleotide, thus slowing the rates of glycolysis, electron transport, and subsequent ATP formation [189]. Exposure of neurons to high concentrations of peroxynitrite more often leads to rapid necrosis due to acute, severe cellular energetic derangements [18; 190]. In contrast, lower concentrations of peroxynitrite can lead to delayed, apoptotic neuronal death [191]. Peroxynitrite-induced apoptosis is similar to other forms of oxidant/free radical mediated apoptosis in being dependent on activation of caspases-2, -3, -8 and -9 [192; 193; 194]. Mitochondria are key sites of cellular death and constitute a primary locus for the intracellular formation and reactions of peroxynitrite [80]. Peroxynitrite-mediated inactivation of mitochondrial MnSOD favors more peroxynitrite formation, resulting in positive feedback processes that promote mitochondrial dysfunction and the triggering of apoptotic signaling of cell death, including activation of PARP and caspases [195; 196; 197; 198]. As discussed previously, peroxynitrite also causes neuronal death via nitration of MnSOD following activation of neurons by glial cell-derived cytokines [96].
Previous reports have implicated apoptosis in antinociceptive tolerance and associated hypersensitivity. Indeed, chronic morphine exposure causes apoptosis in the spinal cord dorsal horn as determined by in situ terminal deoxynucleotidyl transferase (TdT)-mediated dUPT-biotin nick-end labeling (TUNEL) staining, upregulation of the pro-apoptotic caspase-3 and Bax proteins, and downregulation of the anti-apoptotic Bcl-2 protein [136; 187; 188]. Caspase-3 inhibitors that block apoptosis prevent the development of morphine hyperalgesia and antinociceptive tolerance [136; 187; 188]. Interestingly in these studies, apoptosis was found in neurons but not glial cells, although morphine can cause glial cell apoptosis [199]. The mechanisms of this morphine-induced apoptosis remain unclear. However, a role for peroxynitrite exists since its spinal inhibition during the development of antinociceptive tolerance to morphine, blocks oxidative DNA damage and PARP activation [17]. Taken together, these results provide a likely link between peroxynitrite, apoptosis and tolerance. Spinal PARP activation is seen during neuropathic pain and morphine tolerance where it induces excitotoxic transynaptic morphological changes in superficial dorsal horn “dark neurons” [129; 132]. Preventing PARP activation with PARP inhibitors or with peroxynitrite decomposition catalysts inhibits the development of morphine antinociceptive tolerance [136; 187]. It is therefore likely that the beneficial effects of peroxynitrite decomposition catalysts occur by attenuating neuronal and/or glial apoptosis during opiate-induced tolerance driven by PARP and caspase activation and nitration of MnSOD.
Concluding remarks and looking ahead
Considerable evidence over the years has supported the roles of ·NO and O2·- as precursors of peroxynitrite, in the development of morphine antinociceptive tolerance. Since the rate of interaction between ·NO and O2·- to form peroxynitrite is faster than the dismutation of O2·- by superoxide dismutase, peroxynitrite formation from O2·- and ·NO is the likely signaling molecule involved in antinociceptive tolerance [17] as in pain of several etiologies [23; 24; 89; 200; 201] (Figure 1). Because studies have only recently begun to unravel the role of peroxynitrite in antinociceptive tolerance and pain, few data are available to help understand the molecular and biochemical pathways engaged by this nitro-oxidative species. To date we know that peroxynitrite contributes to peripheral and central sensitization by increasing production of pro-inflammatory cytokines, by activating PARP, and modulating the cyclooxygenase pathway to increase the production of proinflammatory and pronociceptive PGE2 (activation of COX-1 and COX-2 and induction of COX-2) [21]. Peroxynitrite is also involved in neuroimmune activation, apoptosis and post-translational nitration and modification of key proteins known to be implicated in central and peripheral sensitization [17; 23; 89] (Figure 2). Additionally, nitroxidative species may be involved more subtly in central sensitization at least in part by sensitizing wide dynamic range neurons in the dorsal horn [202]. Importantly for eventual clinical management, the peroxynitrite decomposition catalysts evaluated to date apparently synergize with non-selective COX-1/COX-2 inhibitors and selective COX-2 inhibitors, and do so at greatly reduced doses. This synergism should minimize the obvious side effects of either drug class when coadministered [21]. Considering the many molecular, biochemical, and pharmacological similarities between opiate-mediated antinociceptive tolerance and the hypersensitivity associated with chronic neuropathic pain, the broader implication of our proposed studies is that peroxynitrite is a viable therapeutic target in both disease states (Figure 1). We believe that continued research in this field will soon provide a valid pharmacological basis for developing peroxynitrite-based therapeutic targets as adjuncts or alternatives to opiates (or other analgesics such as NSAIDs) in the management of pain and in particular chronic pain.
Ackowledgements
We would like to thank Dr William Neumann for his thoughts, insights and critique in the preparation of this review article and Dr Andrew Lechner for editorial input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Renfrey S, Downton C, Featherstone J. The painful reality. Nat Rev Drug Discov. 2003;2:175–6. doi: 10.1038/nrd1038. [DOI] [PubMed] [Google Scholar]
- [2].Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- [4].Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–9. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- [5].Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr., Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–48. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- [6].Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- [7].Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem. 2007;282:6324–37. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- [8].Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- [9].Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr., Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- [10].Taylor DA, Fleming WW. Unifying perspectives of the mechanisms underlying the development of tolerance and physical dependence to opioids. J Pharmacol Exp Ther. 2001;297:11–8. [PubMed] [Google Scholar]
- [11].Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- [12].Rothman RB. A review of the role of anti-opioid peptides in morphine tolerance and dependence. Synapse. 1992;12:129–38. doi: 10.1002/syn.890120206. [DOI] [PubMed] [Google Scholar]
- [13].Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- [14].Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–80. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Conno F, Caraceni A, Martini C, Spoldi E, Salvetti M, Ventafridda V. Hyperalgesia and myoclonus with intrathecal infusion of high-dose morphine. Pain. 1991;47:337–9. doi: 10.1016/0304-3959(91)90225-M. [DOI] [PubMed] [Google Scholar]
- [16].Devulder J. Hyperalgesia induced by high-dose intrathecal sufentanil in neuropathic pain. J Neurosurg Anesthesiol. 1997;9:146–8. doi: 10.1097/00008506-199704000-00007. [DOI] [PubMed] [Google Scholar]
- [17].Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–9. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- [19].Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11:204–14. [PubMed] [Google Scholar]
- [21].Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008 doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- [22].Muscoli C, Sacco I, Alecce W, Palma E, Nistico R, Costa N, Clementi F, Rotiroti D, Romeo F, Salvemini D, Mehta JL, Mollace V. The protective effect of superoxide dismutase mimetic M40401 on balloon injury-related neointima formation: role of the lectin-like oxidized low-density lipoprotein receptor-1. J Pharmacol Exp Ther. 2004;311:44–50. doi: 10.1124/jpet.104.068205. [DOI] [PubMed] [Google Scholar]
- [23].Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–78. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- [24].Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–73. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- [25].Pasternak GW. Nitric oxide and opioid tolerance. NIDA Res Monogr. 1995;147:182–94. [PubMed] [Google Scholar]
- [26].Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–7. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- [27].Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci U S A. 1993;90:5162–6. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kolesnikov YA, Pick CG, Pasternak GW. NG-nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol. 1992;221:399–400. doi: 10.1016/0014-2999(92)90732-j. [DOI] [PubMed] [Google Scholar]
- [29].Bhargava HN, Bian JT. Effects of acute administration of L-arginine on morphine antinociception and morphine distribution in central and peripheral tissues of mice. Pharmacol Biochem Behav. 1998;61:29–33. doi: 10.1016/s0091-3057(98)00067-7. [DOI] [PubMed] [Google Scholar]
- [30].Bhargava HN, Kumar S, Barjavel MJ. Kinetic properties of nitric oxide synthase in cerebral cortex and cerebellum of morphine tolerant mice. Pharmacology. 1998;56:252–6. doi: 10.1159/000028205. [DOI] [PubMed] [Google Scholar]
- [31].Bian JT, Bhargava HN. Effect of chronic administration of L-arginine, NG-nitro-L-arginine or their combination on morphine concentration in peripheral tissues and urine of the mouse. Gen Pharmacol. 1998;30:753–7. doi: 10.1016/s0306-3623(97)00336-4. [DOI] [PubMed] [Google Scholar]
- [32].Heinzen EL, Pollack GM. The development of morphine antinociceptive tolerance in nitric oxide synthase-deficient mice. Biochem Pharmacol. 2004;67:735–41. doi: 10.1016/j.bcp.2003.08.046. [DOI] [PubMed] [Google Scholar]
- [33].Heinzen EL, Booth RG, Pollack GM. Neuronal nitric oxide modulates morphine antinociceptive tolerance by enhancing constitutive activity of the mu-opioid receptor. Biochem Pharmacol. 2005;69:679–88. doi: 10.1016/j.bcp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [34].Liang DY, Clark JD. Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett. 2004;365:73–7. doi: 10.1016/j.neulet.2004.04.054. [DOI] [PubMed] [Google Scholar]
- [35].Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–9. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [36].Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–46. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu W, Wang CH, Cui Y, Mo LQ, Zhi JL, Sun SN, Wang YL, Yu HM, Zhao CM, Feng JQ, Chen PX. Inhibition of neuronal nitric oxide synthase antagonizes morphine antinociceptive tolerance by decreasing activation of p38 MAPK in the spinal microglia. Neurosci Lett. 2006;410:174–7. doi: 10.1016/j.neulet.2006.08.091. [DOI] [PubMed] [Google Scholar]
- [38].Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–43. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- [39].Abdel-Zaher AO, Hamdy MM, Aly SA, Abdel-Hady RH, Abdel-Rahman S. Attenuation of morphine tolerance and dependence by aminoguanidine in mice. Eur J Pharmacol. 2006;540:60–6. doi: 10.1016/j.ejphar.2006.03.059. [DOI] [PubMed] [Google Scholar]
- [40].Bhat RS, Bhaskaran M, Mongia A, Hitosugi N, Singhal PC. Morphine-induced macrophage apoptosis: oxidative stress and strategies for modulation. J Leukoc Biol. 2004;75:1131–8. doi: 10.1189/jlb.1203639. [DOI] [PubMed] [Google Scholar]
- [41].Kapasi AA, Coscia SA, Pandya MP, Singhal PC. Morphine modulates HIV-1 gp160-induced murine macrophage and human monocyte apoptosis by disparate ways. J Neuroimmunol. 2004;148:86–96. doi: 10.1016/j.jneuroim.2003.11.015. [DOI] [PubMed] [Google Scholar]
- [42].Singhal PC, Pamarthi M, Shah R, Chandra D, Gibbons N. Morphine stimulates superoxide formation by glomerular mesangial cells. Inflammation. 1994;18:293–9. doi: 10.1007/BF01534270. [DOI] [PubMed] [Google Scholar]
- [43].Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–5. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- [44].Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain. 1994;56:69–75. doi: 10.1016/0304-3959(94)90151-1. [DOI] [PubMed] [Google Scholar]
- [45].Powell KJ, Hosokawa A, Bell A, Sutak M, Milne B, Quirion R, Jhamandas K. Comparative effects of cyclo-oxygenase and nitric oxide synthase inhibition on the development and reversal of spinal opioid tolerance. Br J Pharmacol. 1999;127:631–44. doi: 10.1038/sj.bjp.0702587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- [47].Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther. 2005;313:1239–47. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- [48].Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bianchi M, Maggi R, Pimpinelli F, Rubino T, Parolaro D, Poli V, Ciliberto G, Panerai AE, Sacerdote P. Presence of a reduced opioid response in interleukin-6 knock out mice. Eur J Neurosci. 1999;11:1501–7. doi: 10.1046/j.1460-9568.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- [51].Powell KJ, Quirion R, Jhamandas K. Inhibition of neurokinin-1-substance P receptor and prostanoid activity prevents and reverses the development of morphine tolerance in vivo and the morphine-induced increase in CGRP expression in cultured dorsal root ganglion neurons. Eur J Neurosci. 2003;18:1572–83. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- [52].Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–6. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- [53].Possel H, Noack H, Keilhoff G, Wolf G. Life imaging of peroxynitrite in rat microglial and astroglial cells: Role of superoxide and antioxidants. Glia. 2002;38:339–50. doi: 10.1002/glia.10066. [DOI] [PubMed] [Google Scholar]
- [54].Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Liu Y, Casares F, Goligorsky MS. Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem. 1995;270:30290–3. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- [55].Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–7. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- [56].Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–36. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- [57].Mollace V, Colasanti M, Rodino P, Lauro GM, Rotiroti D, Nistico G. NMDA-dependent prostaglandin E2 release by human cultured astroglial cells is driven by nitric oxide. Biochem Biophys Res Commun. 1995;215:793–9. doi: 10.1006/bbrc.1995.2533. [DOI] [PubMed] [Google Scholar]
- [58].Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–41. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mander P, Brown GC. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J Neuroinflammation. 2005;2:20. doi: 10.1186/1742-2094-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bal-Price A, Matthias A, Brown GC. Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem. 2002;80:73–80. doi: 10.1046/j.0022-3042.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- [61].Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–4. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- [62].Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–75. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–84. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- [64].Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–8. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- [66].Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide autoaugments superoxide formation and upregulates gp91(phox) expression in porcine pulmonary artery endothelial cells: inhibition by iloprost. Eur J Pharmacol. 2006;538:108–14. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- [68].Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–60. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–8. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- [70].Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- [71].Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 2006;531:264–9. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- [72].Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–8. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- [73].Supinski G, Stofan D, Nethery D, Szweda L, DiMarco A. Apocynin improves diaphragmatic function after endotoxin administration. J Appl Physiol. 1999;87:776–82. doi: 10.1152/jappl.1999.87.2.776. [DOI] [PubMed] [Google Scholar]
- [74].Kawai J, Ando K, Tojo A, Shimosawa T, Takahashi K, Onozato ML, Yamasaki M, Ogita T, Nakaoka T, Fujita T. Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation. 2004;109:1147–53. doi: 10.1161/01.CIR.0000117231.40057.6D. [DOI] [PubMed] [Google Scholar]
- [75].Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004;313:812–7. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- [76].Rachmilewitz D, Okon E, Karmeli F. Sulphydryl blocker induced small intestinal inflammation in rats: a new model mimicking Crohn’s disease. Gut. 1997;41:358–65. doi: 10.1136/gut.41.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–11. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- [78].Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–24. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- [79].Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–8. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383:401–9. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- [82].Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- [83].Redondo-Horcajo M, Lamas S. Oxidative and nitrosative stress in kidney disease: a case for cyclosporine A. J Nephrol. 2005;18:453–7. [PubMed] [Google Scholar]
- [84].Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–9. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–51. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- [86].Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol. 2006;290:H2220–7. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- [87].Nilakantan V, Halligan NL, Nguyen TK, Hilton G, Khanna AK, Roza AM, Johnson CP, Adams MB, Griffith OW, Pieper GM. Post-translational modification of manganese superoxide dismutase in acutely rejecting cardiac transplants: role of inducible nitric oxide synthase. J Heart Lung Transplant. 2005;24:1591–9. doi: 10.1016/j.healun.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [88].Pittman KM, MacMillan-Crow LA, Peters BP, Allen JB. Nitration of manganese superoxide dismutase during ocular inflammation. Exp Eye Res. 2002;74:463–71. doi: 10.1006/exer.2002.1141. [DOI] [PubMed] [Google Scholar]
- [89].Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [90].Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–15. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- [91].Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–6. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- [92].Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon ), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of notriggered activation of PKCepsilon. J Biol Chem. 2002;277:15021–7. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- [93].McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- [94].MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–8. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- [95].Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–88. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- [96].Tangpong J, Sompol P, Vore M, Clair W, St, Butterfield DA, Clair DK., St Tumor necrosis factor alpha-mediated nitric oxide production enhances manganese superoxide dismutase nitration and mitochondrial dysfunction in primary neurons: an insight into the role of glial cells. Neuroscience. 2008;151:622–9. doi: 10.1016/j.neuroscience.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mishra OP, Delivoria-Papadopoulos M. Cellular mechanisms of hypoxic injury in the developing brain. Brain Res Bull. 1999;48:233–8. doi: 10.1016/s0361-9230(98)00170-1. [DOI] [PubMed] [Google Scholar]
- [98].Zanelli SA, Ashraf QM, Delivoria-Papadopoulos M, Mishra OP. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci Lett. 2000;296:5–8. doi: 10.1016/s0304-3940(00)01608-6. [DOI] [PubMed] [Google Scholar]
- [99].Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience. 2002;112:869–77. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- [100].Aoyama K, Matsumura N, Watabe M, Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci. 2008;27:20–30. doi: 10.1111/j.1460-9568.2007.05979.x. [DOI] [PubMed] [Google Scholar]
- [101].Trotti D, Aoki M, Pasinelli P, Berger UV, Danbolt NC, Brown RH, Jr., Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276:576–82. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- [102].Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr., Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2:848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- [103].Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–9. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- [104].Gorg B, Wettstein M, Metzger S, Schliess F, Haussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–73. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- [105].Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. Faseb J. 1994;8:745–52. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- [106].Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol. 2004;189:222–30. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- [107].Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci U S A. 2000;97:5610–5. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–51. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Semba J, Wakuta MS. Regional differences in the effects of glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid on extracellular amino acids and dopamine in rat brain: an in vivo microdialysis study. Gen Pharmacol. 1998;31:399–404. doi: 10.1016/s0306-3623(98)00047-0. [DOI] [PubMed] [Google Scholar]
- [110].Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- [111].Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15:461–73. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- [112].Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- [113].Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–32. [PubMed] [Google Scholar]
- [114].Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- [115].Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–71. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- [116].Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- [117].Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- [118].Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–9. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- [120].Liaw WJ, Stephens RL, Jr., Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [121].Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29:222–32. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [122].Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- [124].Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–11. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- [125].Swan JH, Meldrum BS. Protection by NMDA antagonists against selective cell loss following transient ischaemia. J Cereb Blood Flow Metab. 1990;10:343–51. doi: 10.1038/jcbfm.1990.63. [DOI] [PubMed] [Google Scholar]
- [126].Moncada C, Lekieffre D, Arvin B, Meldrum B. Effect of NO synthase inhibition on NMDA- and ischaemia-induced hippocampal lesions. Neuroreport. 1992;3:530–2. [PubMed] [Google Scholar]
- [127].Catania MV, Hollingsworth Z, Penney JB, Young AB. Phospholipase A2 modulates different subtypes of excitatory amino acid receptors: autoradiographic evidence. J Neurochem. 1993;60:236–45. doi: 10.1111/j.1471-4159.1993.tb05843.x. [DOI] [PubMed] [Google Scholar]
- [128].Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–8. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- [129].Mao J, Price DD, Zhu J, Lu J, Mayer DJ. The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain. 1997;72:355–66. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- [130].Kawamura T, Akira T, Watanabe M, Kagitani Y. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997;36:1023–30. doi: 10.1016/s0028-3908(97)00096-8. [DOI] [PubMed] [Google Scholar]
- [131].Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001;64:168–73. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- [132].Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–6. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22:7650–61. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vorwerk CK, Naskar R, Schuettauf F, Quinto K, Zurakowski D, Gochenauer G, Robinson MB, Mackler SA, Dreyer EB. Depression of retinal glutamate transporter function leads to elevated intravitreal glutamate levels and ganglion cell death. Invest Ophthalmol Vis Sci. 2000;41:3615–21. [PubMed] [Google Scholar]
- [135].Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001;905:254–8. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- [136].Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- [138].Wen ZH, Wu GJ, Chang YC, Wang JJ, Wong CS. Dexamethasone modulates the development of morphine tolerance and expression of glutamate transporters in rats. Neuroscience. 2005;133:807–17. doi: 10.1016/j.neuroscience.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [139].Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124:77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [140].Schulz JB, Matthews RT, Muqit MM, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–9. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- [141].Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s disease. Biochem Pharmacol. 2002;64:1037–48. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- [142].Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–9. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–9. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- [144].Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001;902:156–63. doi: 10.1016/s0006-8993(01)02342-3. [DOI] [PubMed] [Google Scholar]
- [145].Himi T, Ikeda M, Yasuhara T, Nishida M, Morita I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J Neural Transm. 2003;110:1337–48. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- [146].Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–23. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kennedy AJ, Voaden MJ, Marshall J. Glutamate metabolism in the frog retina. Nature. 1974;252:50–2. doi: 10.1038/252050a0. [DOI] [PubMed] [Google Scholar]
- [148].Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123–42. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- [149].Suarez I, Bodega G, Arilla E, Fernandez B. Region-selective glutamine synthetase expression in the rat central nervous system following portocaval anastomosis. Neuropathol Appl Neurobiol. 1997;23:254–61. [PubMed] [Google Scholar]
- [150].Suarez I, Bodega G, Fernandez B. Modulation of glutamate transporters (GLAST, GLT-1 and EAAC1) in the rat cerebellum following portocaval anastomosis. Brain Res. 2000;859:293–302. doi: 10.1016/s0006-8993(00)01993-4. [DOI] [PubMed] [Google Scholar]
- [151].Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. Faseb J. 2005;19:1878–80. doi: 10.1096/fj.05-3748fje. [DOI] [PubMed] [Google Scholar]
- [152].Reddy DS, Kulkarni SK. Chronic neurosteroid treatment prevents the development of morphine tolerance and attenuates abstinence behavior in mice. Eur J Pharmacol. 1997;337:19–25. doi: 10.1016/s0014-2999(97)01294-6. [DOI] [PubMed] [Google Scholar]
- [153].Salvemini D. Analgesic methods using synthetic catalysts for the dismutation of superoxide radicals. 6,180,620 US patent. 2001
- [154].Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–9. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- [155].Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- [156].Droy-Lefaix MT, Drouet Y, Geraud G, Hosford D, Braquet P. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Radic Res Commun. 1991;12-13(Pt 2):725–35. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- [157].Haglind E, Xia G, Rylander R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- [158].McInnis J, Wang C, Anastasio N, Hultman M, Ye Y, Salvemini D, Johnson KM. The role of superoxide and nuclear factor-kappaB signaling in N-methyl-D-aspartate-induced necrosis and apoptosis. J Pharmacol Exp Ther. 2002;301:478–87. doi: 10.1124/jpet.301.2.478. [DOI] [PubMed] [Google Scholar]
- [159].Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–93. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- [160].Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Ndengele M, Salvemini D. Superoxide-related signaling cascade mediates nuclear factor-kappaB activation in acute inflammation. Antioxid Redox Signal. 2004;6:699–704. doi: 10.1089/1523086041361659. [DOI] [PubMed] [Google Scholar]
- [161].Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- [162].Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem. 2002;277:2330–5. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- [163].Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–27. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Haddad JJ, Land SC. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br J Pharmacol. 2002;135:520–36. doi: 10.1038/sj.bjp.0704467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Salvemini D, Riley DP, Lennon PJ, Wang ZQ, Currie MG, Macarthur H, Misko TP. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br J Pharmacol. 1999;127:685–92. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Macarthur H, Couri DM, Wilken GH, Westfall TC, Lechner AJ, Matuschak GM, Chen Z, Salvemini D. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: correlation with preserved circulating catecholamines. Crit Care Med. 2003;31:237–45. doi: 10.1097/00003246-200301000-00037. [DOI] [PubMed] [Google Scholar]
- [167].Macarthur H, Westfall TC, Riley DP, Misko TP, Salvemini D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci U S A. 2000;97:9753–8. doi: 10.1073/pnas.97.17.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wong CS, Hsu MM, Chou R, Chou YY, Tung CS. Intrathecal cyclooxygenase inhibitor administration attenuates morphine antinociceptive tolerance in rats. Br J Anaesth. 2000;85:747–51. doi: 10.1093/bja/85.5.747. [DOI] [PubMed] [Google Scholar]
- [169].Hernandez-Delgadillo GP, Cruz SL. Dipyrone potentiates morphine-induced antinociception in dipyrone-treated and morphine-tolerant rats. Eur J Pharmacol. 2004;502:67–73. doi: 10.1016/j.ejphar.2004.08.032. [DOI] [PubMed] [Google Scholar]
- [170].Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–52. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- [171].Salvemini D, Masferrer JL. Interactions of nitric oxide with cyclooxygenase: in vitro, ex vivo, and in vivo studies. Methods Enzymol. 1996;269:12–25. doi: 10.1016/s0076-6879(96)69005-3. [DOI] [PubMed] [Google Scholar]
- [172].Goodwin DC, Landino LM, Marnett LJ. Reactions of prostaglandin endoperoxide synthase with nitric oxide and peroxynitrite. Drug Metab Rev. 1999;31:273–94. doi: 10.1081/dmr-100101918. [DOI] [PubMed] [Google Scholar]
- [173].Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–4. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Yang T, Zhang A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am J Physiol Renal Physiol. 2006;291:F891–5. doi: 10.1152/ajprenal.00512.2005. [DOI] [PubMed] [Google Scholar]
- [175].Cheng HF, Zhang MZ, Harris RC. Nitric oxide stimulates cyclooxygenase-2 in cultured cTAL cells through a p38-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1391–7. doi: 10.1152/ajprenal.00315.2005. [DOI] [PubMed] [Google Scholar]
- [176].Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. Faseb J. 1999;13:1121–36. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- [177].Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–30. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- [178].Markey CM, Alward A, Weller PE, Marnett LJ. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987;262:6266–79. [PubMed] [Google Scholar]
- [179].Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996;93:15069–74. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Trostchansky A, O’Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R, Rubbo H. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic Biol Med. 2007;42:1029–38. doi: 10.1016/j.freeradbiomed.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [181].von Knethen A, Brune B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. Faseb J. 1997;11:887–95. [PubMed] [Google Scholar]
- [182].Habib A, Bernard C, Lebret M, Creminon C, Esposito B, Tedgui A, Maclouf J. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol. 1997;158:3845–51. [PubMed] [Google Scholar]
- [183].Eligini S, Habib A, Lebret M, Creminon C, Levy-Toledano S, Maclouf J. Induction of cyclo-oxygenase-2 in human endothelial cells by SIN-1 in the absence of prostaglandin production. Br J Pharmacol. 2001;133:1163–71. doi: 10.1038/sj.bjp.0704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Perkins DJ, Kniss DA. Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J Leukoc Biol. 1999;65:792–9. doi: 10.1002/jlb.65.6.792. [DOI] [PubMed] [Google Scholar]
- [185].Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- [186].Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–61. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- [187].Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann N Y Acad Sci. 2001;933:175–84. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- [188].Lim G, Wang S, Lim JA, Mao J. Activity of adenylyl cyclase and protein kinase A contributes to morphine-induced spinal apoptosis. Neurosci Lett. 2005;389:104–8. doi: 10.1016/j.neulet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- [189].Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- [190].Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140-141:113–24. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- [191].Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–21. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [192].Shacka JJ, Sahawneh MA, Gonzalez JD, Ye YZ, D’Alessandro TL, Estevez AG. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13:1506–14. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- [193].Zhuang S, Simon G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am J Physiol Cell Physiol. 2000;279:C341–51. doi: 10.1152/ajpcell.2000.279.2.C341. [DOI] [PubMed] [Google Scholar]
- [194].Virag L, Marmer DJ, Szabo C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic Biol Med. 1998;25:1075–82. doi: 10.1016/s0891-5849(98)00139-7. [DOI] [PubMed] [Google Scholar]
- [195].Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–36. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- [196].MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31:1603–8. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- [197].Yamakura F, Matsumoto T, Fujimura T, Taka H, Murayama K, Imai T, Uchida K. Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochim Biophys Acta. 2001;1548:38–46. doi: 10.1016/s0167-4838(01)00212-6. [DOI] [PubMed] [Google Scholar]
- [198].Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–9. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- [199].Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–36. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- [200].Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–71. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008 doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]