Summary
Stress granules are cytoplasmic mRNA-silencing foci that form transiently during the stress response. Stress granules harbor abortive translation initiation complexes and are in dynamic equilibrium with translating polysomes. Mammalian Staufen 1 (Stau1) is a ubiquitous double-stranded RNA-binding protein associated with polysomes. Here, we show that Stau1 is recruited to stress granules upon induction of endoplasmic reticulum or oxidative stress as well in stress granules induced by translation initiation blockers. We found that stress granules lacking Stau1 formed in cells depleted of this molecule, indicating that Stau1 is not an essential component of stress granules. Moreover, Stau1 knockdown facilitated stress granule formation upon stress induction. Conversely, transient transfection of Stau1 impaired stress granule formation upon stress or pharmacological initiation arrest. The inhibitory capacity of Stau1 mapped to the amino-terminal half of the molecule, a region known to bind to polysomes. We found that the fraction of polysomes remaining upon stress induction was enriched in Stau1, and that Stau1 overexpression stabilized polysomes against stress. We propose that Stau1 is involved in recovery from stress by stabilizing polysomes, thus helping stress granule dissolution.
Keywords: ER stress, P bodies, Staufen, Oxidative stress, Silencing foci, Stress granules
Introduction
The cellular response to stress involves a global silencing of ongoing translation. The transient formation of large cytoplasmic foci, known as stress granules, was recently reported to be a hallmark of the stress response. Stress granules harbor abortive translation initiation complexes that accumulate upon inactivation of eIF2α by specific kinases activated by distinct stressors. Thus, stress granules contain polyadenylated mRNA in a complex with poly-A binding protein (PABP), several initiation factors including eIF4E, eIF4G, eIF3, eIF2B and phosphorylated-eIF2α, and small, but not large, ribosomal subunits (Kimball et al., 2003) (reviewed by Anderson and Kedersha, 2008). In addition, stress granules include a growing number of RNA-binding proteins (RBPs), which modulate stability or translation, as well as act on nuclear splicing and post-splicing events (Gallouzi et al., 2000; Baez and Boccaccio, 2005; Wilczynska et al., 2005; Vessey et al., 2006; Guil et al., 2006; Stöhr et al., 2006; Mazroui et al., 2007; Kawahara et al., 2008) (reviewed by Anderson and Kedersha, 2008). Stress granules are also induced by overexpression of translational repressors, by pharmacological inhibition of translation initiation or by inosine-modified RNA (Thomas et al., 2005; Baez and Boccaccio, 2005; Wilczynska et al., 2005; Dang et al., 2006; Mazroui et al., 2006; Scadden, 2007; Kim et al., 2008). The transient assembly of abortive initiation complexes in stress granules is mediated by the self-aggregation of the RBP T-cell intracellular antigen (TIA-1), TIA-1-related (TIAR) and RasGAP-associated endoribonuclease (G3BP), among others, and requires O-glycosylation of ribosomal proteins (Kedersha et al., 1999; Tourriere et al., 2003; Ohn et al., 2008) (reviewed by Anderson and Kedersha, 2008). Stress granule disassembly is mediated by the chaperone activity of stress-induced Hsp70 and by phosphorylation of specific RBPs (Tourriere et al., 2003; Tsai et al., 2008) (reviewed by Anderson and Kedersha, 2008). Stress granules are highly dynamic and are in equilibrium with translating polysomes, because disruption of polysomes promotes stress granule formation, whereas polysome stabilization prevents stress granule assembly (Kedersha et al., 2000; Mollet et al., 2008) (reviewed by Anderson and Kedersha, 2008). The processing bodies, also termed decapping bodies or glycine-tryptophan (GW) bodies, are related mRNA-silencing foci. Processing bodies are constitutively present, and they also depend on the global translational state of the cell (Cougot et al., 2004; Andrei et al., 2005; Ferraiuolo et al., 2005; Wilczynska et al., 2005) (reviewed by Eulalio et al., 2007; Anderson and Kedersha, 2008).
We have previously reported the presence of the double-stranded RBPs Staufen 1 (Stau1) and Staufen 2 (Stau2) in stress granules formed in brain oligodendrocytes exposed to oxidative stress (Thomas et al., 2005). Whether Staufen molecules participate in stress granule structure or physiology has not been addressed. The ubiquitously expressed mammalian Stau1 binds to ribosomal subunits through protein-protein and protein-RNA interactions and is shown to be associated to polysomes in several cell types (Kiebler et al., 1999; Marion et al., 1999; Duchaine et al., 2002; Luo et al., 2002; Thomas et al., 2005; Baez and Boccaccio, 2005; Dugré-Brisson et al., 2005). In addition, Stau1 is considered to be a global mRNA-binding factor, as it was shown to bind to different RNA motifs, including G-quartets, the β-actin zip code, the Myc 3′UTR; the FMR1 3′UTR, the HIV transactivating response region and the so-called Staufen-binding motif (Rackham and Brown, 2004; Dugré-Brisson et al., 2005; Kim, Y. K. et al., 2005). Recently, at least 7% of cellular mRNAs were shown to be present in Stau1 ribonucleoparticles (RNPs) (Furic et al., 2008). Consistently with this broad spectrum of targets, Staufen was associated with a variety of cytosolic functions. Staufen is involved in mRNA transport in both oocytes and somatic cells in vertebrates, as well as in invertebrates (Ferrandon et al., 1994; Broadus et al., 1998; Kiebler et al., 1999; Micklem et al., 2000; Tang et al., 2001; Belanger et al., 2003; Yoon and Mowry, 2004; Gautrey et al., 2005; Lebeau et al., 2008; Vessey et al., 2008). When bound to the 3′UTR, mammalian Stau1 triggers mRNA decay by recruitment of UPF1, a key molecule for mRNA degradation (Kim, Y. K. et al., 2005). By contrast, it enhances CAP-dependent translation when tethered to the 5′UTR (Dugré-Brisson et al., 2005). In addition to these cytoplasmic functions, nuclear roles for mammalian Stau1 molecules have begun to emerge (Kiebler et al., 2005). Whether Stau1 functions contribute to stress granule physiology is unknown.
In this work we show that, although mammalian Stau1 is always present in stress granules, either induced by cellular stress or by pharmacological inhibition of translation initiation, is not an essential component of these foci. Moreover, we found that Stau1 depletion facilitated stress granule assembly, whereas transfection of Stau1 constructs inhibited their formation. Processing body integrity was moderately affected by Stau1, according to our finding that Stau1 is scarcely recruited to these structures. We show that Stau1 associates with polysomes, protecting them from stress-induced breakdown. We propose that downstream of the stress signaling, the breakdown of polysomes and concomitant stress granule formation are regulated by Stau1.
Results
Presence of Stau1 in stress granules and processing bodies
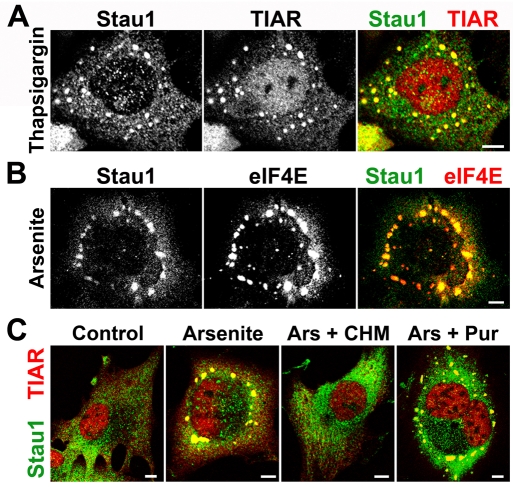
We have previously shown that Stau1 is recruited to stress granules in brain primary cell cultures upon induction of oxidative stress (Thomas et al., 2005). Here, we analyzed the presence of Stau1 in stress granules induced in transformed and non-transformed cell lines of distinct origin. Using a specific antibody against Stau1 (Craig et al., 2005), we found that this molecule was present in stress granules induced in NIH 3T3, HeLa, BHK, COS-7, WI-38, H1299 and U2OS cells, upon exposure to the calcium-pump inhibitor thapsigargin – a known inductor of endoplasmic reticulum stress – as well as in stress granules induced by arsenite, which induces oxidative stress (Fig. 1 and M.G.T., L.J.M.T. and G.L.B., unpublished data). Stau1 was observed to localize in every stress granule identified by staining of the specific marker TIAR or of the eukaryotic initiation factor 4E (eIF4E), also known to be recruited to these foci (Fig. 1A,B). Confocal slicing along the z-axis confirmed the presence of Stau1 inside stress granules and Stau1 was also detected in stress granules induced by heat shock or DTT (G.L.B. and Mariela Loschi, unpublished data). Stress granules are known to be transient, and thus we investigated whether the recruitment of Stau1 to these structures varies during their assembly and dissolution. We found that although thapsigargin-induced stress granules persisted longer than those induced by oxidative stress (see below), Stau1 was always detected in stress granules during their assembly and dissolution (not shown). By contrast, distinct molecules were incorporated to thapsigargin-induced stress granules in a time-dependent manner (see below).
Fig. 1.
Stau1 is recruited to stress granules upon induction of ER or oxidative stress. NIH 3T3 (A) and HeLa cells (B) were exposed to 1 μM thapsigargin or 0.5 mM sodium arsenite for 1 hour and immunostained for Stau1 and the indicated stress granule markers. (C) BHK cells were exposed to arsenite in the presence of cycloheximide (CHM) or puromycin (Pur). Representative cells showing inhibition of stress granule formation by cycloheximide and the absence of effect by puromycin are depicted. In the absence of stress granules, Stau1 remained dispersed in the cytoplasm. Scale bars: 10 μm.
Finally, we tested whether the stress-induced Stau1 aggregates were sensitive to polysome-stabilizing drugs, a distinctive feature of stress granules. As expected, we found that stabilization of polysomes by cycloheximide prevented the formation of foci containing TIAR and Stau1, whereas puromycin elicited no effect or slightly enhanced stress granule formation (Fig. 1C). Thus, stress granule assembly and incorporation of Stau1 to these foci are concomitant with polysome disruption.
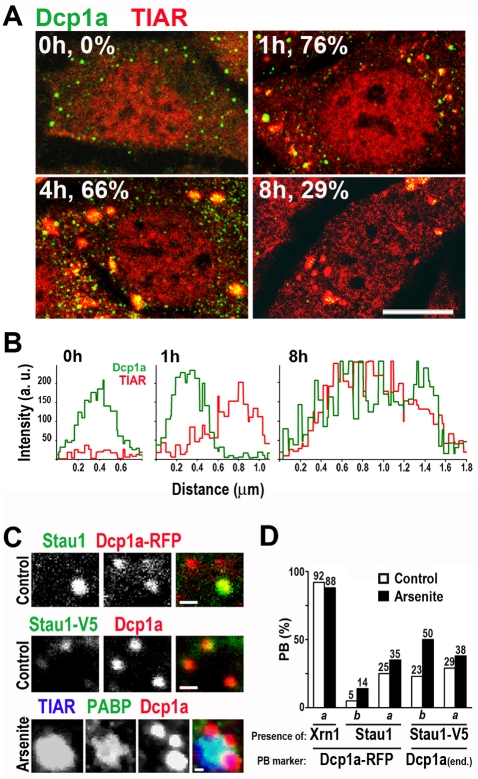
Similarly to stress granules, processing bodies represent dynamic storage compartments for untranslated mRNAs, and are likewise in equilibrium with translating polysomes. In addition, stress granules and processing bodies share certain protein components (reviewed by Eulalio et al., 2007; Anderson and Kedersha, 2008). Here, we investigated the recruitment of Stau1 to normal or stress-induced processing bodies identified by the presence of Dcp1a, which is a marker for processing bodies. First, we investigated the presence of processing bodies upon induction of oxidative or ER stress. In both stress models, stress granules were frequently detected in close contact with processing bodies (Fig. 2), as similarly reported for stress granules induced by the overexpression of G3BP or TIA-1 (Kedersha et al., 2005) (reviewed by Anderson and Kedersha, 2008). As reported before, we found that treatment with arsenite increased the size and number of processing bodies, as well as the number of cells containing processing bodies with a time-course that paralleled that of stress granules (not shown). At all time points analyzed upon arsenite exposure, we found that Dcp1a was excluded from stress granules, whereas the processing body components GW182 and Xrn1 were present in both types of foci (M.G.T. and G.L.B., unpublished results). By contrast, upon continuous treatment with 250 nM thapsigargin, Dcp1a incorporated to stress granules and free processing bodies were no longer detected (4 and 8 hours in Fig. 2A,B). Strikingly, during the stress granule dissolution phase of thapsigargin treatment, processing bodies also vanished; the Dcp1a signal remaining dispersed in the cytoplasm. All these observations suggest that a regulated transfer of molecules between stress granules and processing bodies occurs, and that these foci are differentially modulated by different stressors.
Fig. 2.
Stau1 is not recruited to processing bodies. (A,B) NIH 3T3 cells were continuously exposed to thapsigargin and stained for TIAR and DCP1a at the indicated times. Percentage of cells with stress granules is indicated. Scale bar: 10 μm. The intensity profiles of Dcp1a and TIAR were analyzed by confocal line-scanning of single processing bodies and stress granules (B). Processing bodies were initially detected in close apposition with stress granules (1 hour). At 4 hours, larger stress granules were observed and Dcp1a staining was detected both in processing bodies and as punctuated inclusions inside stress granules. At 8 hours, free processing bodies were absent and Dcp1a signal was detected in the remaining stress granules. (C) Representative processing bodies showing a lack of signal (71-77% of processing bodies) or weak signal (23-29% of processing bodies) of endogenous or transfected Stau1 under resting conditions. Bottom, immunostaining with specific antibodies showed that PABP is excluded from processing bodies upon oxidative stress induction. Scale bars: 1 μm. (D) U2OS (a) or NIH 3T3 cells (b) were transiently transfected with the indicated constructs for 24 hours. After treatment with arsenite for 1 hour, cells were immunostained for TIA-1 and Dcp1a, Stau1 or Xrn1. The presence of Stau1 or Xrn1 in randomly selected processing bodies (PBs) from 10 cells in each condition was evaluated using ×100 confocal images and Z-slice analysis. The percentage of processing bodies containing the indicated markers is plotted.
Next, we followed the distribution of endogenous or transfected Stau1 and Dcp1a molecules in NIH 3T3 and U2OS cells under resting or oxidative stress conditions. Expression of transfected molecules was allowed for short times, because overexpression of Stau1 or Dcp1a affects stress granules and processing bodies (Fenger-Gron et al., 2005) (see below). Endogenous Stau1 was barely detected in processing bodies identified as Dcp1a-RFP foci under resting conditions (Fig. 2C,D), and was scarcely recruited to processing bodies upon stress induction. Similarly, tagged Stau1 was sporadically detected in processing bodies identified by staining of endogenous Dcp1a, and oxidative stress induction moderately increased the proportion of processing bodies containing Stau1-V5 (Fig. 2C,D). As expected, the exoribonuclease Xrn1 was always detected in Dcp1a-RFP foci (Fig. 2D), whereas PABP was completely excluded from processing bodies in any condition tested (Fig. 2C).
In summary, in resting conditions, the ubiquitously expressed Stau1 is mostly associated with polysomes (Marion et al., 1999; Luo et al., 2002; Dugré-Brisson et al., 2005; Thomas et al., 2005) and is mobilized to stress granules concomitantly with polysome disruption upon stress. A proportion of Stau1 remains non-aggregated and is probably retained in the fraction of polysomes that were not disassembled by the stressor (see below).
Stau1 silencing enhances stress granule formation
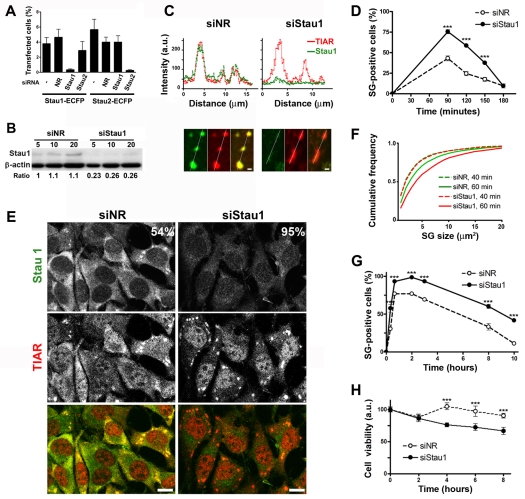
We evaluated the effect of Stau1 depletion on stress granule formation. The selected siRNAs against Stau1 or Stau2 (siStau1 and siStau2) specifically diminished the expression of their target molecules without affecting the expression of the other Staufen homologue or of ECFP constructs (Fig. 3A,B and M.A.D., L.J.M.T. and G.L.B., unpublished observations). Reduction of endogenous Stau1 in NIH 3T3 cells by siStau1 was approximately 75%, as judged by western blot analysis (Fig. 3B). We then analyzed whether silencing of Staufen molecules affects stress granule formation. We found that upon oxidative stress induction, stress granules were assembled in cells with reduced levels of Stau1, and remarkably, Stau1 was barely detectable or completely absent from these stress granules, as visualized by line-scan analysis (Fig. 3C). A quantitative analysis performed at different times upon arsenite exposure revealed that the formation of stress granules was significantly facilitated by Stau1 depletion (Fig. 3D,E). Silencing of Staufen 2 provoked a minor stimulation of stress granule formation in NIH 3T3 cells (M.A.D., M.G.T. and G.L.B., unpublished observations), probably reflecting the fact that this Staufen homolog is expressed at low levels in these cells. We found that siRNAs against human Stau1 also enhanced stress granule formation in U2OS cells (M.G.T. and G.L.B., unpublished observations). For comparison, the effect of siRNA-mediated depletion of selected processing body components on stress granule formation was evaluated. In contrast to the effect of Stau1 knockdown, depletion of Hedls, or of RCK/p54 – a processing body component also recruited to stress granules – moderately impaired the formation of stress granules upon oxidative stress induction (supplementary material Fig. S1A). Staining for Dcp1a showed processing body disruption in resting conditions and partial recovery of processing bodies upon arsenite exposure (supplementary material Fig. S1B). Thus, the enhancing effect on stress granule formation was restricted to the siRNA-mediated knockdown of Staufen molecules.
Fig. 3.
Stau1 depletion facilitates stress granule formation. (A) Stau1-ECFP or Stau2-ECFP constructs were independently transfected into COS-7 cells simultaneously with the indicated siRNAs (NR, non-relevant). Expressing cells and total cells identified by DAPI staining were counted 16 hours after transfection. (B) NIH 3T3 cells were treated with siNR or siStau1 and extracts were analyzed by western blot. Intensity of the Stau1 signal relative to that of β-actin indicates a 75% reduction of Stau1 levels. (C) Line-scan analysis of arsenite-induced stress granules in siNR- or siStau1-treated cells indicates the presence of negligible amounts of Stau1 upon Stau1 depletion. Scale bar: 1 μm. (D) Upon treatment with the indicated siRNAs, cells were continuously exposed to arsenite and cells containing stress granules (SGs) were identified by TIAR staining. A representative experiment out of three is shown where approximately 200 siNR-treated cells and 300 siStau1-treated cells randomly selected from duplicate coverslips were analyzed for each time point. Stress granule formation is facilitated in Stau1-depleted cells; ***P<0.0001 for each data pair. (E) TIAR and Stau1 staining in siNR- and siStau1-treated cells after 1 hour arsenite treatment. Percentage of cells containing stress granules is indicated. (F,G) After treatment with the indicated siRNA, NIH 3T3 cells were exposed to thapsigargin and stained for TIAR and Stau1. (F) stress granule size was evaluated in approximately 1800 stress granules present in 90 cells at 40 or 60 minutes after thapsigargin treatment. (G) Time course of stress granule formation. A representative experiment out of three is depicted. A minimum of 400 cells was analyzed for each point. Stau1 depletion significantly facilitates the formation of stress granules induced by ER stress (***P<0.0003). (H) NIH 3T3 cells were treated with the indicated siRNAs and continuously exposed to 100 nM thapsigargin. Percentage of viable cells was determined in triplicate using the MTT viability assay. A representative experiment out of three is depicted. ***P<0.0001.
We then analyzed whether stress granules induced by ER stress were similarly affected. We found that silencing of Stau1 significantly enhanced the formation of thapsigargin-induced stress granules. As for the oxidative stress model, we found that cells with stronger Stau1 silencing showed a higher incidence of stress granules (supplementary material Fig. S1C). The size of the stress granules was also affected by Stau1 depletion. An increase in the average size from 5.8 μm2 in control cells to 7.2 μm2 in siStau1-treated cells was observed 60 minutes after stress induction (Fig. 3F). Finally, as for the arsenite-stress model, thapsigargin-induced stress granules lasted longer in Stau1-depleted cells (Fig. 3G). Altogether, these observations indicate that Stau1 is not an essential component of stress granules; this molecule rather impairs stress granule formation and facilitates their dissolution. We also analyzed the effect of Stau1 depletion on processing bodies. Both basal and arsenite-induced processing bodies were weakly enhanced by Stau1 depletion (M.G.T. and G.L.B., unpublished results). It has been proposed that stress granules help cell survival in several ways (Kim, W. J. et al., 2005; Kim, W. J. et al., 2007; Mazroui et al., 2007; Yu et al., 2007; Arimoto et al., 2008; Eisinger-Mathason et al., 2008) (reviewed by Anderson and Kedersha, 2008). We assessed cell survival upon induction of ER stress in Stau1-depleted cells. We found that the exposure of siNR-treated cells to 100 nM thapsigargin resulted in a non-lethal stimulus, whereas it provoked the death of Stau1-depleted cells (Fig. 3H). These results highlight the relevance of Stau1 as a protective molecule against the stress stimulus.
Stau1 overexpression impairs stress granule formation
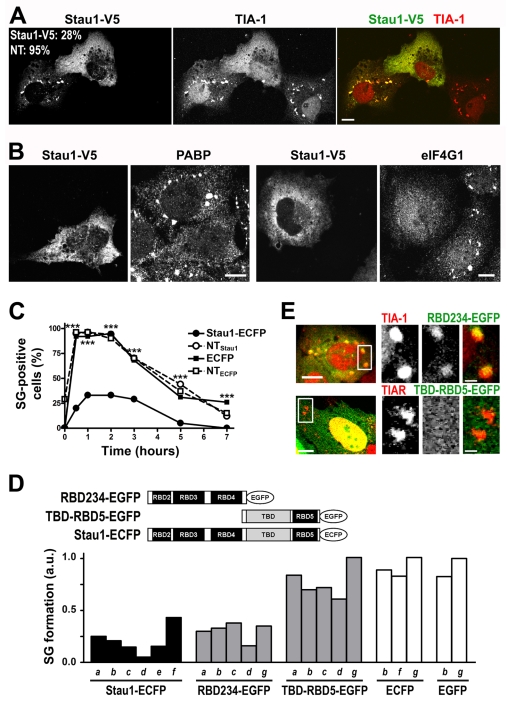
Next, we evaluated the effect of increasing Stau1 levels on stress granule formation. First, we confirmed that transfected tagged Stau1 was incorporated into stress granules. Murine or rat Stau1 fused to V5 or ECFP colocalized with the marker proteins TIA-1 and TIAR, the small ribosomal subunit maker S6, the translation initiation factors eIF4E and eIF4G1, and the RBPs HuR and G3BP in low-expressing cells exposed to arsenite (supplementary material Fig. S2A). Strikingly, we found that a moderate overexpression of Stau1 inhibited stress granule formation upon oxidative stress induction. The stress granule marker molecules TIA-1 and TIAR were detected in the cytoplasm of Stau1-overexpressing cells and showed a non-granular distribution (Fig. 4A). The absence of stress granules was confirmed by the uniform distribution of PABP and eIF4G1 (Fig. 4B), indicating that aggregates containing polyadenylated mRNA or initiation factors were not formed. As expected, overexpression of ECFP had no effect on stress granule formation and, as previously shown (Gilks et al., 2004), transfection of TIA-1-GFP was not inhibitory but rather stimulatory (supplementary material Fig. S2B). The inhibitory capacity of Stau1 correlated with the expression levels. Levels of transfected Stau1 relative to the endogenous protein were estimated as indicated in the Materials and Methods. After 24 hours of expression, cells with three times the normal amount of Stau1 showed a reduction from 86% to 22% in arsenite-induced stress granule formation (supplementary material Fig. S2C). By contrast, and as reported before (Gilks et al., 2004), we found that inhibition of stress granule formation by a fragment containing the prion-like domain of TIA-1 required a massive overexpression obtained after 3 days of transfection (C.C.L. and G.L.B., unpublished results). Stau1-mediated inhibition was partially overridden by increasing concentrations of arsenite (L.J.M.T. and G.L.B., unpublished data), suggesting that the formation of stress granules depends on the balance between Stau1 levels and the strength of the stress stimulus.
Fig. 4.
Stau1 overexpression inhibits stress granule formation. (A,B) COS-7 cells were transfected with Stau1-V5 and exposed to arsenite 24 hours after transfection. Representative cells showing the presence of stress granules in non-expressing or low expressing cells and their absence upon moderate Stau1-V5 overexpression are shown. Percentage of transfected or non-transfected cells containing stress granules is indicated. Scale bars: 10 μm. (C) NIH 3T3 cells were continuously exposed to thapsigargin 16 hours after transfection with Stau1-ECFP or ECFP. Cells with moderate level of expression (less than four times the endogenous levels) were analyzed for stress granule formation. A minimum of 120 transfected cells or neighboring non-transfected cells (NTECFP and NTStau1) from duplicate coverslips were analyzed. ***P<0.0001 for Stau1-ECFP expressing cells. (D) U2OS (a-c); NIH 3T3 (d); H1299 (e) Cos7 (f) or HeLa cells (g) were transfected with the indicated Stau1 construct and exposed to 250 nM or 500 nM thapsigargin (a and b); or to 0.1, 0.2 or 0.25 mM arsenite (c, d and e, respectively); or to 0.5 mM arsenite (f and g). Approximately 200 transfected and non-transfected cells were counted in each case. Stress granule formation is expressed as the ratio of the percentage of transfected stress granule-forming cells relative to that of neighboring non-transfected cells. The incidence of stress granules in non-transfected cells was as follows: a, 32%; b, 54%; c, 41%, d, 86%; e, 35%; f, 97% and g, 86%. Differences between averaged independent experiments (two in b and f) or between replicate coverslips (a-g) were less than 10% in all cases. (E) U2OS cells transfected with the indicated constructs were exposed to thapsigargin (upper panel) or arsenite (bottom panel). Representative stress granules showing inclusion of RBD234-EGFP or exclusion of TBD-RBD5-EGFP are shown. Scale bars: 10 μm (left panels) and 2 μm (insets).
We also analyzed the effect of Stau1 transfection on the formation of stress granules induced by ER stress. A time-course analysis indicated that cells overexpressing Stau1 had a reduced response to thapsigargin relative to non-transfected cells or ECFP-transfected cells (Fig. 4C). Thus, the regulation of stress granule formation by Stau1 was independent of which eIF2α kinase was involved in triggering their assembly.
The inhibitory capacity of several Staufen constructs was compared. Murine Stau1 fused to V5 or rat Stau1 fused to ECFP had comparable effects (Fig. 4A-D). There were no differences between the inhibitory capacity of a mutant Stau1 that was unable to bind protein phosphatase 1 (Monshausen et al., 2002) and that of the wild-type construct (L.J.M.T. and G.L.B., unpublished results). Stau2 had a moderate effect on the assembly of stress granules induced by arsenite or thapsigargin (L.J.M.T., C.C.L. and G.L.B., unpublished results). Stau1 is a modular protein with four dsRNA-binding domains (RBD2 to RBD5) and a tubulin-binding domain (TBD) (Fig. 4D). The N-terminal half of the molecule mediates the association with polysomes (Luo et al., 2002), and we found that this region, including up to RBD4 (RBD234) (Fig. 4D) retained the inhibitory capacity of the full-length molecule. The formation of stress granules upon treatment with arsenite or thapsigargin was significantly impaired in NIH 3T3 or U2OS cells expressing this construct (Fig. 4D). By contrast, a construct lacking this region, named TBD-RBD5, had no significant effect on the assembly of stress granules induced either by oxidative or ER stress (Fig. 4D). Stau1-ECFP, RBD234-EGFP and TBD-RBD5-EGFP were expressed at similar levels as judged by western blot analysis (results not shown). We found that, like the full-length Stau1, the RBD234 fragment was recruited to the stress granules in the reduced number of cells that succeed in forming these foci (Fig. 4E). By contrast, the TBD-RBD5 was not recruited to stress granules (Fig. 4E), in agreement with the observation that the RBD5 has a very low RNA-binding activity (Wickham et al., 1999). Altogether, these results suggest that the Stau1-mediated inhibition of stress granule formation is linked to its association with polysomes.
Stau1 regulates stress granule formation downstream of the phosphorylation of eIF2α
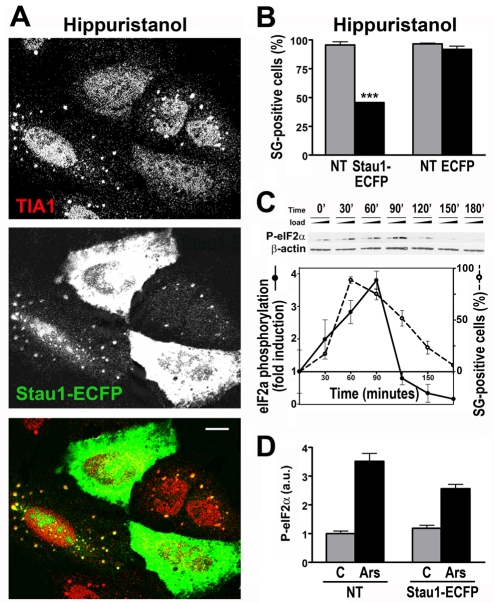
We have previously shown that Stau1-containing stress granules are induced by edeine, a drug that blocks the recruitment of the large ribosome subunit (Thomas et al., 2005). More recently, it was demonstrated that unlike stress-induced stress granules, the phosphorylation of eIF2α is not required when stress granules are assembled by initiation blockers such as hippuristanol (Mazouri et al., 2006; Anderson and Kedersha, 2008). Here, we evaluate the effect of Stau1 on the formation of stress granules triggered by this inhibitor. We found that endogenous or transfected Stau1 was recruited to hippuristanol-induced stress granules and that the assembly of stress granules in Stau1-transfected cells was dramatically reduced from 95% to 46% (Fig. 5A,B). Altogether, these results suggest that both types of stress granules – phospho-eIF2α dependent as well as phospho-eIF2α independent – are similarly downregulated by Stau1.
Fig. 5.
Stau1 modulates stress granule assembly downstream of eIF2α phosphorylation. (A) U20S cells were transfected with Stau1-ECFP and exposed to 1 μM hippuristanol for 60 minutes. (B) Stress granule formation was evaluated in 350 Stau1-ECFP- or ECFP-transfected cells and in 300 neighboring non-transfected cells (NT) treated as in A. Stau1-ECFP inhibits stress granule formation (***P<0.0001). (C) Cells were continuously exposed to arsenite, stress granules were visualized by TIAR staining and the levels of phosphorylated eIF2α relative to β-actin were determined by western blot of 2.5, 5 or 10 μg total protein. Total levels of eIF2α remained constant (not shown). Phosphorylation of eIF2α peaked simultaneously with stress granule formation and thereafter decayed below basal levels. (D) Levels of phosphorylated or total eIF2α were determined by immunofluorescence in single cells expressing Stau1-ECFP under control conditions (C; n=26) or upon arsenite exposure (Ars; n=51) and in neighboring non-expressing cells (NT) in control (n=32), or stress conditions (n=64).
Accordingly, we found that the stress-induced phosphorylation of eIF2α was not affected by Stau1. First, we analyzed the temporal correlation between eIF2α phosphorylation and stress granule formation. We found that both processes were rapidly triggered by arsenite and reached their maximal induction at about the same time (60-90 minutes) (Fig. 5C). Thereafter, phosphorylation rapidly decayed below basal levels, whereas more than 50% of the cells still contained stress granules (120 minutes in Fig. 5C), suggesting that sustained phosphorylation of eIF2α is not required once stress granules are formed. Next, we evaluated the phosphorylation of eIF2α in single cells by immunofluorescence at 60 minute after stimulus. We found that eIF2α was phosphorylated in cells transfected with Staufen1-ECFP as well as in non-transfected cells, whether or not they contained stress granules (Fig. 5D; supplementary material Fig. S3). Finally, siRNA-mediated depletion of Stau1 had no effect on eIF2α phosphorylation triggered by arsenite (M.G.T., M.A.D. and G.L.B., unpublished results).
Stress granule dissolution is mediated by the stress-induced expression of Hsp70, which helps to revert the aggregation of stress granule-core proteins (Mazroui et al., 2007; Anderson and Kedersha, 2008). We found that Stau1 transfection did not alter Hsp70 levels under resting conditions and allowed arsenite-induced upregulation of this chaperone as in non-transfected cells (C.C.L. and G.L.B., unpublished results). We conclude that the Stau1-dependent regulation of stress granule formation does not involve stress sensing, impairment of the inactivation of the key factor eIF2α or upregulation of the stress granule-dissolving factor Hsp70.
Stau1 stabilizes polysomes against stress-induced breakdown
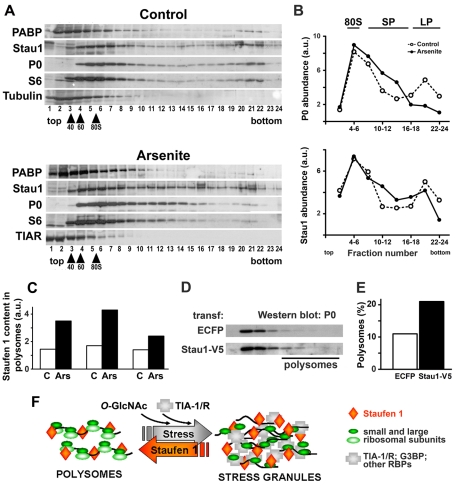
Stau1 is known to associate to polysomes and ribosomal subunits under resting conditions and is recruited to stress granules, which exclude polysomes, upon stress induction. Thus, we investigated the association of Stau1 to polysomes upon induction of stress granule formation. Cell extracts were separated by sedimentation in sucrose gradients and the distribution of Stau1 and of selected marker proteins was analyzed by western blot. As expected, 60 minutes after arsenite exposure, the ribosomal proteins P0 and S6 shifted towards fractions of lower sedimentation (fractions 1-15 in Fig. 6A,B). PABP accompanied the shift (Fig. 6A), reflecting the fact that translation of most messengers is impaired upon stress induction. A similar shift of the global polysome profile was described in related sublethal stress models (Koritzinsky et al., 2006). A quantitative analysis indicated that, upon exposure to arsenite, the proportion of P0 and S6 in the fraction containing large polysomes (fractions 16 to 24) was reduced to 35% of control levels (Fig. 6A,B). In striking contrast, the distribution of Stau1 was much less affected by the exposure to arsenite, the abundance of this molecule in the fast-sedimenting polysomes being moderately reduced to 80% relative to that observed in non-stressed cells.
Fig. 6.
Stau1 associates with stress-resistant polysomes. (A) NIH 3T3 cells were exposed to 0.5 mM arsenite for 1 hour, polysomes were separated in sedimentation gradients and fractions were analyzed by western blot to detect P0, a marker for large ribosomal subunits, S6, a marker for small subunits, PABP, TIAR and Stau1. (B) Distribution of P0 and Stau1 in pooled fractions from the gradient shown in A were evaluated by western blot and relative abundances are represented in the graphs. (C) Three independent experiments were performed as in A, and the distribution of polysomes was evaluated by following the P0 and S6 distributions in the gradient. Left column pair, total number of fractions = 13; polysome-containing fractions = 10-13. Middle and right column pairs, total number of fractions = 24; polysome-containing fractions = 16-24. The content of Stau1 in the polysomal fraction was measured by western blot analysis and is expressed normalized to S6 (left column pair) or P0 (middle and right column pairs). Duplicate western blot analysis of each gradient showed variations less than 10%. On average, the polysomes that remain upon stress induction contain twice the amount of Stau1 than found in polysomes under resting conditions. (D,E) Cells were transfected with ECFP or Stau1-V5 and exposed to 0.5 mM arsenite for 1 hour. The polysome profile was evaluated by monitoring the distribution of P0 in the gradient and the relative amount of polysomes is expressed as the percentage of P0 in the polysomal fraction relative to total. The amount of polysomes recovered after stress induction was increased from 10 to 20% in the presence of Staufen1-V5. (F) A model for the modulation of stress granules by Stau1. Under resting conditions, Stau1 is associated with polysomes by binding to mRNAs and ribosomal subunits. Cellular stress or pharmacological inhibition of 60S ribosomal recruitment provokes the breakdown of polysomes and concomitant accumulation of abortive translation initiation complexes that are aggregated by specific RBPs including TIA-1, TIAR and G3BP and by the O-glycosylation of ribosomal proteins (reviewed by Anderson and Kedersha, 2008; Ohn et al., 2008). Stau1 is recruited to growing stress granules by a piggyback mechanism. Formation of stress granules is counterbalanced by Stau1, which stabilizes polysomes against stress-induced breakdown, thus helping stress granule dissolution. In addition, Hsp70 contributes to stress granule disassembly (Mazroui et al., 2007).
The stress-granule marker protein TIAR was analyzed simultaneously and, as previously described (Kedersha et al., 2002), it was detected mostly in the fractions containing small RNPs, also co-migrating with free ribosomal subunits but never beyond 80S monosomes (Fig. 6A). This observation rules out the possibility that the fast-sedimenting fractions contained intact or fragmented stress granules to which Stau1 would associate. In addition, as Stau1 includes a tubulin-binding domain that associates with microtubules in vitro (Wickham et al., 1999), we investigated the presence of supramolecular complexes containing microtubules. Tubulin (Fig. 6A) and actin (results not shown) were not detected beyond the fractions corresponding to small polysomes, thus excluding the possibility that the fast-sedimenting fractions represent Stau1 complexes associated to cytoskeletal structures.
The mobilization of Stau1 relative to that of the ribosomal markers S6 and P0 upon arsenite was measured in three independent experiments performed similarly. In all cases we found that most polysomes are disrupted upon oxidative stress induction and that the fraction of polysomes recovered after the stress insult contained twice the amount of Stau1 than polysomes extracted from non-stressed cells (Fig. 6C). Next, we evaluated the effect of overexpressed Stau1 on polysome disruption upon stress. U2OS cells were transfected with tagged Stau1 or with ECFP, exposed to arsenite and the polysome profile was analyzed as before, by monitoring the distribution of P0 in sucrose gradients. As expected, arsenite provoked a partial breakdown of polysomes in all cases. However, we found that the amount of polysomes recovered upon stress was significantly larger in Stau1-transfected cells than in ECFP-transfected cells (Fig. 6D,E).
Altogether, our observations suggest that Stau1 associates with polysomes, preventing their disruption and downregulating stress granule formation. To investigate whether this has an effect on the translational blockage triggered by stress, we evaluate the protein synthesis rate in Stau1-depleted cells exposed to oxidative or ER stress. Incorporation of radiolabelled amino acids was measured as indicated in the Materials and Methods at several time points during stress granule formation and dissolution. We found no significant differences in the inhibition of protein synthesis that occurs simultaneously with stress granule formation (supplementary material Fig. S4) (1-2 hours after oxidative stress; 2 hours after ER stress). In addition, the partial recovery of protein synthesis that correlates with stress granule dissolution (supplementary material Fig. 4) (3-5 hours after oxidative stress; 4-8 hours after ER stress) and with Hsp70 translation (M.A.D., C.C.L. and G.L.B., unpublished results) was not significantly affected by Stau1 knockdown. These data indicating that polysome stabilization by Stau1 does not correlate with higher translation rates are suggestive of polysome stalling.
Discussion
Here, we report the downregulation of stress granule formation by the double-stranded RBP Stau1. We found that this ubiquitously expressed protein is recruited to stress granules induced in cell lines, oligodendrocytes and neurons by several stress stimuli, including oxidative stress, ER stress or heat shock (Thomas et al., 2005). Given that Stau1 binds to mRNAs and ribosomal subunits (Kiebler et al., 1999; Marion et al., 1999; Duchaine et al., 2002; Luo et al., 2002; Baez and Boccaccio, 2005; Thomas et al., 2005), we propose that Stau1 is mobilized to stress granules in association with mRNAs and/or small ribosomal subunits, which are always present in stress granules.
Although stress granules always contained Stau1, we found that stress granules lacking this protein were formed in Stau1-depleted cells, indicating that Stau1 is not an essential component of these foci. Moreover, Stau1 knockdown facilitates the formation of stress granules induced by oxidative or ER stress. Conversely, we found that a moderate overexpression of Stau1 impaired stress granule formation. Stau1 is the first stress granule component reported to negatively regulate the assembly of these foci. The modulating effect was observed on stress granules induced either by a stress signal or by hippuristanol, an initiation blocker that does not induce the phosphorylation of eIF2α. Thus, the regulation of stress granule formation by Stau1 occurs downstream of this event.
We propose that upstream of the assembly step mediated by the aggregation domains of TIA-1, TIAR and G3BP, and by post-translational modifications of ribosomal proteins, stress granule formation is regulated by Stau1, probably by affecting the equilibrium between silencing foci and polysomes (Fig. 6F). Supporting this model, a significant proportion of endogenous or overexpressed Stau1 is associated with polysomes (Kiebler et al., 1999; Marion et al., 1999; Duchaine et al., 2002; Luo et al., 2002; Thomas et al., 2005; Baez and Boccaccio, 2005; Dugré-Brisson et al., 2005). In addition, the present studies revealed that the polysomes that remain after the stress-induced breakdown are enriched in Stau1. Our results suggest a direct effect of Stau1 in mediating polysome stability against stress. Supporting this notion, the Stau1 domains directly interacting with ribosomes (RBD2 to RBD4) (Luo et al., 2002) elicited an effect comparable with that of the full-length molecule. How Stau1 modulates polysome stability remains to be investigated. Polysome breakdown upon stress is the consequence of impaired initiation and continuing elongation and termination. Our data suggest that Stau1 leads to polysome stalling by unknown mechanisms. Stau1 may slow elongation, so that loss of ribosome subunits is reduced. Alternatively, Stau1 may impair ribosome release. Interestingly, stalled polysomes have been recently described to accumulate during mitosis and this impairment in elongation is determinant of the lack of stress granules during cell division (Sivan et al., 2007). Whether Stau1 is involved in polysome slowdown and stress granule inhibition during mitosis is unknown.
In addition to its interaction with ribosomes, Stau1 is a general mRNA binding factor and this might contribute to the global effect on polysome stability described here. Stau1 ribonucleoparticles comprise at least 7% cellular messengers, a fraction of them involved in cellular metabolism and ubiquitylation (Furic et al., 2007). The binding motifs and the regulatory relevance of this direct or indirect association have not been addressed and thus, their putative contribution to stress granule regulation remains unsolved. It has been shown that Stau1 triggers mRNA degradation when it is bound downstream of the STOP codon (Kim, Y. K. et al., 2005). The importance of the decay of putatively relevant targets including TIA-1 (Kim, Y. K. et al., 2007) and ARF1 (Kim, Y. K. et al., 2005) requires further investigation. In addition, Stau1 stimulates translation when bound to the 5′UTR of mRNAs (Dugré-Brisson et al., 2005), although the cellular targets have not been identified. We found that the Stau1 domains involved in translation enhancement are required for stress granule disruption, and thus, this may contribute to stress granule regulation.
Finally, it has been reported that Stau1 molecules interact with molecular motors and the cytoskeleton, mediating the transport and anchorage of ribonucleoparticles (Ferrandon et al., 1994; Broadus et al., 1998; Wickham et al., 1999; Kiebler et al., 1999; Micklem et al., 2000; Tang et al., 2001; Belanger et al., 2003; Yoon and Mowry, 2004; Gautrey et al., 2005; Vessey et al., 2008). We believe that the anchorage of ribosomes or mRNAs to the cytoskeleton is not a major factor in impairing the mobilization of the translational apparatus to stress granules, because Stau1 constructs excluding the protein domains thought to be involved in the interaction with the cytoskeleton are still able to inhibit stress granule formation. It has been proposed that stress granule aggregation requires the activity of motor molecules (Kwon et al., 2007; Anderson and Kedersha, 2008), so it might be that stress granule dissolution also requires active transport. Staufen molecules were implicated in RNA transport in several cell types and thus, mammalian Stau1 might help to the motor-mediated transport of mRNPs from discrete silencing foci to dispersed polysomes.
Given that Stau1 is a modulator of stress granule formation, whether Stau1 is regulated by cellular stress is a relevant issue. We found that Stau1 levels remained constant during acute oxidative stress in NIH 3T3 cells (M.A.D. and G.L.B., unpublished results). Whether Stau1 expression responds to other stress models remains to be investigated. Mammalian Staufen molecules contain several putative phosphorylation sites, and it has been shown that phosphorylation of Xenopus Staufen modulates its binding to intracellular structures (Allison et al., 2004). Whether differences in the expression levels, splicing variants or phosphorylation state of Stau1 and Stau2 modulate stress granule formation remains an open question.
The formation of stress granules is usually transient and stress granules and related silencing foci containing polyadenylated mRNA form persistently when cell viability is compromised (Kayali et al., 2005; Jamison et al., 2008; Anderson and Kedersha, 2008). We found that cell survival upon stress is affected in Stau1-depleted cells and this might be connected to the differential persistence of stress granules. In addition to their relevance in regulating translation and mRNA stability upon stress (Stohr et al., 2006; Mazroui et al., 2007; Anderson and Kedersha, 2008), stress granules are believed to indirectly affect transcription, inflammation and cell survival by sequestration of specific molecules (Yu et al., 2007; Kim, W. J. et al., 2007; Arimoto et al., 2008; Eisinger-Mathason et al., 2008). Moreover, viral infections affect stress granule assembly, thus modulating the cell defense mechanisms (Anderson and Kedersha, 2008). The contribution of the modulation of stress granule assembly by Stau1 to these important cell responses remains to be investigated.
Materials and Methods
Plasmids
The following plasmids were used: Stau1-ECFP, pECFP-N1 (Clontech, Palo Alto, CA) carrying a cDNA encoding rat Stau1 (AF290989), gift from Stefan Kindler (University of Hamburg, Germany); Stau1-V5, murine Stau1 (AF061942) cloned in pCDNA6.0 (Invitrogen, Carlsbad, CA); RBD234-EGFP and TBD-RBD5-EGFP generated by PCR-cloning of the 1-816 nt. and the 814-1464 nt. fragments respectively from the murine AF061942 in the XhoI and BamHI sites of pECFP-N1.
Cell transfection, drug treatment and immunofluorescence
NIH 3T3, COS-7 and HeLa cells from ATCC were grown in DMEM or MEM (Sigma) supplemented with 10% fetal bovine serum (Natocor, Córdoba, Argentina), penicillin and streptomycin (Sigma). Plasmid transfection was performed using Lipofectamine 2000 (Invitrogen). Thapsigargin (used at 250 nM unless otherwise indicated) from a DMSO stock, sodium arsenite (used at 0.25 mM unless otherwise indicated); cycloheximide and puromycin (used at 0.1 mg/ml) and hippuristanol (used at 1 μM) from stock aqueous solutions were added to conditioned medium. Stress stimuli were pulsed (1 hour) or continuous as indicated.
For immunofluorescence, cells were fixed in 4% paraformaldehyde, 4% sucrose in PBS at 37°C; permeabilized in 0.1% Triton X-100 in PBS and blocked in 1% Blocking Reagent (Boehringer Mannheim). Primary antibodies were diluted as follows: rabbit polyclonal antibodies against Stau1, RLS1 (Thomas et al., 2005), anti-PABP (provided by Evita Mohr, University of Hamburg, Germany), anti-Dcp1 and anti Xrn1 (gift from Jens Lykke Andersen, University of Colorado, CO), 1:500; anti-S6 (Cell Signaling, Beverly, MA), anti-eIF4G1 (Abcam, Cambridge, UK); anti-eIF2α and anti-phospho-eIF2α (Stressgen, Victoria, British Columbia, Canada) 1:100; goat polyclonal anti-TIA-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 1:100. Monoclonal antibodies anti-TIAR; anti eIF4E and G3BP (BD Biosciences, San José, CA); anti-HuR and anti-hPABP (ImmuQuest, Cleveland, UK), 1:100, and anti-V5 (Invitrogen), 1:500. Secondary antibodies were from Molecular Probes or Jackson ImmunoResearch Laboratories (West Grove, PA). Cells were mounted in Mowiol 4-88 (Calbiochem, EMD Biosciences, San Diego, CA).
siRNA treatment
The following siRNA sequences were used: anti-Stau1 (siStau1), 5′-aactgccatgatagcccgaga-3′; anti-Staufen 2 (siStau2), 5′-aacaaaggatggagtggtcca-3′; anti-Hedls (siHedls, Cat. No. 004397, Dharmacon, Chicago IL); anti-Rck/p54 (siRck/p54, Cat. No. 0143295, Dharmacon), and non-relevant siRNA (siNR), 5′-uagcgacuaaacacaucaauu-3′, siStau1 and siNR carrying the siSTABLE chemical modification (Dharmacon). Cells grown at 50% confluence were treated with 100 nM siRNA and TransIT-TKO (Dharmacon) following manufacturer's instructions, and analyzed 48 hours later. When simultaneous plasmid transfection was required, the siRNA was incorporated into the lipofectamine-transfection procedure and cells were analyzed 16 hours later.
Confocal microscopy, cell counting, stress granule size and intensity measurements
Images were acquired with a LSM 5 PASCAL confocal microscope (Carl Zeiss, Oberkochen, Germany). Equipment adjustment was assessed by using 1 μm FocalCheck fluorescent microspheres (Molecular Probes). Line intensity profiles were plotted with the `Intensity Profile' tool. Pictures were exported to Adobe Photoshop software for cropping. Neither filters nor gamma-adjustments were applied. To estimate the expression levels of ECFP-tagged Stau1, NIH 3T3 cells were transfected with ECFP or Stau1-ECFP, and stained with the RLS1 antibody and a Cy3-labeled secondary antibody. Relative expression levels were determined in single cells where the Stau1 immunofluorescence intensity was linear with the fluorescence intensity of the ECFP moiety. Stress granules were identified by TIAR staining and cells were scored as positive when they had at least three foci of a minimal size of 0.5 μm, because cells with less than five stress granules were infrequent in all tested conditions and stress granules are usually 0.5-5 μm in size. Cell counting was performed manually with the help of the `Cell Counter' tool of the ImageJ software (NIH) on ×40 or ×100 micrographs. P-values according to Fisher's exact test were determined using Instat software (GraphPad Software, San Diego, CA). Error bars represent s.e. from at least two replicates. Stress granule size was measured with the `Analyze Particles' tool of the ImageJ software on ×100 confocal micrographs.
Sedimentation velocity centrifugation
Cells were harvested in CSK buffer (Thomas et al., 2005) supplemented with 0.25 mM sucrose, 1% Triton X-100, and protease inhibitor cocktail (Sigma), homogenized and centrifuged for 10 minutes at 5000 r.p.m. to obtain post-nuclear extracts. Phosphatase inhibitors (50 mM orthovanadate, 2.5 mM cypermetrine, 0.2 mM okadaic acid, 1 mM β-glycerophosphate and 1M NaF) were included when required. Samples of 0.5 to 1.5 mg protein (determined by the Bicinchoninic Acid Protein Kit assay, Sigma) were loaded onto continuous 13 ml sucrose gradients (linear 20-60% w/v in CSKB) and centrifuged at 220,000 g for 2 hours. The polysomal profile was monitored by absorbance at 254 nm. Protein from 0.5 ml fractions was precipitated in 10% trichloroacetic acid, washed twice with cold acetone and analyzed by western blot.
Western blot
Protein from total, post-nuclear cell extracts or gradient fractions were analyzed. Briefly, protein was resuspended in Laemmli sample buffer, separated by SDS-PAGE, and electrotransferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA). Primary antibodies were used as follows: anti-Stau1 RLS1, 1:5000; rabbit anti-PABP, anti-S6, anti-eIF2α and anti-phospho-eIF2α; 1:1000, mouse monoclonal anti-TIAR, anti-β actin (Sigma) and anti-β tubulin (Sigma), 1:1000; human anti-P0 (Immunovision, Springdale, AR) 1:10,000.
Detection of peroxidase-coupled anti-V5 antibody (1:5000) (Invitrogen) or of secondary antibodies (Sigma) was performed by autoradiography, using the LumiGlo system (Cell Signaling) and Hyperfilm (Amersham Biosciences) or in a chemiluminescence reader (STORM840, Amersham). ImageQuant software was used for quantitative measurements, using the `volume report' and the `local average' tools. Alternatively, autoradiographs were scanned and signal intensity assessed with ImageJ (NIH) software.
Protein synthesis assay
L-amino acids [14C(U)] (Perkin Elmer, Boston MA) were added to cells plated in 10 mm wells. At the indicated times, supernatant was carefully removed and cells where washed once with warm PBS. Cells were lysed in 0.2 ml RIPA buffer containing protease inhibitor cocktail and the trichloroacetic acid (TCA)-insoluble radioactivity was determined in duplicates. Triplicates wells were evaluated for each time point.
MTT assay for cell viability
Cell viability was evaluated using MTT [3(4,5-cimethylthiazol-2-yl)-2,5-diphenil tetrazolium bromide]. At the end of the indicated treatment, cells were incubated with 0.5 g/ml MTT during 2 hours at 37°C until the formation of dark formazan crystals. After lysis with acid isopropanol (0.04 N HCl in isopropanol) absorbance at 570 nm was measured. Linearity of the response was evaluated in each experiment by measuring serial dilutions of cells. All the measures were done in triplicate wells.
Supplementary Material
We thank María Jimena Ortega and Neda Berardone (Instituto Leloir) for their assistance in confocal microscopy analysis; Mariela Loschi (Instituto Leloir) for sharing unpublished data and for her critical reading of the manuscript, Nancy Kedersha (Harvard Medical Scholl); Jens Lykke Andersen (University of Colorado, USA); Evita Mohr and Stefan Kindler (University of Hamburg, Germany) for providing useful antibodies and constructs. J. Pelletier (Department of Biochemistry, McGill University, Montreal, Quebec, Canada), for generously providing hippuristanol. We are grateful to David R. Colman (The Montreal Neurological Institute, McGill University, Canada) for his constant support. This work was supported by the following grants: PICT 01-08691, Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina, and The Wadsworth Foundation (USA) to G.L.B. and 1R03 TW 006037-01A1, National Institutes of Health, USA, to D.R.C. G.L.B. is a member of the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina; M.G.T. and L.J.M.T. are recipients of a fellowship from CONICET and of the Luis Federico Leloir award, respectively. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/4/563/DC1
References
- Allison, R., Czaplinski, K., Git, A., Adegbenro, E., Stennard, F., Houliston, E. and Standart, N. (2004). Two distinct Staufen isoforms in Xenopus are vegetally localized during oogenesis. RNA 10, 1751-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, P. and Kedersha, N. (2008). Stress granules: the Tao of RNA triage. Trends Biochem Sci. 33, 141-150. [DOI] [PubMed] [Google Scholar]
- Andrei, M. A., Ingelfinger, D., Heintzmann, R., Achsel, T., Rivera-Pomar, R. and Luhrmann, R. (2005). A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11, 717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto, K., Fukuda, H., Imajoh-Ohmi, S., Saito, H. and Takekawa, M. (2008). Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 10, 1324-1332. [DOI] [PubMed] [Google Scholar]
- Baez, M. V. and Boccaccio, G. L. (2005). Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J. Biol. Chem. 280, 43131-43140. [DOI] [PubMed] [Google Scholar]
- Belanger, G., Stocksley, M. A., Vandromme, M., Schaeffer, L., Furic, L., DesGroseillers, L. and Jasmin, B. J. (2003). Localization of the RNA-binding proteins Staufen1 and Staufen2 at the mammalian neuromuscular junction. J. Neurochem. 86, 669-677. [DOI] [PubMed] [Google Scholar]
- Broadus, J., Fuerstenberg, S. and Doe, C. Q. (1998). Staufen-dependent localization of prospero mRNA contributes to neuroblast daughter-cell fate. Nature 391, 792-795. [DOI] [PubMed] [Google Scholar]
- Cougot, N., Babajko, S. and Seraphin, B. (2004). Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 165, 31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, P. O., Berguer, P. M., Ainciart, N., Zylberman, V., Thomas, M. G., Martinez Tosar, L. J., Bulloj, A., Boccaccio, G. L. and Goldbaum, F. A. (2005). Multiple display of a protein domain on a bacterial polymeric scaffold. Proteins 61, 1089-1100. [DOI] [PubMed] [Google Scholar]
- Dang, Y., Kedersha, N., Low, W. K., Romo, D., Gorospe, M., Randal, K., Anderson, P. and Liu, J. O. (2006). Eukaryotic initiation factor 2alpha independent pathway of stress granule induction by the natural product pateamine A. J. Biol. Chem. 281, 32870-32878. [DOI] [PubMed] [Google Scholar]
- Duchaine, T. F., Hemraj, I., Furic, L., Deitinghoff, A., Kiebler, M. A. and DesGroseillers, L. (2002). Staufen2 isoforms localize to the somatodendritic domain of neurons and interact with different organelles. J. Cell Sci. 115, 3285-3295. [DOI] [PubMed] [Google Scholar]
- Dugré-Brisson, S., Elvira, G., Boulay, K., Chatel-Chaix, L., Mouland, A. J. and DesGroseillers, L. (2005). Interaction of Staufen1 with the 5′ end of mRNA facilitates translation of these RNAs. Nucleic Acids Res. 33, 4797-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger-Mathason, T. S., Andrade, J., Groehler, A. L., Clark, D. E., Muratore-Schroeder, T. L., Pasic, L., Smith, J. A., Shabanowitz, J., Hunt, D. F., Macara, I. G. et al. (2008). Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol. Cell 31, 722-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio, A., Behm-Ansmant, I. and Izaurralde, E. (2007). P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell. Biol. 8, 9-22. [DOI] [PubMed] [Google Scholar]
- Fenger-Grøn, M., Fillman, C., Norrild, B. and Lykke-Andersen, J. (2005). Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell. 20, 905-915. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo, M. A., Basak, S., Dostie, J., Murray, E. L., Schoenberg, D. R. and Sonenberg, N. (2005). A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 170, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon, D., Elphick, L., Nusslein-Volhard, C. and St Johnston, D. (1994). Staufen protein associates with the 3′UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell 79, 1221-1232. [DOI] [PubMed] [Google Scholar]
- Furic, L., Maher-Laporte, M. and DesGroseillers, L. (2008). A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA 14, 324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi, I. E., Brennan, C. M., Stenberg, M. G., Swanson, M. S., Eversole, A., Maizels, N. and Steitz, J. A. (2000). HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl. Acad. Sci. USA 97, 3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautrey, H., McConnell, J., Hall, J. and Hesketh, J. (2005). Polarised distribution of the RNA-binding protein Staufen in differentiated intestinal epithelial cells. FEBS Lett. 579, 2226-2230. [DOI] [PubMed] [Google Scholar]
- Gilks, N., Kedersha, N., Ayodele, M., Shen, L., Stoecklin, G., Dember, L. M. and Anderson, P. (2004). Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil, S., Long, J. C. and Caceres, J. F. (2006). hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell. Biol. 26, 5744-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison, J. T., Kayali, F., Rudolph, J., Marshall, M., Kimball, S. R. and Degracia, D. J. (2008). Persistent redistribution of poly-adenylated mRNAs correlates with translation arrest and cell death following global brain ischemia and reperfusion. Neuroscience 154, 504-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara, H., Imai, T., Imataka, H., Tsujimoto, M., Matsumoto, K. and Okano, H. (2008). Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. J. Cell Biol. 181, 639-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali, F., Montie, H. L., Rafols, J. A. and DeGracia, D. J. (2005). Prolonged translation arrest in reperfused hippocampal cornu Ammonis 1 is mediated by stress granules. Neuroscience 134, 1223-1245. [DOI] [PubMed] [Google Scholar]
- Kedersha, N. L., Gupta, M., Li, W., Miller, I. and Anderson, P. (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha, N., Cho, M. R., Li, W., Yacono, P. W., Chen, S., Gilks, N., Golan, D. E. and Anderson, P. (2000). Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha, N., Chen, S., Gilks, N., Li, W., Miller, I. J., Stahl, J. and Anderson, P. (2002). Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha, N., Stoecklin, G., Ayodele, M., Yacono, P., Lykke-Andersen, J., Fritzler, M. J., Scheuner, D., Kaufman, R. J., Golan, D. E. and Anderson, P. (2005). Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler, M. A., Hemraj, I., Verkade, P., Kohrmann, M., Fortes, P., Marion, R. M., Ortin, J. and Dotti, C. G. (1999). The mammalian Staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J. Neurosci. 19, 288-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler, M. A., Jansen, R. P., Dahm, R. and Macchi, P. (2005). A putative nuclear function for mammalian Staufen. Trends Biochem. Sci. 30, 228-231. [DOI] [PubMed] [Google Scholar]
- Kim, J. E., Ryu, I., Kim, W. J., Song, O. K., Ryu, J., Kwon, M. Y., Kim, J. H. and Jang, S. K. (2008). Proline-rich transcript in brain protein induces stress granule formation. Mol. Cell. Biol. 28, 803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. J., Back, S. H., Kim, V., Ryu, I. and Jang, S. K. (2005). Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell. Biol. 25, 2450-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. J., Kim, J. H. and Jang, S. K. (2007). Anti-inflammatory lipid mediator 15d-PGJ2 inhibits translation through inactivation of eIF4A. EMBO J. 26, 5020-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. K., Furic, L., Desgroseillers, L. and Maquat, L. E. (2005). Mammalian Staufen1 recruits Upf1 to specific mRNA 3′UTRs so as to elicit mRNA decay. Cell. 120, 195-208. [DOI] [PubMed] [Google Scholar]
- Kim, Y. K., Furic, L., Parisien, M., Major, F., DesGroseillers, L. and Maquat, L. E. (2007). Staufen1 regulates diverse classes of mammalian transcripts. EMBO J. 26, 2670-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, S. R., Horetsky, R. L., Ron, D., Jefferson, L. S. and Harding, H. P. (2003). Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284, 273-284. [DOI] [PubMed] [Google Scholar]
- Koritzinsky, M., Magagnin, M. G., van den Beucken, T., Seigneuric, R., Savelkouls, K., Dostie, J., Pyronnet, S., Kaufman, R. J., Weppler, S. A., Voncken, J. W. et al. (2006). Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25, 1114-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, S., Zhang, Y. and Matthias, P. (2007). The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 21, 3381-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau, G., Maher-Laporte, M., Topolnik, L., Laurent, C. E., Sossin, W., Desgroseillers, L. and Lacaille, J. C. (2008). Staufen1 regulation of protein synthesis-dependent long-term potentiation and synaptic function in hippocampal pyramidal cells. Mol. Cell. Biol. 28, 2896-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M., Duchaine, T. F. and DesGroseillers, L. (2002). Molecular mapping of the determinants involved in human Staufen-ribosome association. Biochem. J. 365, 817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion, R. M., Fortes, P., Beloso, A., Dotti, C. and Ortin, J. (1999). A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum. Mol. Cell. Biol. 19, 2212-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui, R., Sukarieh, R., Bordeleau, M. E., Kaufman, R. J., Northcote, P., Tanaka, J., Gallouzi, I. and Pelletier, J. (2006). Inhibition of ribosome recruitment induces stress granule formation independent of eIF2-alpha phosphorylation. Mol. Biol. Cell 17, 4212-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui, R., Di Marco, S., Kaufman, R. J. and Gallouzi, I. E. (2007). Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol. Biol. Cell 18, 2603-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem, D. R., Adams, J., Grunert, S. and St Johnston, D. (2000). Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 19, 1366-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet, S., Cougot, N., Wilczynska, A., Dautry, F., Kress, M., Bertrand, E. and Weil, D. (2008). Translationally repressed mRNA transiently cycles through stress granules during stress. Mol. Biol. Cell 19, 4469-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen, M., Rehbein, M., Richter, D. and Kindler, S. (2002). The RNA-binding protein Staufen from rat brain interacts with protein phosphatase-1. J. Neurochem. 81, 557-564. [DOI] [PubMed] [Google Scholar]
- Ohn, T., Kedersha, N., Hickman, T., Tisdale, S. and Anderson, P. (2008). A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat. Cell Biol. 10, 1224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackham, O. and Brown, C. M. (2004). Visualization of RNA-protein interactions in living cells: FMRP and IMP1 interact on mRNAs. EMBO J. 23, 3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden, A. D. (2007). Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol. Cell 28, 491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan, G., Kedersha, N. and Elroy-Stein, O. (2007). Ribosomal slowdown mediates translational arrest during cellular division. Mol. Cell. Biol. 27, 6639-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr, N., Lederer, M., Reinke, C., Meyer, S., Hatzfeld, M., Singer, R. H. and Hüttelmaier, S. (2006). ZBP1 regulates mRNA stability during cellular stress. J. Cell Biol. 175, 527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S. J., Meulemans, D., Vazquez, L., Colaco, N. and Schuman, E. (2001). A role for a rat homolog of Staufen in the transport of RNA to neuronal dendrites. Neuron 32, 463-475. [DOI] [PubMed] [Google Scholar]
- Thomas, M. G., Martinez Tosar, L. J., Loschi, M., Pasquini, J. M., Correale, J., Kindler, S. and Boccaccio, G. L. (2005). Staufen recruitment into stress granules does not affect early mRNA transport in oligodendrocytes. Mol. Biol. Cell 16, 405-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere, H., Chebli, K., Zekri, L., Courselaud, B., Blanchard, J. M., Bertrand, E. and Tazi, J. (2003). The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823-831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tsai, N. P., Ho, P. C. and Wei, L. N. (2008). Regulation of stress granule dynamics by Grb7 and FAK signalling pathway. EMBO J. 27, 715-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey, J. P., Vaccani, A., Xie, Y., Dahm, R., Karra, D., Kiebler, M. A. and Macchi, P. (2006). Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J. Neurosci. 26, 6496-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey, J. P., Macchi, P., Stein, J. M., Mikl, M., Hawker, K. N., Vogelsang, P., Wieczorek, K., Vendra, G., Riefler, J., Tbing, F. et al. (2008). A loss of function allele for murine Staufen1 leads to impairment of dendritic Staufen1-RNP delivery and dendritic spine morphogenesis. Proc. Natl. Acad. Sci. USA 105, 16374-16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, L., Duchaine, T., Luo, M., Nabi, I. R. and DesGroseillers, L. (1999). Mammalian Staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol. Cell. Biol. 19, 2220-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska, A., Aigueperse, C., Kress, M., Dautry, F. and Weil, D. (2005). The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118, 981-992. [DOI] [PubMed] [Google Scholar]
- Yoon, Y. J. and Mowry, K. L. (2004). Xenopus Staufen is a component of a ribonucleoprotein complex containing Vg1 RNA and kinesin. Development. 131, 3035-3045. [DOI] [PubMed] [Google Scholar]
- Yu, C., York, B., Wang, S., Feng, Q., Xu, J. and O'Malley, B. W. (2007). An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol. Cell. 25, 765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.