Programmed cell death, or apoptosis, is important for the development and homeostasis of tissues. Too little cell death can result in autoimmune diseases or cancer, whereas excessive cell death can lead to debilitating degenerative diseases of the heart or nervous system. The realization that apoptosis was genetically controlled first arose when it was observed that certain mutants of the model organism Caenorhabditis elegans caused failure of apoptosis in cells that normally undergo this process during development (Hengartner et al., 1992). Subsequently, it was found that proteins that are encoded by the mutant genes discovered in C. elegans shared homology with mammalian proteins, including B-cell CLL/lymphoma 2 (Bcl-2) (Hengartner and Horvitz, 1994). Further study in mammals revealed that there is an intrinsic apoptotic pathway that involves the mitochondria and an extrinsic apoptotic pathway that involves death receptors. The mitochondrial pathway of apoptosis in mammals, on which this poster article is focused, is regulated by members of the Bcl-2 family of proteins.
Figure 1.
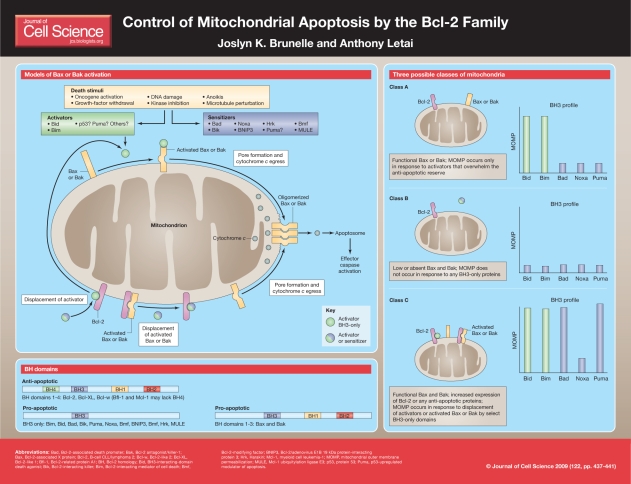
Proteins of the Bcl-2 family have either pro- or anti-apoptotic activities and regulate the mitochondrial pathway of apoptosis by controlling the permeabilization of the outer mitochondrial membrane. In response to many types of stress or damage, certain members of the Bcl-2 family, known as BH3-only proteins (see below), are activated. Certain BH3-only proteins cause the activation of the pro-apoptotic proteins Bcl-2-associated X protein (Bax) or Bcl-2 antagonist/killer-1 (Bak) at the mitochondrion. Activated Bax and Bak homo-oligomerize and participate in the formation of pores in the outer mitochondrial membrane through which pro-apoptotic molecules escape, including second mitochondria-derived activator of caspase (Smac) (also known as Diablo) and cytochrome c. Release of cytochrome c leads to the activation of caspases, which are proteases that cleave key cellular proteins. This leads to many of the morphological characteristics of apoptosis, including condensed nuclei, DNA laddering and exposure of phosphatidylserine to the outer leaflet of the plasma membrane.
Expression of Bcl-2 or other related anti-apoptotic proteins, including myeloid cell leukemia-1 (Mcl-1), Bcl-2-like 1 (Bcl-XL), Bcl-2-like 2 (Bcl-w) and Bcl-2-related protein A1 (Bfl-1), block cell death in response to many varieties of insult by preventing the activation and homo-oligomerization of both Bax and Bak. Anti-apoptotic proteins perform their anti-death function by sequestering BH3-only proteins or activated, monomeric Bax and Bak. Cells that survive continuous, permanent death signaling owing to the presence of Bcl-2 are dependent on Bcl-2 for their survival. It is known that certain cancer cells depend upon Bcl-2 and other anti-apoptotic proteins for survival. We have found that dependence on anti-apoptotic proteins can be identified in cancer cells using a strategy that we call BH3 profiling (see below and Box 1). In cancer cells that are dependent on Bcl-2, the Bcl-2 protein binds pro-apoptotic BH3-only proteins such as Bcl-2-interacting mediator of cell death (Bim). We describe such cells as being `primed for death'. Molecular therapies that are targeted to anti-apoptotic proteins such as Bcl-2 can induce apoptosis in primed cancer cells; one such Bcl-2 antagonist, Abbott (ABT)-737, has shown impressive success in killing leukemia and lymphoma cells (Table 1).
Box 1. The BH3 profiling technique
BH3 profiling is a technique that uses BH3 domains of BH3-only proteins to apply a standardized death signal to mitochondria. This allows for the comparison of how readily different mitochondria, and hence cells, undergo apoptosis. Each anti-apoptotic protein of the Bcl-2 family has a distinct pattern of binding to certain BH3-only proteins. Peptides are designed using the amino-acid sequence (approximately 20 amino acids) of the corresponding BH3-only protein. Mitochondria are isolated from the cell line or patient sample. Peptides are incubated with the mitochondria and mitochondrial outer-membrane permeabilization (MOMP) is measured. The resulting pattern of peptides that do or do not cause MOMP is the readout of the assay.
BH3 profiling can also distinguish among three classes of apoptotic block that are used by cancer cells to survive. A class A block indicates that functional activator BH3-only proteins are present at relatively low levels. In this case, the BH3-only protein activators Bid and Bim, but not any of the BH3-only protein sensitizer peptides, would cause MOMP. In a class B block, the pro-apoptotic proteins Bax and/or Bak are absent or not functional. In this case, none of the BH3-only peptides would cause MOMP as Bax and/or Bak are required for their effect. A class C block indicates that anti-apoptotic proteins are present and primed with BH3-only protein activators, or activated Bax or Bak. In this case, one can compare the pattern of the BH3-only sensitizer peptides that cause MOMP with the binding code for the specific anti-apoptotic proteins to determine which anti-apoptotic proteins are primarily responsible for maintaining survival.
Table 1.
Current clinical strategies to target proteins of the Bcl-2 family
| Drug | Company | Clinical phase | Function | Additional comments |
|---|---|---|---|---|
| ABT-263 | Abbott Laboratories | Phase I and I/II clinical trials in NHL, CLL and SCLC | BH3-mimetic small molecule targeting Bcl-2, Bcl-XL and Bcl-w | ABT-263 is an orally available compound that is closely related to ABT-737 |
| Obatoclax (GX15-070) | Gemin X | Multiple phase I and II clinical trials in hematological malignancies and non-small-cell lung cancer | BH3-mimetic small molecule | Might be a pan-inhibitor of anti-apoptotic proteins |
| Oblimersen | Genta | Many clinical trials including phase III in melanoma and CLL | Antisense DNA targeting Bcl-2 | Clinical activity marginal, but not clear that drug is reducing Bcl-2 levels in vivo |
| AT-101 | Ascenta Therapeutics | Phase II clinical trials in a variety of cancers | BH3-mimetic small molecule | AT-101 is the negative enantiomer of gossypol |
CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin's lymphoma; SCLC, small-cell lung cancer
In this brief Cell Science at a Glance poster article, we discuss how proteins of the Bcl-2 family control the crucial event in the commitment to apoptosis – the permeabilization of the mitochondrial outer membrane. In addition, we discuss recently developed methods for probing how cancer cells manipulate members of the Bcl-2 family to block apoptosis. Understanding these principles is key to understanding how certain insults and derangements commit selected cells to death, but spare others.
Members of the Bcl-2 family
The Bcl-2 family can be divided into pro-apoptotic and anti-apoptotic proteins. These proteins contain one or more Bcl-2 homology (BH) domains, which share sequence homology and are important for heterodimeric interactions among members of the Bcl-2 family (Chittenden et al., 1995; Danial and Korsmeyer, 2004). Most anti-apoptotic proteins contain BH domains 1-4. Pro-apoptotic proteins can be divided into two groups according to function and the number of BH domains possessed. Bax and Bak are pro-apoptotic proteins that contain BH domains 1-3 and are known as multidomain pro-apoptotic or effector proteins. The remaining pro-apoptotic proteins contain only the third BH domain and are known as BH3-only proteins. BH3-only proteins act as upstream sentinels of cellular damage and derangement. They can be activated by many noxious stimuli – including DNA damage, growth-factor withdrawal and oncogene activation – via mechanisms that include transcriptional upregulation, subcellular localization and/or post-translational modifications. For example, p53-upregulated modulator of apoptosis (Puma) and Noxa (the latin word for damage; also known as PMAIP1) are transcriptionally upregulated by p53 in response to DNA damage (Nakano and Vousden, 2001; Oda et al., 2000). In some cells, Bim can be sequestered within the cytoskeleton, to be released only in response to certain death stimuli (Puthalakath et al., 1999). BH3-interacting-domain death agonist (Bid) is activated by cleavage by caspase 8 to form truncated Bid (tBID) (Li et al., 1998), whereas Bcl-2-associated death promoter (Bad) is activated by dephosphorylation (Zha et al., 1996). The relatively numerous pro- and anti-apoptotic members of this family engage in complex interactions with each other to ultimately decide whether a cell will commit to death by controlling permeabilization of the mitochondrial outer membrane.
Mitochondrial permeabilization
Mitochondrial outer membrane permeabilization (MOMP), which releases numerous pro-apoptotic proteins into the cytosol, is the pivotal event in the intrinsic apoptotic pathway. Bax and Bak double-knockout cells fail to undergo MOMP in response to many different death stimuli, including staurosporine, ultraviolet (UV) radiation, growth-factor deprivation, DNA damage and endoplasmic reticulum stress (Wei et al., 2001). Apoptosis that is caused by BH3-only proteins absolutely requires Bax and Bak (Kuwana et al., 2005; Lindsten et al., 2000; Wei et al., 2000; Wei et al., 2001). Bax proteins can be found as monomers in the cytosol or loosely associated with the outer mitochondrial membrane when not activated. Bax translocates to and inserts into the mitochondrial outer membrane during the activation process (Billen et al., 2008; Hsu et al., 1997; Wolter et al., 1997). Bak is inserted into the outer mitochondrial membrane even when not activated (Wei et al., 2000).
One of the steps that is involved in the activation of Bax and Bak is a conformational change that exposes the N-terminus of the proteins, which is otherwise hidden in the inactive state (Yethon et al., 2003). This activated conformation can be recognized by conformation-specific antibodies, such as 6A7, which is specific for Bax (Hsu and Youle, 1997). Following activation, Bax and Bak form homo-oligomers that can be visualized via western blotting following chemical crosslinking (Wei et al., 2000). Bax and Bak oligomers participate in forming pores in and cause permeabilization of the outer mitochondrial membrane, leading to the release of the contents of the mitochondrial intermembrane space, including cytochrome c and Smac, into the cytosol (Wang, 2001). These contents drive the activation of caspases, which are proteases that cleave and disable crucial proteins throughout the cell.
Bax and Bak activation
Given the lethal consequences of Bax and Bak activation, understanding how their activation is controlled is key to understanding how a cell makes the decision to undergo apoptosis. Two models of Bax and Bak activation exist: the indirect and the direct models. According to the indirect model, Bax and Bak must always be bound by anti-apoptotic proteins to prevent their activation. BH3-only proteins provoke death solely by binding to anti-apoptotic proteins, causing release and activation of Bax and Bak (Willis et al., 2007). A prediction of this model is that, for a cell to survive, all Bax and Bak proteins must be bound to an anti-apoptotic protein such as Bcl-2 and/or Mcl-1. However, co-immunoprecipitation of anti-apoptotic proteins with Bax and Bak demonstrates that usually only a small minority of Bak is so bound. Furthermore, co-immunoprecipitation is often carried out in the presence of detergents such as Triton X-100 or Tergitol-type nonyl phenoxylpolyethoxylethanol-40 (NP-40). These detergents can artificially induce Bax and Bak conformations that mimic an activated state and stimulate artifactual binding to anti-apoptotic proteins (Hsu and Youle, 1997). This can result in an overestimation of the amount of Bax that is actually bound to anti-apoptotic proteins. Artificial activation of Bax and Bak does not occur in buffers that contain detergents such as 3-[3-(Cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate (CHAPS). However, it might be that Bax and Bak can alternatively interact with proteins that remove the requirement for sequestration by anti-apoptotic proteins (Cheng et al., 2003). More recent iterations of this model include the concept that the sequestered forms of Bax and Bak are those fractions of the total Bax and Bak population that are already activated, perhaps spontaneously, or perhaps by other unspecified means (Fletcher et al., 2008).
According to the direct model of Bax and Bak activation, activator proteins (which include Bid, Bim and possibly others such as Puma and p53) directly interact with and induce conformational changes in Bax and Bak. Studies using full-length proteins, lipid membranes and real-time fluorescence resonance energy transfer (FRET) analyses has provided convincing evidence of such an interaction and for its role in membrane permeabilization (Lovell et al., 2008). Anti-apoptotic proteins prevent death by binding and sequestering such pro-apoptotic activator BH3-only proteins, and also by binding any monomeric, activated Bax and Bak proteins that might be present. In this model, BH3-only proteins are further divided into activator and sensitizer categories (Letai et al., 2002). Sensitizers cannot activate Bax and Bak directly, but can bind anti-apoptotic proteins and cause the release of activator BH3-only proteins, leading to activation of Bax and Bak. A prediction of this model is that the deletion of all activators would result in a profound block in apoptosis that is equivalent to the loss of Bax and Bak. By contrast, however, a combined knockout of Bid and Bim results in relatively minor defects in apoptosis (Willis et al., 2007), but it is likely that additional factors other than Bid and Bim can act as activators. In fact, there are data that support the role of Puma, p53 and heat as activators of Bax and Bak (Chipuk et al., 2004; Kim et al., 2006; Pagliari et al., 2005), and possibly others remain undiscovered. The important point is that the activation of Bax and Bak might be affected by factors outside of the Bcl-2 family of proteins. Very recent results have demonstrated a structure of a complex of the BH3 domain of Bim with Bax (Gavathiotis et al., 2008). Surprisingly, the interaction takes place on the Bax surface distal from the hydrophobic pocket formed by the BH1, BH2 and BH3 domains. The analogous pocket is used by anti-apoptotic proteins to bind BH3 domains.
The gulf between the two models is not unbridgeable. As long as one is willing to accept that there are activated subsets of Bax and Bak that are required to kill, and that must be sequestered by anti-apoptotic proteins to maintain survival, a unifying model can be constructed. In this model, activated Bax and Bak are responsible for the permeabilization of membranes. They achieve the activated state either by interacting with activator proteins, by spontaneously activating, or via other unknown means. Anti-apoptotic proteins inhibit death by sequestering activator proteins or activated Bax and Bak. In addition to activating Bax and Bak (a property possessed by only a subset of BH3-only proteins), BH3-only proteins cause death by displacing activators and Bax and Bak from anti-apoptotic proteins, permitting progression of the death signal. This model is summarized in the poster accompanying this article.
Specificity in the interaction between anti-apoptotic and BH3-only proteins
Mammalian anti-apoptotic proteins include Bcl-2, Bcl-XL, Mcl-1, Bcl-w and Bfl-1. Anti-apoptotic proteins bind and sequester pro-apoptotic proteins, including activator BH3-only proteins and Bax and Bak, to prevent apoptosis. Sensitizer proteins provoke apoptosis by competitively inhibiting this interaction. Each anti-apoptotic protein has its own pattern of interaction with sensitizer proteins (Certo et al., 2006; Chen et al., 2005; Kuwana et al., 2005; Opferman et al., 2003). For example, Bad selectively binds Bcl-2, Bcl-XL and Bcl-w; Noxa specifically binds Mcl-1; and Harakiri (Hrk) is specific for Bcl-XL. Bim, Bid and Puma can bind to all the anti-apoptotic proteins. Mcl-1 has additional unique characteristics. Mcl-1 ubiquitylation ligase E3 (MULE) contains a BH3 domain that specifically binds Mcl-1, which leads to Mcl-1 ubiquitylation and degradation (Zhong et al., 2005). This interaction, in addition to the numerous PEST sequences [which have many proline (P), glutamic acid (E), serine (S) and threonine (T) residues (Kozopas et al., 1993)] possessed by Mcl-1, might account for the short half-life of the Mcl-1 protein. The specificity of these interactions can be exploited to deduce important elements of the control of apoptosis by individual cell types using a procedure called BH3 profiling.
BH3 profiling
The dependence of a cell or mitochondrion on any one of the anti-apoptotic proteins can be detected by a technique called BH3 profiling (see also Box 1) (Certo et al., 2006; Deng et al., 2007). To perform BH3 profiling, mitochondria are exposed to a panel of BH3-domain peptides from BH3-only proteins and MOMP is measured. On the basis of which peptides induce MOMP, one can discern whether there is dependence on an individual anti-apoptotic protein. For example, a response pattern that includes Bim, Bid, Noxa, Puma and Bmf suggests dependence on Mcl-1. Alternatively, a response pattern that includes Bim, Bid, Bad, Puma and Bcl-2-modifying factor (Bmf) suggests dependence on Bcl-2 or Bcl-w. Cells that are dependent on anti-apoptotic proteins share the characteristic of being `primed for death'. The anti-apoptotic proteins of such cells are already burdened with significant amounts of activator BH3-only proteins, such as Bim (Del Gaizo Moore et al., 2007), and can be killed merely by inhibiting anti-apoptotic function. This, in turn, releases the bound pre-existing pro-apoptotic proteins and provokes apoptosis. We have found that many cancer cells and cell lines have this `primed' phenotype, but limited results in normal tissues suggests that this is less likely to be the case in normal tissues. A strategy of Bcl-2 antagonism is being employed in clinical trials of agents such as ABT-263, as some cancers are dependent on Bcl-2 for survival (Oltersdorf et al., 2005; Tse et al., 2008).
Future directions
Although many important discoveries have been made regarding the roles of members of the Bcl-2 family in the mitochondrial apoptosis pathway, important questions remain. Are there other important activator and sensitizer proteins? Are there undiscovered mechanisms for holding Bax and Bak at bay? How do the noxious stimuli we routinely use to kill cells in culture and in vivo interact with proteins of the Bcl-2 family to cause apoptosis? Finally, can direct inhibition of anti-apoptotic proteins be an effective tool in treating cancer? We await the results of clinical trials of drugs such as ABT-263 (Table 1) to answer this key question.
The authors gratefully acknowledge support from NIH grants R01 CA129974 and P01 CA068484. A.L. is a Leukemia and Lymphoma Society Scholar. Deposited in PMC for release after 12 months.
References
- Billen, L. P., Kokoski, C. L., Lovell, J. F., Leber, B. and Andrews, D. W. (2008). Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 6, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo, M., Del Gaizo Moore, V., Nishino, M., Wei, G., Korsmeyer, S., Armstrong, S. A. and Letai, A. (2006). Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351-365. [DOI] [PubMed] [Google Scholar]
- Chen, L., Willis, S. N., Wei, A., Smith, B. J., Fletcher, J. I., Hinds, M. G., Colman, P. M., Day, C. L., Adams, J. M. and Huang, D. C. (2005). Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393-403. [DOI] [PubMed] [Google Scholar]
- Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J. and Korsmeyer, S. J. (2003). VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513-517. [DOI] [PubMed] [Google Scholar]
- Chipuk, J. E., Kuwana, T., Bouchier-Hayes, L., Droin, N. M., Newmeyer, D. D., Schuler, M. and Green, D. R. (2004). Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010-1014. [DOI] [PubMed] [Google Scholar]
- Chittenden, T., Flemington, C., Houghton, A. B., Ebb, R. G., Gallo, G. J., Elangovan, B., Chinnadurai, G. and Lutz, R. J. (1995). A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 14, 5589-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial, N. N. and Korsmeyer, S. J. (2004). Cell death: critical control points. Cell 116, 205-219. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore, V., Brown, J. R., Certo, M., Love, T. M., Novina, C. D. and Letai, A. (2007). Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 117, 112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, J., Carlson, N., Takeyama, K., Dal Cin, P., Shipp, M. and Letai, A. (2007). BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12, 171-185. [DOI] [PubMed] [Google Scholar]
- Fletcher, J. I., Meusburger, S., Hawkins, C. J., Riglar, D. T., Lee, E. F., Fairlie, W. D., Huang, D. C. and Adams, J. M. (2008). Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. USA 105, 18081-18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis, E., Suzuki, M., Davis, M. L., Pitter, K., Bird, G. H., Katz, S. G., Tu, H. C., Kim, H., Cheng, E. H., Tjandra, N. et al. (2008). BAX activation is initiated at a novel interaction site. Nature 455, 1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner, M. O. and Horvitz, H. R. (1994). C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76, 665-676. [DOI] [PubMed] [Google Scholar]
- Hengartner, M. O., Ellis, R. E. and Horvitz, H. R. (1992). Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356, 494-499. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. T. and Youle, R. J. (1997). Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272, 13829-13834. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. T., Wolter, K. G. and Youle, R. J. (1997). Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94, 3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Rafiuddin-Shah, M., Tu, H. C., Jeffers, J. R., Zambetti, G. P., Hsieh, J. J. and Cheng, E. H. (2006). Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 8, 1348-1358. [DOI] [PubMed] [Google Scholar]
- Kozopas, K. M., Yang, T., Buchan, H. L., Zhou, P. and Craig, R. W. (1993). MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 90, 3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana, T., Bouchier-Hayes, L., Chipuk, J. E., Bonzon, C., Sullivan, B. A., Green, D. R. and Newmeyer, D. D. (2005). BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525-535. [DOI] [PubMed] [Google Scholar]
- Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S. and Korsmeyer, S. J. (2002). Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183-192. [DOI] [PubMed] [Google Scholar]
- Li, H., Zhu, H., Xu, C. J. and Yuan, J. (1998). Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491-501. [DOI] [PubMed] [Google Scholar]
- Lindsten, T., Ross, A. J., King, A., Zong, W. X., Rathmell, J. C., Shiels, H. A., Ulrich, E., Waymire, K. G., Mahar, P., Frauwirth, K. et al. (2000). The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell, J. F., Billen, L. P., Bindner, S., Shamas-Din, A., Fradin, C., Leber, B. and Andrews, D. W. (2008). Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074-1084. [DOI] [PubMed] [Google Scholar]
- Nakano, K. and Vousden, K. H. (2001). PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683-694. [DOI] [PubMed] [Google Scholar]
- Oda, E., Ohki, R., Murasawa, H., Nemoto, J., Shibue, T., Yamashita, T., Tokino, T., Taniguchi, T. and Tanaka, N. (2000). Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053-1058. [DOI] [PubMed] [Google Scholar]
- Oltersdorf, T., Elmore, S. W., Shoemaker, A. R., Armstrong, R. C., Augeri, D. J., Belli, B. A., Bruncko, M., Deckwerth, T. L., Dinges, J., Hajduk, P. J. et al. (2005). An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677-681. [DOI] [PubMed] [Google Scholar]
- Opferman, J. T., Letai, A., Beard, C., Sorcinelli, M. D., Ong, C. C. and Korsmeyer, S. J. (2003). Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671-676. [DOI] [PubMed] [Google Scholar]
- Pagliari, L. J., Kuwana, T., Bonzon, C., Newmeyer, D. D., Tu, S., Beere, H. M. and Green, D. R. (2005). The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Natl. Acad. Sci. USA 102, 17975-17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath, H., Huang, D. C., O'Reilly, L. A., King, S. M. and Strasser, A. (1999). The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287-296. [DOI] [PubMed] [Google Scholar]
- Tse, C., Shoemaker, A. R., Adickes, J., Anderson, M. G., Chen, J., Jin, S., Johnson, E. F., Marsh, K. C., Mitten, M. J., Nimmer, P. et al. (2008). ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421-3428. [DOI] [PubMed] [Google Scholar]
- Wang, X. (2001). The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922-2933. [PubMed] [Google Scholar]
- Wei, M. C., Lindsten, T., Mootha, V. K., Weiler, S., Gross, A., Ashiya, M., Thompson, C. B. and Korsmeyer, S. J. (2000). tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060-2071. [PMC free article] [PubMed] [Google Scholar]
- Wei, M. C., Zong, W. X., Cheng, E. H., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K. A., MacGregor, G. R., Thompson, C. B. and Korsmeyer, S. J. (2001). Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S. N., Fletcher, J. I., Kaufmann, T., van Delft, M. F., Chen, L., Czabotar, P. E., Ierino, H., Lee, E. F., Fairlie, W. D., Bouillet, P. et al. (2007). Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856-859. [DOI] [PubMed] [Google Scholar]
- Wolter, K. G., Hsu, Y. T., Smith, C. L., Nechushtan, A., Xi, X. G. and Youle, R. J. (1997). Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yethon, J. A., Epand, R. F., Leber, B., Epand, R. M. and Andrews, D. W. (2003). Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 278, 48935-48941. [DOI] [PubMed] [Google Scholar]
- Zha, J., Harada, H., Yang, E., Jockel, J. and Korsmeyer, S. J. (1996). Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87, 619-628. [DOI] [PubMed] [Google Scholar]
- Zhong, Q., Gao, W., Du, F. and Wang, X. (2005). Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085-1095. [DOI] [PubMed] [Google Scholar]