Abstract
Objective
The synovial fibroblast, or fibroblast-like synoviocyte (FLS), has a central role in pannus invasion and destruction of cartilage and bone in rheumatoid arthritis (RA). However, regulation of the FLS remains incompletely understood. The aim of this study was to determine whether the invasive properties of FLS are genetically regulated by arthritis severity loci.
Methods
DA rats (arthritis susceptible) and rat strains congenic for arthritis-protective intervals were studied. Primary FLS cell lines were generated from each strain and used in a well-established FLS invasion model through a collagen-rich barrier. Cells or culture supernatants were analyzed for gene expression, activity of different matrix metalloproteinases (MMPs), cytoskeleton integrity, and cell proliferation.
Results
The median number of FLS from DA.F344(Cia5d) rats that invaded through the collagen-rich barrier was reduced 86.5% compared with the median number of invading FLS from DA rats. Histologic examination showed that DA.F344(Cia5d) rats preserved a normal joint without pannus, hyperplasia, or erosions. FLS from DA.F344(Cia5d) rats produced significantly lower levels of active MMP-2 compared with FLS from DA rats, but the levels of proMMP-2 and MMP-2 messenger RNA in DA.F344(Cia5d) rats were similar to those in DA rats. Treatment of FLS from DA rats with an MMP-2 inhibitor reduced cell invasion to a level similar to that in DA.F344(Cia5d) rats, demonstrating that MMP-2 activity accounted for the difference between FLS from these 2 strains. Analysis of MMP-2–activating pathways revealed increased levels of soluble membrane type 1 (MT1)–MMP in DA rats compared with DA.F344(Cia5d) rats.
Conclusion
These data represent the first evidence for a genetic component in the regulation of FLS invasion. A gene located within the Cia5d interval accounts for this effect and operates via the regulation of soluble MT1-MMP production and MMP-2 activation. These observations suggest novel potential pathways for prognostication and therapy.
Rheumatoid arthritis (RA) is one of the most common chronic autoimmune diseases. RA affects ~1% of the population and is commonly associated with disability and deformities (1,2). RA is a complex trait with a significant genetic contribution to disease susceptibility and severity (1). The basic joint pathology in RA is characterized by pronounced synovial hyperplasia, which is also called pannus. Pannus produces several proinflammatory cytokines and proteases and, like a malignant tumor, invades and destroys cartilage and bone (2–4).
Complex cell–cell interactions (5,6), paracrine and autocrine factors such as cytokines and growth factors (7,8), NF-κB (9), and angiogenesis (10) are involved in pannus formation. Synovial fibroblasts, also called fibroblast-like synoviocytes (FLS), have been considered key players in this process, and their numbers are significantly increased in the hyperplastic synovial pannus of patients with RA and in rodent models of arthritis (2). RA FLS invade cartilage (11) and produce increased amounts of several proteolytic enzymes that further contribute to joint destruction (3,4). The invasive properties of RA FLS have also been studied in vitro and associated with radiographic damage in RA, under-scoring their direct clinical relevance (12). Nonetheless, the regulation of the invasive properties of FLS remains incompletely understood.
We have been working with unique congenic strains generated between arthritis-susceptible DA rats and arthritis-resistant F344 and ACI rats studied for pristane-induced arthritis (PIA) (13–15). In these congenic strains, a significantly milder form of arthritis develops, and normal joint architecture is preserved, with nearly no synovial hyperplasia and no cartilage or bone erosions (14,15). These observations led us to hypothesize that arthritis susceptibility and severity genes located within quantitative trait loci could modulate disease via regulation of the invasive properties of synovial pannus and, more specifically, of FLS.
This study is the first to provide evidence that the invasive properties of FLS are genetically regulated. We further implicate the arthritis quantitative trait locus Cia5d in the control of FLS invasion via regulation of the production of soluble membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-2 activation.
MATERIALS AND METHODS
Rats
DA (DA/BklArbNsi; arthritis-susceptible), F344, and ACI (F344/Hsd and ACI/Hsd, respectively; arthritis-resistant [Harlan, Indianapolis, IN]) inbred rat strains were used in the breeding of the congenic and subcongenic strains. The genotype-guided breeding strategy was previously described in detail (13–15). The DA.F344(Cia5d) strain is a subcongenic strain derived from the DA.F344(Cia5) congenic strain, which was previously shown to regulate PIA more significantly than collagen-induced arthritis (15). All experiments involving animals were reviewed and approved by the Feinstein Institute for Medical Research Institutional Animal Care and Use Committee. Animals were housed in a pathogen-free environment, with 12-hour light–dark cycles and free access to food and water.
Initiation of PIA
Eight-to-twelve-week–old rats received 150 µl of pristane by intradermal injection. The dose was divided into 2 injection sites at the base of the tail (14,16).
Arthritis severity scoring
Arthritis scoring was performed on days 14, 18, and 21 after the initiation of PIA, using a previously described system that evaluates the ankles, mid-feet, wrists, midforepaws, and metacarpophalangeal, metatarsophalangeal, and interphalangeal joints, generating a score ranging from 0 to 80 per rat per day (14,17). Scores obtained until day 21 were added and used as the arthritis severity index. The median arthritis severity index score for each group was used for analysis. On day 21 postinduction, the rats were euthanized, and synovial tissue was collected from the ankles for isolation of FLS. A separate group of DA and DA.F344(Cia5d) rats with PIA was followed up for 32 days; the joints of these rats were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for histologic analysis.
Isolation and culture of primary FLS
FLS were isolated by enzymatic digestion of the synovial tissue. Briefly, tissues were minced and incubated with a solution containing 0.15 mg/ml DNase, 0.15 mg/ml hyaluronidase (type I-S), and 1 mg/ml collagenase (type IA) (Sigma-Aldrich, St. Louis, MO) in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) for 1 hour at 37°C. Cells were washed and resuspended in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen), 30 mg/ml glutamine, 250 µg/ml amphotericin B (Sigma-Aldrich), and 20 µg/ml gentamicin (Invitrogen). After overnight culture, nonadherent cells were removed, and adherent cells were cultured. All experiments were performed with cells obtained after passage 4 (>95% FLS purity).
Invasion assay
In vitro invasion of FLS was assayed in a transwell system using Matrigel-coated inserts from Becton Dickinson (Franklin Lakes, NJ) and Chemicon (Temecula, CA). In the initial experiments, chambers from Becton Dickinson were used, but due to manufacturing quality problems with subsequent lots of inserts from Becton Dickinson, we switched to Chemicon inserts. At 70–80% confluency, cells were harvested by trypsin–EDTA digestion and washed with serum-free DMEM. Cells (1.5 × 104) were resuspended in 300 µl of serum-free DMEM and plated on the upper compartment of the Matrigel-coated inserts. The lower compartment was filled with DMEM with 10% FBS, and the plates were incubated at 37°C for 24 hours. After 24 hours, the upper surface of the insert was wiped with cotton swabs to remove noninvading cells and the Matrigel layer.
For the Becton Dickinson inserts, the bottom surface of the insert was stained with crystal violet, and the number of cells that invaded through Matrigel was counted in 3 random fields at 100× magnification. For the Chemicon inserts, the bottom surface of the insert, which contained the cells that invaded through Matrigel, was stained with the kit staining solution and resolubilized with 10% acetic acid, and the solution absorbance was read at 570 nm, according to the protocol suggested by the manufacturer. Experiments with Becton Dickinson inserts were done in duplicate, and those with Chemicon inserts were performed in triplicate. When indicated, the MMP inhibitors GM6001 (25 µM) and SB-3CT (500 µM) (both from Chemicon) were added to the upper chamber for overnight incubation. The same amount of diluent (DMSO) was used as control.
Zymography
Gelatin and casein zymography was performed according to previously described methods (18,19). Briefly, FLS were cultured on Matrigel-coated plates or Petri dishes, and the protein content in the supernatants was quantified. The same amount of total protein per cell line supernatant was used in each experiment. Protein was mixed with Tris–glycine–sodium dodecyl sulfate (SDS) sample buffer (Invitrogen), loaded into a zymogram precasted gel (Invitrogen), and run for 90 minutes at 125V. After electrophoresis, gels were treated with renaturing buffer (Invitrogen), followed by incubation in developing buffer (Invitrogen) at 37°C overnight. Gels were stained with SimplyBlue SafeStain (Invitrogen) for 1 hour at room temperature and washed. Areas of protease activity appeared as clear bands against a dark-blue background.
Plasma membrane preparation
FLS cultured on Matrigel-coated Petri dishes were collected by scraping and collagenase digestion (20). Cells were resuspended in ice-cold phosphate buffered saline (PBS) with a protease inhibitor cocktail containing 4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, bestatin hydrochloride, E-64, leupeptin, and pepstatin (Sigma-Aldrich), washed, and then subjected to 3 cycles of freeze–thaw. The lysates were sonicated for 3 seconds, and plasma membranes were pelleted by centrifugation for 30 minutes at 14,000 revolutions per minute, at 4°C. The membrane pellets were washed once, resuspended in PBS, and the total amount of protein in each sample was measured.
Quantitative real-time polymerase chain reaction (PCR)
FLS cell lines were cultured on Matrigel-coated Petri dishes (Becton Dickinson) until reaching 70–80% confluency, a point of intense transcriptional activity. Cells were then washed twice with PBS, and the collagen matrix was digested with collagenase D (Sigma-Aldrich). Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) and digested with DNase (Qiagen). RNA was quantified with spectrophotometry (NanoDrop Technologies, Wilmington, DE), and its integrity was determined with the 2100 Bioanalyzer (Agilent, Palo Alto, CA). Total RNA (200 ng) from each sample was used for complementary DNA synthesis using the SuperScript III kit (Invitrogen). The primer and probe sequences for interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα), as well as the quantitative PCR methods, have been previously described (14). Primers, TaqMan probe sequences for IL-6 and transforming growth factor β1 (TGFβ1) (Applied Biosystems, Foster City, CA), and probe sequences for MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-13, MT1–MMP, and MT2-MMP (Exiqon, Woburn, MA) are available online at www.NSLIJ-GENETICS.ORG/GULKO. GAPDH was used as endogenous control. Samples were run in duplicate, and the mean values were used for analysis. Data were analyzed using Sequence Detection System software, version 1.9.1 (Applied Biosystems). Results were obtained as threshold cycle values (Ct). The relative expression of all the genes was adjusted for GAPDH in each sample (ΔCt).
Western blot analysis
FLS (70–80% confluency) were plated on Matrigel-coated Petri dishes overnight. The supernatants were collected and concentrated with Microcon YM-10 columns (Millipore, Bedford, MA). The same amount of total proteins (5 µg) was loaded in 3–8% SDS–polyacrylamide gel electrophoresis (Invitrogen) under reducing conditions. Proteins were transferred to a nitrocellulose membrane (Millipore). Membranes were blocked overnight with 5% nonfat milk (Sigma-Aldrich) at 4°C, then incubated with mouse anti–MT1-MMP monoclonal antibody (clone 113-5B7; Chemicon) in PBS with 0.1% Tween 20 for 1 hour at room temperature. Horseradish peroxidase–conjugated goat anti-mouse IgG was used as secondary antibody and incubated for 1 hour at room temperature. Protein bands were detected by enhanced chemiluminescence (ECL Plus; Amersham Biosciences, Piscataway, NJ) and visualized using Kodak X-OMAT films.
Statistical analysis
Mean values (for normally distributed data) were analyzed by Student’s t-test, and median values were compared between groups with the nonparametric Mann-Whitney test, using SigmaStat, version 3.0 (SPSS, Chicago, IL).
RESULTS
Cia4, Cia5d, and Cia10 define regions that regulate arthritis severity
All 3 congenic strains of rats used in this study (Figure 1A) were previously shown to develop a significantly milder form of arthritis compared with DA rats (13–15), and the results of our study supported those observations (Table 1).
Figure 1.
Map of the congenic intervals, and the effect of the Cia5d locus on fibroblast-like synoviocyte (FLS) invasion. A, Markers used in the breeding of DA.ACI(Cia10), DA.F344(Cia4), and DA.F344(Cia5d) rats are shown, along with their respective positions on chromosomes 2, 7, and 10, and genotypes. Numbers inside the bars represent the position in the chromosome (megabases). B, FLS from DA.F344(Cia5d) rats (passage range 6–9 [median 7.5]) had a significant reduction of 86.5% in the median number of invading cells, compared with FLS from DA rats (passage range 6–16 [median 8]). The median number of invading cells from DA.F344(Cia4) rats and that of invading cells from DA.ACI(Cia10) rats were not significantly lower than that for DA rats. (Experiments were performed with BD Matrigel invasion chambers.) * = P ≤ 0.001 versus DA rats, by Mann-Whitney test. C, The increased invasive properties of FLS from DA rats require exposure to pristane, as FLS from naive DA rats have invading properties similar to those of FLS from naive rats and DA.F344(Cia5d) rats with pristane-induced arthritis (PIA). Therefore, F344 alleles at the Cia5d interval render cells resistant to pristane-induced increased invasive properties. (Experiments were performed with Chemicon Matrigel invasion chambers. Samples were run on 2 separate days, and absorbance was normalized according to a set of controls/duplicates run on both days.) * = P = 0.018; # = P = 0.002 versus DA rats with PIA, by Mann-Whitney test. Bars in B and C are the 25th and 75th percentiles. D and E, Ankle joints from DA rats (D) and DA.F344(Cia5d) rats (E) were collected on day 32 after initiation of PIA. Synovial hyperplasia was extensive in DA rats, with cartilage and bone erosion, while synovium in DA.F344(Cia5d) rats was normal, with no erosions (arrows). (Hematoxylin and eosin stained; original magnification × 100.)
Table 1.
ASI scores on day 21 after initiation of PIA in rats*
| Rat strain | Median (25%–75% CI) ASI |
P, versus DA rats |
|---|---|---|
| DA (n = 14) | 37.5 (16.0–85.0) | – |
| F344 (n = 7) | 0 | <0.001 |
| DA.F344(Cia4) (n = 4) | 2.5 (1.0–4.0) | 0.013 |
| DA.F344(Cia5d) (n = 10) | 5.0 (2.0–20.0) | 0.005 |
| DA.ACI(Cia10) (n = 8) | 3.0 (1.0–5.5) | 0.002 |
ASI = arthritis severity index; PIA = pristane-induced arthritis; CI = confidence interval.
FLS derived from DA.F344(Cia5d) rats have decreased in vitro invasive properties compared with those of FLS derived from DA rats with PIA
Primary FLS cell lines derived from DA, DA.F344(Cia4), DA.F344(Cia5d), and DA.ACI(Cia10) were studied in a 24-hour assay of invasion through Matrigel-coated inserts. This Matrigel invasion assay has been used in studies with RA FLS cell lines (21,22). Furthermore, the invasive properties of FLS on Matrigel correlate with radiographic damage in RA, underscoring the clinical relevance of this phenotype (12). The experiments were initially performed with Becton Dickinson Matrigel invasion chambers. The presence of F344 alleles at Cia5d, as in FLS from the DA.F344(Cia5d) rat strain, caused a reduction of 86.5% in the median numbers of FLS that invaded through the Matrigel layer compared with the median number of invading DA FLS (6.0 versus 44.7 [P ≤ 0.001, by Mann-Whitney test]) (Figure 1B). Although PIA was significantly milder in DA.F344(Cia4) and DA.ACI(Cia10) rats than in DA rats (Table 1), there was no significant difference between these strains in the numbers of invading cells, demonstrating the specificity of the DA.F344(Cia5d) effect in invasion. Each FLS cell line was analyzed in duplicate, and experiments were performed at least twice. These observations demonstrate that a gene contained within the Cia5d locus on rat chromosome 10 is an important new regulator of FLS invasion.
A colorimetric-based Matrigel invasion assay (Chemicon) was also used, and the results confirmed the reduced invasive properties of FLS from DA.F344 (Cia5d) rats with PIA (n = 8) compared with FLS from DA rats with PIA (n = 12) (P = 0.002, by Mann-Whitney test) (Figure 1C).
FLS derived from naive DA rats do not have increased invasive properties
FLS were collected from naive DA rats (n = 5) and naive DA.F344(Cia5d) rats (n = 3) and cultured in the same conditions as those used for FLS obtained from rats with PIA. FLS obtained from naive DA rats and naive DA.F344(Cia5d) rats had similar invasive properties (Figure 1C). The median absorbance of the invading cells obtained from naive DA and naive DA.F344(Cia5d) FLS was similar to that of FLS obtained from DA.F344(Cia5d) rats with PIA but significantly lower than that of FLS obtained from DA rats with PIA (P = 0.018, by Mann-Whitney test) (Figure 1C). These observations demonstrate that the difference in FLS invasion between FLS from DA rats with PIA and FLS from DA.F344(Cia5d) rats with PIA identifies a phenotype that requires a gene–environment interaction, in this case exposure to pristane. The presence of F344 alleles at Cia5d was enough to prevent induction of this phenotype.
Differences in invasive properties between FLS derived from DA and DA.F344(Cia5d) rats are retained after several passages in culture
Significant differences in invasion were detected in experiments using early passages of FLS from DA rats (passage range 6–16 [median 8]) and DA.F344(Cia5d) rats (passage range 6–9 [median 7.5]) (Figure 1B), as well as in experiments using later passages (for DA, passage range 4–62 [median 22]; for DA.F344(Cia5d), passage range 5–55 [median 46]) (Figure 1C). A complete description of FLS passages in each experiment/figure and the differences in invasion and levels of activated MMP-2 is available online at www.NSLIJ-GENETICS.ORG/GULKO.
No invasion or destruction of cartilage and bone by synovial tissue from DA.F344(Cia5d) rats with PIA
Ankle joints were collected from DA and DA.F344(Cia5d) rats after 32 days of PIA. Histologic analysis revealed pronounced synovial hyperplasia with cartilage and bone invasion in DA (Figure 1D), while nearly no synovial hyperplasia was observed in tissues from DA.F344(Cia5d) rats (Figure 1E). More importantly, in agreement with the FLS invasion data, synovial tissue from DA.F344(Cia5d) rats did not invade or destroy cartilage or bone, demonstrating a direct correlation between the in vitro phenotype and in vivo findings.
Proliferation, levels of cytokine messenger RNA (mRNA), or actin cytoskeleton characteristics do not explain the differences in the invasive properties of FLS from DA and DA.F344(Cia5d) rats
FLS cell lines from DA and those from DA.F344(Cia5d) rats had similar proliferative characteristics, similar levels of expression of IL-1β, IL-6, TGFβ1, and TNFα (by quantitative PCR), and similar actin cytoskeleton characteristics, as measured by fluorescent phalloidin staining and cell spreading. Detailed results are available online at www.NSLIJ-GENETICS.ORG/GULKO.
MMP-dependent differences between the invasive properties of FLS from DA and DA.F344(Cia5d) rats
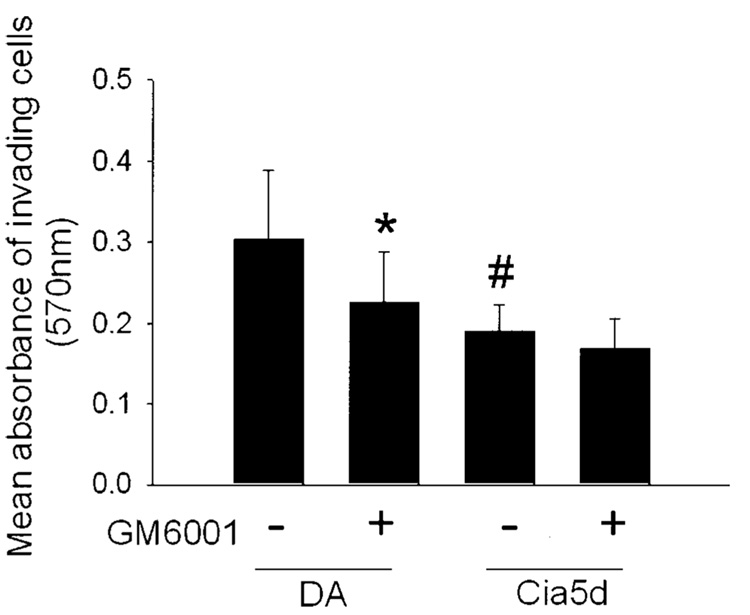
The Matrigel invasion assays were performed in the presence or absence of 25 µM GM6001, a general inhibitor of MMPs that is known to inhibit the activity of MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13. In the presence of GM6001, the invasive properties of FLS from DA (n = 12) were significantly reduced, to levels similar to those of FLS derived from DA.F344(Cia5d) (Figure 2). The mean ± SD absorbance at 570 nm of invading cells from DA rats was 0.303 ± 0.08; in the presence of GM6001, the level decreased to 0.225 ± 0.06 (P = 0.018). For invading cells from DA.F344(Cia5d), the mean ± SD absorbance at 570 nm was 0.191 ± 0.03 (P = 0.004, by t-test). The invasion through Matrigel of FLS from DA.F344(Cia5d) was not significantly affected by MMP inhibition with GM6001 (mean ± SD absorbance at 570 nm 0.167 ± 0.03). These observations demonstrated that MMPs are critical for the difference in the invasive properties between FLS obtained from DA and FLS obtained from DA.F344(Cia5d) rats.
Figure 2.
Effect of the matrix metalloproteinase inhibitor GM6001 on the invasive properties of fibroblast-like synoviocytes (FLS) derived from DA rats and FLS from DA.F344(Cia5d) rats. FLS from DA and DA.F344(Cia5d) were plated on Matrigel-coated invasion chambers, with or without 25 µM GM6001, for 24 hours. The absorbance of invading cells from DA (n = 12) was significantly reduced in the presence of GM6001. The absorbance of invading cells from DA.F344(Cia5d) without GM6001 (n = 7) was significantly lower than that of cells from DA without GM6001 and was similar to that of cells from DA treated with GM6001. Bars show the mean and SD. * = P = 0.018; # = P = 0.004 versus FLS from DA without GM6001, by t-test.
Similar levels of MMPs and similar collagenase and MMP-3 (stromelysin 1) activity in FLS from DA and DA.F344(Cia5d) rats
FLS cell lines were cultured on Matrigel to recreate the environment of the invasion chamber. MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, MT1-MMP, and MT2-MMP were detected by quantitative PCR in all FLS cell lines, but no significant differences in gene expression between DA rats and DA.F344(Cia5d) rats were observed (Figure 3A). These results suggest that although the Cia5d-regulated invasive phenotype is dependent on MMPs, the MMP effect is not controlled at the mRNA level. MMP-8 was not expressed by rat FLS.
Figure 3.
Levels of matrix metalloproteinase (MMP) mRNA, collagenase activity, and MMP-3 activity in fibroblast-like synoviocytes (FLS) from DA rats and DA.F344(Cia5d) rats. A, Messenger RNA levels of MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, membrane type 1 (MT1)–MMP, and MT2-MMP were not significantly different in FLS cell lines from DA rats and FLS cell lines from DA.F344(Cia5d) rats, as determined by quantitative polymerase chain reaction. B, Collagenase activity was nearly identical in the supernatants of FLS cultures from DA rats (n=7) and DA.F344(Cia5d) rats (n = 7). Values in A and B are the mean and SD. C, Casein zymography revealed similar levels of MMP-3 in the supernatants of FLS cultures from DA rats (n = 4) and DA.F344(Cia5d) rats (n = 4). ΔCt = change in threshold cycle.
It was considered that the MMP-mediated difference in invasive properties could be regulated at the level of protein activation. Collagenase activity (MMP-1, MMP-8, and MMP-13) was measured in the supernatants of FLS cell lines. Because no MMP-8 mRNA was detected in rat FLS cell lines, the results shown in Figure 3B are considered to reflect MMP-1 and MMP-13 activity. No significant differences were detected between FLS cell lines from DA (n = 7) and those from DA.F344(Cia5d) rats (n = 7) (Figure 3B).
GM6001 also inhibits MMP-3 (stromelysin 1) activity, and, therefore, MMP-3 activity was quantified using a casein zymogram gel. No significant differences in the levels of proMMP-3 or active MMP-3 were detected between FLS from DA and FLS from DA.F344(Cia5d) rats, thereby excluding MMP-3 as the mediator of invasive differences between FLS (Figure 3C).
Correlation between MMP-2 activation and the invasive properties of FLS
Gelatinases (MMP-2 and MMP-9) were expressed by FLS from DA and FLS from DA.F344(Cia5d) rats (Figure 3A); both of these gelatinases are known to be inhibited by GM6001. To address the role of gelatinase activation on the difference in invasive properties between DA and DA.F344(Cia5d) rat FLS, 4 µg of total protein per FLS cell line culture supernatant was analyzed in a gelatin zymogram. Supernatants from DA FLS had significantly higher levels of active MMP-2 (64 kd) compared with supernatants from DA.F344(Cia5d) FLS (Figure 4A). Levels of proMMP-2 (72 kd) were similar in both rat strains, demonstrating that the difference was not in the overall synthesis of proMMP-2 but instead in its activation. FLS from arthritis-resistant F344 rats also had reduced levels of active MMP-2 (Figure 4B). These observations demonstrated that the Cia5d gene controls FLS invasion via the regulation of MMP-2 activation, and that F344-derived alleles at this locus reduce its activation. MMP-9 was not detected in gelatin zymograms.
Figure 4.
Reduced MMP-2 activity in DA.F344(Cia5d) explains the reduced invasive properties of FLS. A, Gelatin zymography showed significantly lower levels of active MMP-2 in supernatants of DA.F344(Cia5d) FLS (n = 6) compared with DA FLS (n = 7) after 24 hours in culture. The results are representative of experiments involving 11 DA and 8 DA.F344(Cia5d) rats, in which 4 µg of protein per FLS cell line supernatant per lane was loaded. B, Gelatin zymography of supernatants of DA, DA.F344(Cia5d), and F344 FLS showed reduced levels of activated MMP-2 in DA.F344(Cia5d) and F344 rats compared with DA rats (8 µg of protein per FLS cell line supernatant per lane was loaded). C, Gelatin zymography of supernatants of naive DA FLS (n = 5) and naive DA.F344(Cia5d) FLS (n = 4) demonstrated low levels of active MMP-2, similar to those in FLS from DA.F344(Cia5d) rats with pristane-induced arthritis (PIA) but significantly lower than those in FLS from DA rats with PIA (8 µg of total protein per cell line culture supernatant was loaded). D, Treatment with the MMP-2 inhibitor SB-3CT (compared with DMSO as control) significantly reduced the invasive properties of FLS from DA rats to levels similar to those of FLS from untreated DA.F344(Cia5d). DA.F344(Cia5d) FLS treated with SB-3CT also exhibited a modest reduction in invasive properties, consistent with low MMP-2 activation. Values are the mean and SD results for 5 different cell lines per group. * = P ≤ 0.001 versus untreated DA rats; # = P = 0.027 versus untreated DA rats; ¶ = P = 0.008 versus untreated DA.F344(Cia5d) rats, by t-test. See Figure 3 for other definitions.
Reduced levels of active MMP-2 in FLS from naive DA rats are consistent with the invasive properties of FLS
Although the supernatants of FLS derived from DA rats with PIA had increased levels of active MMP-2, FLS from naive DA rats had significantly lower levels, which were similar to those in FLS from both naive DA.F344(Cia5d) and DA.F344(Cia5d) rats with PIA (Figure 4C). Therefore, the MMP-2 activation data for FLS from rats with PIA and FLS from naive rats matched the FLS invasion data shown in Figure 1C. These observations were replicated with cell lines from different passages (available online at www.NSLIJ-GENETICS.ORG/GULKO) and represent the first demonstration that pristane induces long-lasting changes in FLS MMP-2 activation, and that this process is genetically regulated by a gene within the Cia5d locus.
Inhibition of MMP-2 suppresses DA rat FLS invasion
Treatment of FLS from DA rats with the selective gelatinase inhibitor SB-3CT (500 µM) significantly reduced the number of invading cells (the mean ± SD of the absorbance at 570 nm of invading cells from DA rats without inhibitor was 0.307 ± 0.09, and that of DA rats treated with SB-3CT was 0.105 ± 0.02; P ≤ 0.001, by t-test) (Figure 4D) and did not affect cell viability (data not shown). The mean ± SD absorbance of invading SB-3CT–treated FLS from DA rats was also lower than that of FLS from untreated DA.F344(Cia5d) rats (0.186 ± 0.05; P = 0.008, by t-test) and similar to that of SB-3CT–treated FLS from DA.F344(Cia5d) rats. The invasive properties of SB-3CT–treated FLS from DA.F344(Cia5d) rats were slightly reduced compared with those of untreated FLS, which is consistent with the observation that FLS from DA.F344(Cia5d) rats produced active MMP-2 (Figure 4A), albeit at significantly lower levels than in FLS from DA rats (the mean ± SD absorbance at 570 nm of invading cells from DA.F344(Cia5d) rats without inhibitor was 0.186 ± 0.05, compared with 0.106 ± 0.01 in SB-3CT–treated FLS from DA.F344(Cia5d) rats; P = 0.008, by t-test) (Figure 4D).
MMP-9 protein was not detected in the zymograms; therefore, the reduced invasion associated with SB-3CT treatment was attributed to inhibition of MMP-2 activity.
Similar levels of tissue inhibitor of metalloproteinases 2 (TIMP-2), urokinase plasminogen activator (uPA), and tissue plasminogen activator (tPA) activity in FLS from DA and DA.F344(Cia5d)
Three of the main pathways of MMP-2 activation include 1) the formation of a ternary complex between MT1-MMP, TIMP-2, and proMMP-2 (which cleaves proMMP-2 into its active form) and activation of the plasmin system via 2) uPA and 3) tPA. The levels of TIMP-2 measured in 600 µg of total protein per FLS culture supernatant from DA and DA.F344(Cia5d) were nearly identical (data not shown). The activity of uPA and tPA measured in the supernatants of FLS cultures from DA and DA.F344(Cia5d) revealed no significant differences (data not shown). Therefore, neither TIMP-2 nor the 2 pathways of plasminogen activation explain the differences in levels of active MMP-2 and invasion detected in DA and DA.F344(Cia5d) rats.
Increased levels of soluble MT1-MMP coincide with increased levels of active MMP-2 in DA rats
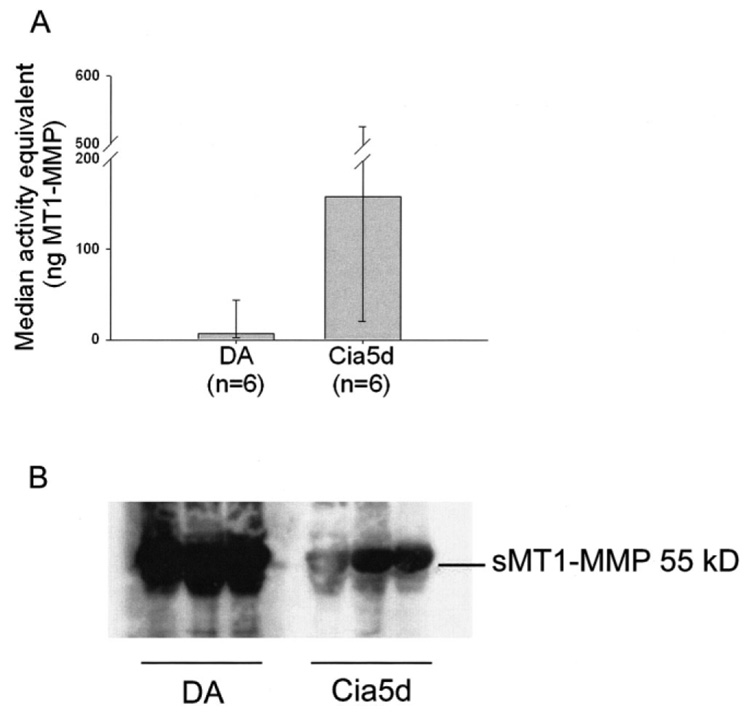
MT1-MMP activity was significantly reduced in the plasma membrane of FLS from DA rats after 24 hours of culture (Figure 5A), the time point at which levels of active MMP-2 were increased. Therefore, the differences in MMP-2 activation and invasive properties were not explained by the membrane-bound activity of MT1-MMP. It was considered that this discrepancy could be explained by increased cleavage of plasma membrane MT1-MMP, which would generate increased levels of its MMP-2–activating soluble form (23,24). Twenty-four–hour FLS culture supernatants from DA (n = 6) and DA.F344(Cia5d) (n = 6) were analyzed by Western blotting, using a monoclonal antibody against the catalytic domain of MT1-MMP. Supernatants from DA had significantly higher levels of soluble MT1-MMP compared with those from DA.F344(Cia5d) rats (Figure 5B), thus providing an explanation for the increased MMP-2 activation.
Figure 5.
Soluble and plasma membrane type 1 matrix metalloproteinase (MT1-MMP) in fibroblast-like synoviocytes (FLS). A, DA FLS cultured for 24 hours had reduced MT1-MMP activity in plasma membranes, compared with DA.F344(Cia5d) FLS. Values are the median and 25th and 75th percentiles. P = 0.052, by Mann-Whitney test. B, Western blotting showed increased levels of soluble MT1-MMP (sMT1-MMP) in supernatants of DA rat FLS (n = 3) compared with DA.F344(Cia5d) rat FLS (n = 3) cultured on Matrigel-coated Petri dishes (5 µg of protein loaded per lane). The data for sMT1-MMP were replicated in 3 additional FLS cell culture supernatants per strain.
DISCUSSION
RA histology is typically characterized by pronounced synovial hyperplasia, also called pannus. RA pannus produces proinflammatory cytokines and proteases and invades cartilage and bone, leading to joint destruction and deformities (2). The FLS is a key player in RA pannus and joint pathology and has increased invasive properties compared with osteoarthritis, even after several passages in vitro (11,21). Furthermore, the increased invasive properties of RA FLS have been associated with increased radiographic joint destruction (12), underscoring the relevance of this in vitro phenotype to disease outcome. We observed that several of our congenic strains protected from PIA preserved a nearly normal joint architecture with no synovial hyperplasia or cartilage and bone destruction, and considered that the increased invasive properties of RA FLS would be reproduced in FLS obtained from DA rats with PIA. Furthermore, we hypothesized that at least part of the invasive characteristics would be genetically regulated by one of the arthritis severity quantitative trait loci genes. In the present study, we demonstrate for the first time that FLS invasive properties are genetically controlled and implicate the arthritis severity quantitative trait locus Cia5d in their regulation.
FLS were isolated from synovial tissue obtained from the ankle joints of DA and congenic rat strains previously shown to develop significantly milder arthritis (13–15). After 3 or more passages, these primary cell lines were nearly 100% CD90-positive FLS (data not shown). We used the same in vitro model system originally tested in RA, in which FLS invade through a collagen-rich barrier, Matrigel (12,21,22). FLS obtained from DA and from 2 arthritis-protected congenic rat strains, DA.F344(Cia4) and DA.ACI(Cia10), had similarly increased numbers of invading cells, while FLS from DA.F344(Cia5d) had significantly lower numbers of invading cells. This observation demonstrates that F344 alleles specifically at the Cia5d region on rat chromosome 10 reduce FLS invasion. FLS proliferation was similar in DA rats and DA.F344(Cia5d) rats; therefore, proliferation did not explain the differences in invasion, which is consistent with studies of RA FLS (25) and human cancer cell lines (26,27).
Cell migration and invasion critically depend on actin cytoskeleton dynamics and functional integrity. We could not detect any difference between DA and DA.F344(Cia5d) rat FLS in this regard, using different sensitive assays (28). Quantitative PCR analysis also revealed no significant differences in mRNA levels of 7 MMPs and 4 cytokines known to increase in vitro FLS responses. The difference in invasion was dependent on MMPs, since treatment with the MMP inhibitor GM6001 reduced invasion of DA FLS to levels similar to those of DA.F344(Cia5d) FLS. However, collagenase and MMP-3 (stromelysin 1) activities were similar in the supernatants of FLS cultures from both strains and therefore did not explain the difference in invasion. GM6001 also inhibits the activity of MMP-2 and MMP-9 (gelatinases). MMP-9 protein was not detected, suggesting that the difference was attributable to MMP-2. In fact, levels of active MMP-2 were significantly higher in DA compared with DA.F344(Cia5d) rats. Treatment with the MMP-2 inhibitor SB-3CT reduced the invasive properties of FLS from DA rats to levels similar to those of FLS from DA.F344(Cia5d) rats, thus confirming that MMP-2 accounts for the difference in cell invasion. To our knowledge, this is the first study in which an arthritis quantitative trait locus has been implicated in the regulation of MMP-2 activation.
The MMP-2 gene is not contained within the Cia5d interval and was not considered a candidate gene. Instead, we considered that DA or F344 alleles at a gene located within the Cia5d locus were differentially regulating the pathways that control MMP-2 activation, namely uPA, tPA, TIMP-2, and MT1-MMP (29,30). There was no significant difference in the levels of TIMP-2 or uPA and tPA activity between DA and DA.F344(Cia5d) rats, excluding these pahways.
MT1-MMP is a cell surface MMP that forms a complex with TIMP-2 and has a major role in the activation of MMP-2 (30). Unexpectedly, the plasma membrane activity of MT1-MMP was lower in DA FLS (cultured for 24 hours) than in DA.F344(Cia5d) FLS. However, we detected significantly increased levels of soluble MT1-MMP in DA FLS culture supernatants. Therefore, we considered that the decreased MT1-MMP plasma membrane activity in DA FLS could be attributable to increased shedding of MT1-MMP. It is not known which protease controls cleavage of plasma membrane MT1-MMP, but soluble MT1-MMP is capable of activating MMP-2 (23,24). Our data support this concept and suggest that Cia5d identifies a novel gene that controls MMP-2 activation via the regulation of MT1-MMP shedding from the plasma membrane. This study is the first to demonstrate that increased production of soluble MT1-MMP is associated with FLS invasion and arthritis and the first to show that it is genetically regulated. It is conceivable that soluble MT1-MMP could be more resistant to degradation and inactivation than is the plasma membrane form, leading to prolonged and more pronounced MMP-2 activation.
MMP-2 digests gelatin, fibronectin, laminin, type IV collagen, type V collagen, cartilage proteoglycans, and elastin (31) and is expressed in increased amounts by RA FLS at both the mRNA (32) and protein levels (3,33). Furthermore, increased expression of active MMP-2 in synovial tissue obtained from patients with RA correlates with increased erosive changes (34), a parameter associated with worse outcome and increased risk of development of joint deformities. To our knowledge, no MMP-2–specific inhibitor has been used in arthritis. However, an MMP-2/MMP-9/MMP-3 inhibitor significantly reduced cartilage erosive changes in a model of monarticular arthritis in rats (35). Taken together, our observations can be directly related to RA FLS and their role in invasion and disease severity. Additionally, the suppression of monarthritis reported in a rodent study that used a gelatinase inhibitor (35) supports our concept that modulating MMP-2 activity, perhaps via the Cia5d gene, is a feasible way to treat disease.
Although the FLS invasive phenotype is genetically controlled, it required an environmental trigger, in this case treatment with pristane, to be fully activated in DA rats. This is consistent with the current understanding of RA pathogenesis. Specifically, although there is a strong genetic contribution to RA susceptibility, stochastic and environmental events such as smoking (36), the use of decaffeinated coffee (37), and exposure to mineral oils (38) are required for the development of disease. It remains incompletely understood how those environmental factors and pristane operate to regulate the expression of arthritis. Orally administered pristane is distributed to lymphoid tissues, muscle, and fat and crosses the placenta (39), and it could reach the synovial tissue.
Clearly, exposure to pristane changed the FLS cell phenotype in a fundamental manner, as the differences in invasive properties remained unchanged in DA and DA.F344(Cia5d) cells after >20 passages in culture and several cell duplications. Therefore, once triggered, the FLS invasive phenotype and its associated increase in MMP-2 activation become independent from other cells and molecules that are present in the joint environment. This might explain the progressive joint destruction described in patients with RA even in the absence of clinical or laboratory evidence of joint inflammation or disease activity (40,41). Of additional interest, MMP-2 also regulates the invasive properties of cancers such as gliomas and bladder cancer (42,43), raising the possibility that Cia5d will be relevant for cancer biology and treatment.
While new biologic therapies developed during the past 9 years have significantly improved disease control and the quality of living for patients with RA (44–47), complete remission is still rarely achieved (48), and better therapies are needed. In the present study, we identified a potentially novel pathway for therapeutic intervention. Modulation of the Cia5d gene has the potential to reduce levels of soluble MT1-MMP and decrease MMP-2 activation, thus reducing the invasive properties of FLS and likely cartilage and bone destruction.
Acknowledgments
Dr. Gulko’s work was supported by the NIH (grants R01-AR-46213 and R01-AR-052439 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and grant R01-AI-54348 from the National Institute of Allergy and Infectious Diseases).
REFERENCES
- 1.Gregersen PK, Plenge RM, Gulko PS. Genetics of rheumatoid arthritis. In: Firestein G, Panayi G, Wollheim FA, editors. Rheumatoid arthritis. 2nd ed. New York: Oxford University Press; 2006. pp. 3–14. [Google Scholar]
- 2.Gulko PS, Winchester RJ. Rheumatoid arthritis. In: Austen KF, Frank MM, Atkinson JP, Cantor H, editors. Samter’s immunologic diseases. 6th ed. Baltimore: Lippincott, Williams & Wilkins; 2001. pp. 427–463. [Google Scholar]
- 3.Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts: purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990;194:721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells: regulation by tumor necrosis factor-α and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- 5.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 6.Yellin MJ, Winikoff S, Fortune SM, Baum D, Crow MK, Lederman S, et al. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J Leukoc Biol. 1995;58:209–216. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, et al. Transforming growth factor β 1 (TGF-β 1) induced neutrophil recruitment to synovial tissues: implications for TGF-β-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173:1121–1132. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shealy DJ, Wooley PH, Emmell E, Volk A, Rosenberg A, Treacy G, et al. Anti-TNF-α antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Res. 2002;4:R7. doi: 10.1186/ar430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, et al. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storgard CM, Stupack DG, Jonczyk A, Goodman SL, Fox RI, Cheresh DA. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 12.Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, Breedveld FC, Toes RE, Huizinga TW. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:1999–2002. doi: 10.1002/art.21118. [DOI] [PubMed] [Google Scholar]
- 13.Remmers EF, Joe B, Griffiths MM, Dobbins DE, Dracheva SV, Hashiramoto A, et al. Modulation of multiple experimental arthritis models by collagen-induced arthritis quantitative trait loci isolated in congenic rat lines: different effects of non-major histocompatibility complex quantitative trait loci in males and females. Arthritis Rheum. 2002;46:2225–2234. doi: 10.1002/art.10439. [DOI] [PubMed] [Google Scholar]
- 14.Brenner M, Meng H, Yarlett N, Griffiths M, Remmers E, Wilder R, et al. The non-major histocompatibility complex quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum. 2005;52:322–332. doi: 10.1002/art.20782. [DOI] [PubMed] [Google Scholar]
- 15.Brenner M, Meng HC, Yarlett NC, Joe B, Griffiths MM, Remmers EF, et al. The non-MHC quantitative trait locus Cia5 contains three major arthritis genes that differentially regulate disease severity, pannus formation, and joint damage in collagen-and pristane-induced arthritis. J Immunol. 2005;174:7864–7903. doi: 10.4049/jimmunol.174.12.7894. [DOI] [PubMed] [Google Scholar]
- 16.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–1683. [PMC free article] [PubMed] [Google Scholar]
- 17.Gulko PS, Kawahito Y, Remmers EF, Reese VR, Wang J, Dracheva SV, et al. Identification of a new non-major histocompatibility complex genetic locus on chromosome 2 that controls disease severity in collagen-induced arthritis in rats. Arthritis Rheum. 1998;41:2122–2131. doi: 10.1002/1529-0131(199812)41:12<2122::AID-ART7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Michael IP, Sotiropoulou G, Pampalakis G, Magklara A, Ghosh M, Wasney G, et al. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J Biol Chem. 2005;280:14628–14635. doi: 10.1074/jbc.M408132200. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh MC, Grass L, Soosaipillai A, Sotiropoulou G, Diamandis EP. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 2004;25:193–199. doi: 10.1159/000081102. [DOI] [PubMed] [Google Scholar]
- 20.Zigrino P, Drescher C, Mauch C. Collagen-induced proMMP-2 activation by MT1-MMP in human dermal fibroblasts and the possible role of α2β1 integrins. Eur J Cell Biol. 2001;80:68–77. doi: 10.1078/0171-9335-00134. [DOI] [PubMed] [Google Scholar]
- 21.Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61:975–980. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan A, Akhtar M, Brenner M, Zheng Y, Gulko PS, Symons M. The GTPase rac regulates the proliferation and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Mol Med. 2007;13:297–304. doi: 10.2119/2007-00025.Chan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toth M, Hernandez-Barrantes S, Osenkowski P, Bernardo MM, Gervasi DC, Shimura Y, et al. Complex pattern of membrane type 1 matrix metalloproteinase shedding: regulation by autocatalytic cells surface inactivation of active enzyme. J Biol Chem. 2002;277:26340–26350. doi: 10.1074/jbc.M200655200. [DOI] [PubMed] [Google Scholar]
- 24.Kazes I, Delarue F, Hagege J, Bouzhir-Sima L, Rondeau E, Sraer JD, et al. Soluble latent membrane-type 1 matrix metalloprotease secreted by human mesangial cells is activated by urokinase. Kidney Int. 1998;54:1976–1984. doi: 10.1046/j.1523-1755.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- 25.Seemayer CA, Kuchen S, Kuenzler P, Rihoskova V, Rethage J, Aicher WK, et al. Cartilage destruction mediated by synovial fibroblasts does not depend on proliferation in rheumatoid arthritis. Am J Pathol. 2003;162:1549–1557. doi: 10.1016/S0002-9440(10)64289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, et al. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear β-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001;159:1613–1617. doi: 10.1016/s0002-9440(10)63007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoshyomn S, Lew S, DeMattia J, Singer EB, Penar PL. Brain tumor invasion rate measured in vitro does not correlate with Ki-67 expression. J Neurooncol. 1999;45:111–116. doi: 10.1023/a:1006375316331. [DOI] [PubMed] [Google Scholar]
- 28.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, Garbisa S, et al. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. Embo J. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 31.Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579–6587. [PubMed] [Google Scholar]
- 32.Pap T, Franz JK, Hummel KM, Jeisy E, Gay R, Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2:59–64. doi: 10.1186/ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migita K, Eguchi K, Kawabe Y, Ichinose Y, Tsukada T, Aoyagi T, et al. TNF-α-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology. 1996;89:553–557. doi: 10.1046/j.1365-2567.1996.d01-789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldbach-Mansky R, Lee JM, Hoxworth JM, Smith D, Duray P, Schumacher RH, Jr, et al. Active synovial matrix metalloproteinase-2 is associated with radiographic erosions in patients with early synovitis. Arthritis Res. 2000;2:145–153. doi: 10.1186/ar79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada T, Arima N, Shindo M, Sugama K, Sasaguri Y. Suppression of adjuvant arthritis of rats by a novel matrix metalloproteinase-inhibitor. Br J Pharmacol. 2000;131:1513–1520. doi: 10.1038/sj.bjp.0703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis: results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–735. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 37.Mikuls TR, Cerhan JR, Criswell LA, Merlino L, Mudano AS, Burma M, et al. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2002;46:83–91. doi: 10.1002/1529-0131(200201)46:1<83::AID-ART10042>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Sverdrup B, Kallberg H, Bengtsson C, Lundberg I, Padyukov L, Alfredsson L, et al. Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res Ther. 2005;7:R1296–R1303. doi: 10.1186/ar1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrett LR, Chung JG, Byers PE, Cuchens MA. Dietary effects of pristane on rat lymphoid tissues. Agents Actions. 1989;28:272–278. doi: 10.1007/BF01967414. [DOI] [PubMed] [Google Scholar]
- 40.Mulherin D, Fitzgerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol. 1996;35:1263–1268. doi: 10.1093/rheumatology/35.12.1263. [DOI] [PubMed] [Google Scholar]
- 41.Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 42.Nakada M, Okada Y, Yamashita J. The role of matrix metalloproteinases in glioma invasion. Front Biosci. 2003;8:e261–e269. doi: 10.2741/1016. [DOI] [PubMed] [Google Scholar]
- 43.Cockett MI, Murphy G, Birch ML, O’Connell JP, Crabbe T, Millican AT, et al. Matrix metalloproteinases and metastatic cancer. Biochem Soc Symp. 1998;63:295–313. [PubMed] [Google Scholar]
- 44.Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG. Long-term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4-year extended study. Ann Rheum Dis. 2006;65:753–759. doi: 10.1136/ard.2005.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen SB, Woolley JM, Chan W. Interleukin 1 receptor antagonist anakinra improves functional status in patients with rheumatoid arthritis. J Rheumatol. 2003;30:225–231. [PubMed] [Google Scholar]
- 46.Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46:3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 47.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K. Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57:935–942. doi: 10.1002/art.22895. [DOI] [PubMed] [Google Scholar]