Abstract
We report that 9 d of uncontrolled experimental diabetes induced by streptozotocin (STZ) in rats is an endogenous chronic stressor that produces retraction and simplification of apical dendrites of hippocampal CA3 pyramidal neurons, an effect also observed in nondiabetic rats after 21 d of repeated restraint stress or chronic corticosterone (Cort) treatment. Diabetes also induces morphological changes in the presynaptic mossy fiber terminals (MFT) that form excitatory synaptic contacts with the proximal CA3 apical dendrites. One effect, synaptic vesicle depletion, occurs in diabetes as well as after repeated stress and Cort treatment. However, diabetes produced other MFT structural changes that differ qualitatively and quantitatively from other treatments. Furthermore, whereas 7 d of repeated stress was insufficient to produce dendritic or synaptic remodeling in nondiabetic rats, it potentiated both dendritic atrophy and MFT synaptic vesicle depletion in STZ rats. These changes occurred in concert with adrenal hypertrophy and elevated basal Cort release as well as hypersensitivity and defective shutoff of Cort secretion after stress. Thus, as an endogenous stressor, STZ diabetes not only accelerates the effects of exogenous stress to alter hippocampal morphology; it also produces structural changes that overlap only partially with those produced by stress and Cort in the nondiabetic state.
Adrenal glucocorticoids (GCs) are major mediators of the adaptive response to stress, and they also play a crucial role in feedback mechanisms that contain or protect against defense reactions that are activated by stress, such as inflammation (1, 2). The process of adaptation to environmental demands, also called “allostasis” (3, 4), functions best when stress mediators such as those associated with the activation of the hypothalamo–pituitary–adrenal (HPA) axis are turned on efficiently when needed and turned off again when not needed (5). When HPA activity is elevated chronically or managed inefficiently, various forms of pathophysiology are accelerated, such as bone mineral loss, loss of muscle protein mass, insulin resistance, and dysfunction of the hippocampal region of the brain (5, 6). This cost to the body has been termed “allostatic load” (4, 5). MRI studies in stress-related disorders, such as posttraumatic stress disorder, recurrent depressive illness, Cushing's syndrome, and mild cognitive impairment in aging patients, have revealed atrophy of the human hippocampus accompanied by deficits in declarative and spatial memory (7–11), in line with the recognized role of the hippocampus in cognitive processes (12). However, the exact nature of the atrophy is unclear, whether it is caused by permanent damage or by a potentially reversible form of plasticity.
In experimental animals, severe and prolonged stress or GC administration has been reported to cause brain damage associated with hippocampal neuronal loss (see ref. 6 for review). We have described previously that shorter periods of repeated stress alter hippocampal morphology in a largely reversible manner. This is particularly so for the apical dendritic arborizations of CA3 pyramidal neurons and their presynaptic mossy fiber terminals (MFT), on which less severe stress or GC administration can dynamically reorganize their structure (see ref. 13 for review). In rats and primitive primates, 3–4 wk of daily restraint or psychosocial stress are needed to cause CA3 apical dendritic retraction and simplification of its branching pattern (14, 15). The effect is reversible within 7–10 days after the last stress session (16). The hippocampal morphological changes induced by stress are mediated by interactions between GC secretion, excitatory amino acid, and serotonergic and GABAergic neurotransmission (14, 17) and are also correlated with deficits in hippocampal dependent memory (see ref. 5; for review, see ref. 18).
In contrast to the relatively slow effects of repeated restraint stress, another form of adaptation to adverse external conditions, namely hibernation in ground squirrels, also causes rapid CA3 dendritic remodeling and presynaptic rearrangement in MFT that are evident after the onset of hypothermia and deep torpor; moreover, these neuroanatomical changes are reversed only a few hours after the animals awaken and regain euthermic conditions (19). If extreme metabolic adjustments and the subsequent arrest or sharp decrease of certain metabolic processes alone can induce and accelerate significantly the onset of hippocampal remodeling, then an additional stressful challenge could result in a synergistic effect or could even become a threat to the viability of the metabolically impaired hippocampal neurons. Metabolic diseases such as diabetes and obesity have been associated with increased vulnerability to stress (20) and cognitive dysfunction (21).
Therefore, we investigated whether a preexisting metabolic imbalance can induce hippocampal dendritic and synaptic remodeling and shorten the required 3 wk of daily exposure to stress to observe similar or perhaps more profound hippocampal morphological rearrangements. We tested this hypothesis in rats injected with the diabetogenic agent streptozotocin (STZ) that were subjected to only 1 wk of daily stress exposure, a paradigm that fails to produce any hippocampal morphological change in nondiabetic rats. We chose the STZ-induced diabetes because it is a well-characterized experimental model of insulinopenic Type I diabetes mellitus (22–24) and provides a relevant example of endogenous chronic stress (25, 26). We compare the results of STZ diabetes, which elevates GC levels, with the effects of repeated stress and repeated GC treatment of nondiabetic rats. Although dendritic remodeling is a common feature of all three treatments, changes in the presynaptic MFTs are at least partially unique to each treatment.
Materials and Methods
Experimental Animals.
Adult male Sprague–Dawley rats (Charles River Breeding Laboratories) weighing 200–250 g were housed in groups of three with ad libitum access to food and water. Animals were maintained in a temperature-controlled room with a light/dark cycle of 12:12 h. Experiments were performed during the light period of the cycle and were conducted in accordance with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Induction of Experimental Diabetes.
Rats were fasted the night before drug administration. The next morning, animals were anesthetized with Ketacet/Promace [Aveco/Fort Dodge Labs (Fort Dodge, IA) 10:1.7; 0.1 ml per 100 g of body weight, i.m.]. A single i.v. injection of STZ (Sigma; 70 mg per kilogram of body weight) dissolved in citrate buffer (0.1 M, pH 4.2) was delivered through the femoral vein. Control animals were injected with vehicle only. After confirmation of the development of diabetes by measuring fasting plasma glucose concentration (see below), the 7-d restraint stress paradigm was initiated. Half of the vehicle-injected controls and diabetic animals were subjected to 6-h restraint stress daily for 7 d in wire mesh restrainers secured at the head and tail ends with clips. The animals were returned to their home cages during the stress session. Experimental groups [n = 8 consisted of (i) nonstressed vehicle-injected group (Controls); (ii) nonstressed STZ-treated group; (iii) stressed vehicle-injected group; and (iv) stressed STZ-treated diabetic group. On completion of the stress session on day 7, rats were given access to food for several hours before an overnight fast. Eighteen hours after the final stress session, rats were weighed, and blood was collected for plasma glucose analysis to confirm that diabetic parameters had been maintained in the STZ-treated rats.
Oral Administration of Corticosterone (Cort).
Six rats were given Cort (Aldrich) in the drinking water (400 μg/ml of tap water) for 3 wk. Controls (n = 6) received tap water containing 2.4% of ethanol. On day 22, rats were perfused and their brain processed for electron microscopy, as described below.
Plasma Glucose Analysis.
The development of diabetes was verified 48 h after STZ administration by measuring fasting glucose concentrations by using the glucose oxidase method [Glucose (trinder) kit, Sigma]. Blood samples were obtained from the tip of the tail and collected in heparinized tubes. Rats were considered diabetic and included in the study if they had plasma glucose levels >350 mg/dl. An aliquot of plasma sample was used to determine Cort levels (see below). In addition, fasting serum glucose concentrations were determined 18 h after the final restraint stress session to confirm that hyperglycemia was maintained during the 7-d stress paradigm.
Plasma Cort Assay.
Plasma Cort was measured by RIA by using a rabbit antiserum raised against corticosterone-3-oxime-BSA (Endocrine Sciences, Tarzana, CA). The antiserum had very low crossreactivity with other major steroids. Assay sensitivity was 10 pg of Cort, and coefficients of variation within and between assays were 5 and 10%, respectively. Blood samples were obtained on days 4 and 6 of the stress paradigm on Cont + stress and STZ + stress animals at 0, 1, and 3 h after the beginning of the restraint stress session. Additional blood samples were withdrawn from nonstressed rats to estimate Cort levels of control and diabetic rats in resting conditions.
Electron Microscopy.
The day after the last restraint session for control and experimentally diabetic rats or on day 22 for rats that received oral Cort, animals were anesthetized with Metofane (Pitman–Moore, Mundelein, IL) and transcardially perfused with 100 ml of saline followed by 200 ml of a fixative containing 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (PB, pH = 7.4). Adrenal glands, thymus, and spleen were removed, cleaned, and weighed. Two hours after perfusion, brains were removed from the skulls and stored overnight in the fixative solution at 4°C. Coronal sections (100 μm thick) through the dorsal hippocampus were cut on a Vibratome and washed in PB. Some of the sections were processed for Golgi impregnation (see below). For electron microscopy, the sections were postfixed in 2% osmium tetroxide in PB for 2 h and partially dehydrated through an ascending series of ethanol concentrations (50 and 70%). Sections were stained with 2% uranyl acetate in 70% ethanol and dehydrated further with 95 and 100% ethanol. Ethanol was then replaced with propylen oxide, and sections were flat embedded in Durcupan (Fluka). The MFT zone (stratum lucidum) was trimmed, and ultrathin silver sections were cut on a Reichert ultramicrotome and mounted on single-slot copper grids coated with formvar film. Sections were counterstained with Reynolds' lead citrate, and the final preparations were examined and photographed with a Jeol 100 CX electron microscope. Photographs (10 per subject) were taken randomly from the stratum lucidum area at a primary magnification of ×5,400. Prints (×13,500) were used to trace MFT as well as mitochondria (Mit) and spine profiles. An average of 60 MFT per block per experimental animal were analyzed.
Data Analysis.
The total MFT area as well as the areas occupied by Mit and spine profiles were measured with the aid of a Zeiss Interactive Digitizing System. Synaptic vesicle density was estimated by using the intersection method, positioning an unbiased counting frame with squares of known area (0.2 cm2) at the coordinates of a quadratic lattice superimposed on the micrographs containing the MFT. The number of vesicles per MFT unit area was averaged from counts obtained from five frames, and counts were adjusted to consider section thickness and lost caps by using a variation of Floderus's equation (27, 28)
Each variable was averaged across MFT to obtain a single mean value per animal, and a two-tailed unpaired Student's t test was applied to assess statistical significance.
Golgi Staining Procedure.
Sections were processed according to a modified version of the “single”-section Golgi impregnation procedure (29). Briefly, brain sections were incubated in 3% potassium dichromate in distilled water overnight, then rinsed in distilled water and mounted onto plain slides, and a coverslip was glued over the sections at the four corners. These slide assemblies were incubated in 1.5% silver nitrate in distilled water overnight in the dark. The next day, the slide assemblies were dismantled, and tissue sections were rinsed in distilled water and then dehydrated in 95% ethanol followed by absolute ethanol. The sections were then cleared in clearing solvent (Stephens Scientific, Riverdale, NJ) mounted onto gelatinized slides and coverslipped under Permount (Fisher Scientific).
Data Analysis.
Slides were coded before quantitative analysis; the code was not broken until the analysis was complete. To be selected for analysis, Golgi-impregnated neurons had to possess the following characteristics: (i) location in the CA3 subregion of the dorsal hippocampus; (ii) dark and consistent impregnation throughout the extent of all of the dendrites; (iii) relative isolation from neighboring impregnated cells that could interfere with analysis; and (iv) a cell body in the middle third of the tissue section to avoid analysis of impregnated neurons, which extended largely into other sections. For each brain, six to eight pyramidal cells from CA3c were selected. Because this hippocampal subregion contains different subtypes of pyramidal neurons with different degrees of apical branching patterns, special care was taken in including the same number of neuronal subtypes across experimental animals and groups within each experiment. Each selected neuron was traced at ×400 by using a light microscope with a camera lucida drawing tube attachment, and the number of dendritic branch (bifurcation) points within a 100-μm thick section of each dendritic tree was determined for each selected neuron. The length of the dendrites present in a 100-μm-thick section was determined for each dendritic tree by using a Zeiss Interactive Digitizing Analysis System. Means were determined for both branch points and dendritic length for each brain, and the resulting values were subjected to a one-way ANOVA followed by Tukey post hoc comparisons. A probability level of P < 0.05 was used as the criterion for statistical significance.
Results
Verification of STZ Induction of Experimental Diabetes.
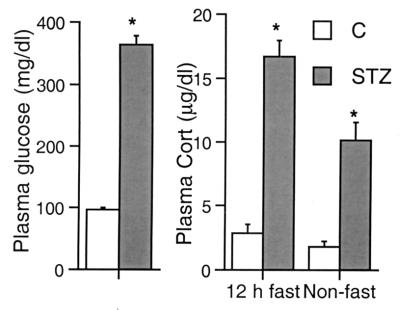
Two days after STZ administration, rats were severely diabetic, as indicated by their plasma glucose levels, which, after an overnight fast, were greatly elevated over those of controls (Fig. 1). STZ-injected rats also exhibited clear signs of overt diabetes, namely polidypsia, polyuria, and hyperphagia. In addition, diabetic rats were characteristically hypoactive and lethargic.
Figure 1.
Fasting plasma glucose (Left) and fasting and nonfasting plasma Cort (Right) levels in control and diabetic rats. Two days after the administration of STZ, rats showed a marked hyperglycemia and significantly elevated levels of plasma Cort. *, P < 0.001, compared with controls, unpaired two-tailed Student's t test. Bars represent means + SEM.
Experimental Diabetes and Plasma Cort Levels.
Under both fasting and nonfasting conditions, diabetic rats exhibited significantly increased plasma Cort levels in the morning compared with controls (Fig. 1). Fig. 2 depicts plasma Cort levels at 0, 1 and 3 h after collecting a blood sample by cutting the tip of the tails. The similar low plasma Cort levels observed over time in control rats indicates that the rapid procedure, taking less than 30 sec for blood sampling, was not perceived as more than acutely stressful by control animals because it did not trigger a lasting increase in plasma Cort. By using the same repeated sampling procedure, diabetic rats showed an initially elevated basal plasma Cort and a sustained elevation that was evident at both 1 and 3 h (Fig. 2).
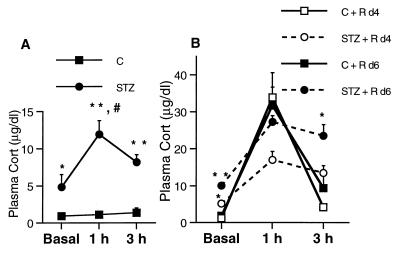
Figure 2.
Plasma Cort levels in control and STZ diabetic animals in resting conditions. Blood samples were collected through tail clipping in less than 30 sec. (A) Control rats showed similar Cort plasma levels at basal, 1-, and 3-h time points. Diabetic rats had increased basal levels of Cort and mounted a stress response that was not shut off by 3 h. #, P < 0.0001 compared with STZ group at the same time point. (B) Plasma Cort time course over 7 d of daily restraint stress of control and STZ diabetic rats. *, P < 0.05 and **, P < 0.001, compared with control group at the same time points. Two-way ANOVA, Tukey post hoc test. Bars represent means + SEM.
Effect of Repeated Stress on Plasma Cort Levels of Control and Diabetic Rats.
The elevated Cort levels observed during repeated sampling in diabetic rats were also seen under conditions of repeated restraint stress. Control rats undergoing restraint showed a similar low basal Cort level immediately before the beginning of the restraint stress sessions on days 4 and 6 (Fig. 2). In contrast, diabetic animals showed a significant increase in basal plasma Cort levels on day 4 that was significantly increased on day 6 [significant main effect of treatment and day and significant interaction between the two factors, F(1,23) = 5.112, P = 0,034, two-way ANOVA]. On days 4 and 6, control animals mounted the expected stress response with the peak in Cort secretion detected after 1 h, declining again after 3 h, thus indicating a normal shutoff of the stress response (Fig. 2), as reported previously (14). In contrast, on day 4, diabetic rats subjected to stress showed increased basal Cort levels and a flattened response to stress that did not shut off after 3 h. On day 6, diabetic rats revealed a sensitization to the stress with exacerbated plasma Cort levels at all of the time points assayed and an inefficient shutoff (Fig. 2, significant main effect of treatment and day of sampling at a 3-h time point, F(1,23) = 20.852; P < 0.001 and F(1,23) = 8.867; P = 0.07, respectively, two-way ANOVA.
Effects of Diabetes and Stress on Body and Organ Weights.
At the end of restraint stress, all restrained animals exhibited a significant reduction in body weight gain (Table 1). Diabetic animals subjected to restraint stress showed body weight loss at the end of the stress regimen. Furthermore, they showed a significant adrenal hypertrophy as well as spleen and thymus atrophy that were potentiated by 1 wk of daily restraint stress. Stress had little effect on organ weights in nondiabetic control animals.
Table 1.
Effect of STZ, 1 wk of daily restraint stress, and 3 wks of oral Cort treatment on rat final body weights (BW) (% of initial BW) and adrenal, spleen, and thymus weights (mg/100 g BW)
| Treatment | Adrenals | Spleen | Thymus | % Initial BW |
|---|---|---|---|---|
| Cont | 16.75 ± 1.16 | 245.31 ± 10.51 | 175.78 ± 17.95 | 132.99 ± 2.12 |
| Cont + R | 15.23 ± 1.50 | 249.63 ± 15.30 | 180.25 ± 16.70 | 104.23 ± 0.06‡ |
| STZ | 21.53 ± 1.45* | 161.80 ± 15.47† | 39.75 ± 13.22‡ | 98.51 ± 3.18‡ |
| STZ + R | 27.36 ± 0.95‡ | 110.79 ± 11.96‡ | 26.23 ± 5.24‡ | 92.73 ± 1.54‡§ |
| Cort, 400 μg/ml | 5.6 ± 0.30‡ | 116.50 ± 7.40‡ | 58.70 ± 7.60‡ | 117.44 ± 5.91¶ |
*,
, and
, P < 0.05, 0.01, and 0.001, respectively, compared with controls (Cont);
, P < 0.005, compared with restraint-stressed group (Cont + R). No significant changes were detected in organ weights from controls left undisturbed for 1 wk and controls receiving 2.4% ethanol for 3 wks (the percent initial BW for the latter group was 172.47 ± 3.62 g.
, P < 0.01, compared with the latter control group). One-way ANOVA Tukey post hoc test. BW, body weight; R, restraint stress.
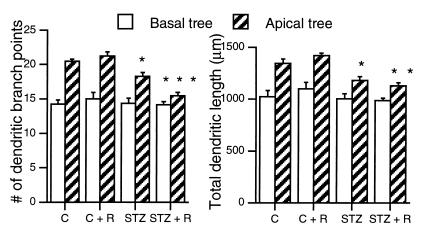
Diabetes Accelerates Stress Effects on Hippocampal CA3 Dendritic Morphology.
The analysis of Golgi-impregnated hippocampal sections of diabetic rats revealed a reduced complexity of the apical dendritic arborizations of CA3 principal neurons, as indicated by the decreased number of branch points and the reduction in total dendritic length (Fig. 3). Seven days of superimposed repeated stress potentiated the dendritic atrophy in diabetic animals. By comparison, control rats failed to show any dendritic reorganization after 7 d of repeated restraint stress. The length and number of branch points of the basal dendritic arborizations were unaffected by the stress paradigm, diabetes, or the combination of both treatments.
Figure 3.
Effect of 7 d of daily restraint stress on the number of dendritic branch points and total dendritic length of CA3 pyramidal neurons from control and STZ rats. *, **, and *** P < 0.05, 0.01, and 0.001, respectively, compared with controls. One-way ANOVA, Tukey post hoc test. Bars represent means + SEM.
Diabetes Causes a Distinctive Reorganization of MFTs That Is Further Altered by Superimposed Short-Term Stress.
Table 2 shows the quantitative morphology of the MFTs and their postsynaptic elements. Diabetes, stress, or the combination of the two altered neither the average total cross-sectional area of MFT nor the number or area occupied by spine profiles per MFT. Similarly, neither treatment altered the number of Mit profiles per MFT. However, the total area occupied by Mit profiles was increased in STZ diabetic nonstressed rats (Table 2).
Table 2.
Quantification of ultrastructural variables of hippocampal MFT in control, restraint-stressed, diabetic-stressed, and Cort-treated rats
| Morphological parameter | Control | Control + R | STZ | STZ + R | Cort, 400 μg/ml |
|---|---|---|---|---|---|
| Total MFT profile area, μm2 | 5.12 ± 0.24 | 5.40 ± 0.30 | 5.76 ± 0.34 | 5.64 ± 0.41 | 7.24 ± 0.23* |
| Mit profile area/MFT profile, μm2 | 0.28 ± 0.01 | 0.26 ± 0.04 | 0.40 ± 0.04*† | 0.28 ± 0.03 | 0.28 ± 0.04 |
| % decrease synaptic vesicle | — | 2.64 ± 0.18 | 21.05 ± 1.80‡ | 42.99 ± 2.99§ | 39.20 ± 0.31§ (85% of MFT) |
| density | 2.80 ± 0.20 (15% of MFT) |
* and
, P < 0.05 compared with control, control + restraint groups, and STZ + restraint group;
and
, P < 0.05 and 0.01, compared with control and control + restraint groups. One-way ANOVA, Tukey post hoc test. No significant differences between controls and controls receiving 2.4% ethanol (see Materials and Methods) were detected. No significant differences on number or area of spine profiles or number of mitochondria/MFT profile were detected among experimental treatment groups (data not shown). R, restraint stress.
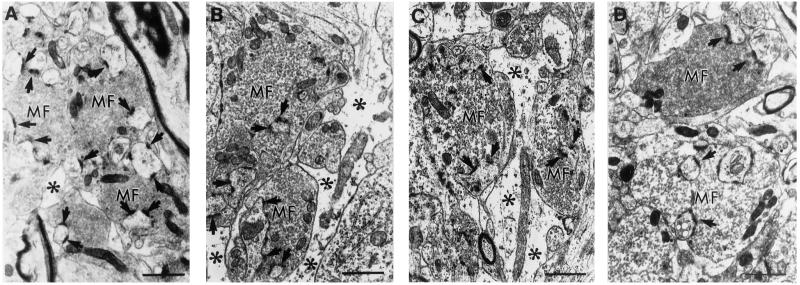
We observed a redistribution of the synaptic vesicle apparatus (depletion and dispersion) in MFT of diabetic rats that was increased by 7 d of restraint stress. Fig. 4 shows the increased dispersion of clear small synaptic vesicles in MFT of STZ and STZ + stress-treated rats compared with controls. The estimation of synaptic vesicle density within the terminals revealed a significant decrease in STZ rats that was potentiated by restraint stress (Table 2). Restraint stress, however, counteracted the increased area occupied by Mit in MFT of STZ rats.
Figure 4.
Ultrastructure of MFT in the stratum lucidum of the dorsal hippocampal CA3 region of control, STZ diabetic, STZ diabetic + stress, and Cort-treated (400 μg/ml) animals. (A) Control MFT (mf) are packed with clear small synaptic vesicles and make asymmetric synaptic contacts (arrows) with the invaginated giant spine profiles from CA3 proximal apical dendrites. (B) MFT of experimentally diabetic animals show a depletion and dispersion of clear small synaptic vesicles (notice the larger intervesicular space). (C) MFT of diabetic animals subjected to 7 d of repeated restraint stress show a potentiation of the depletion and dispersion of synaptic vesicles. Notice the widened glial processes (*) surrounding the MFT in B and C compared with A. (D) MFT of Cort-treated rats for 21 d show depletion and dispersion of synaptic vesicles similar to C. Notice the MFT (Upper) showing a normal distribution of vesicles. (Bars = 1 μm).
The stratum lucidum of diabetic rats showed proliferation of astrocytes with enlarged processes (Fig. 4), and the superimposition of restraint stress accentuated the astroglyosis. Two stressed diabetic animals (the more severely diabetic) showed sporadic degenerating MFT (data not shown).
Exogenous Administration of Cort Mimics the Vesicle Synaptic Dispersion Observed in Stressed Diabetic Rats.
Oral administration of Cort in the drinking water for 21 d caused a significant increase in the cross-sectional area of MFT compared with controls (Table 2). No changes were observed in the area and number of spine or Mit profiles after Cort treatment. However, MFT displayed a dispersion of clear synaptic vesicles with a significant decrease in vesicle density that was very similar to that observed in MFT of diabetic animals subjected to stress.
The dispersion of synaptic vesicles caused by oral Cort administration did not affect equally all MFT studied. In fact, from a total of 360 mossy fiber boutons analyzed, 85% showed the ultrastructural features described above, and the remaining 15% showed a structure very similar to controls and did not appear to be affected by Cort treatment (Table 2 and Fig. 4). This, together with the increased MFT area and lack of increase in area occupied by Mit profiles, distinguished Cort-treated rats from STZ-diabetic ones.
Discussion
We report that uncontrolled experimental diabetes in rats, a model of endogenous allostatic load (see Introduction), causes some of the same morphological changes induced in nondiabetic animals by 3 wk of daily restraint stress or daily Cort administration, namely retraction and simplification of apical dendrites of CA3 pyramidal neurons and depletion of synaptic vesicles of MFT (refs. 28 and 30 and this report). Moreover, the apical dendritic atrophy is potentiated further in diabetic animals by 1 wk of repeated stress, a paradigm that does not affect hippocampal morphology in nondiabetic animals. These morphological changes occurred in concert with an increased reactivity of the HPA axis of STZ rats to superimposed stress that was characterized by hypersensitivity and defective shutoff of the stress response. It is possible that the temporary food deprivation caused by the restraint sessions could have contributed further to the elevated plasma Cort levels in the hyperphagic diabetic rats, although the sessions took place during the nonactive period, when both control and diabetic rats eat less.
We have also found an intriguing dissociation in the effects of experimental diabetes, chronic stress, and chronic Cort treatment on the ultrastructure of MFT. Although all three treatments caused a depletion of clear presynaptic vesicles, STZ-induced diabetes induced a dispersion of vesicles in all MFT that was potentiated by superimposed stress; this contrasts with nondiabetic rats, in which chronic stress caused a clustering of synaptic vesicles in all MFT (28). Moreover, chronic Cort treatment caused a dispersion of synaptic vesicles like that seen in STZ diabetes, but 15% of MFT were totally unaffected by Cort treatment. These differences, together with other unique effects of STZ diabetes and Cort treatment on MFT morphology, indicate that Cort elevation does not alone explain effects of the three treatments on hippocampal structure.
STZ-Treated Rats as a Model of Endogenous Allostatic Load.
In agreement with published reports, diabetic animals showed a sustained stimulation of the HPA axis, with elevated basal plasma Cort levels, adrenal hypertrophy, and spleen and thymus atrophy (25, 31–33). The hyperresponsiveness to superimposed stress is consistent with another report of facilitation of acute stress responses in experimental diabetes (25). Our observation that diabetic rats show a poor shutoff of the stress response suggests insensitivity to feedback mechanisms and is consistent with the reported resistance to the dexamethasone suppression test (25) and down-regulation of GC receptor number in the hippocampus of STZ rats (34).
Experimental diabetes is associated with GC hypersecretion (31, 32), which, in turn, inhibits cellular glucose uptake in the periphery and also in neurons and astrocytes of brain regions such as the hippocampus (1, 6). Interestingly, the exogenous administration of Cort to normoglycemic animals mimics the hippocampal dendritic atrophy observed in chronically stressed rats (30) as well as STZ rats (this report). However, both exogenous Cort administration and experimental diabetes induce a different type of MFT synaptic depletion from that observed after chronic restraint stress, namely dispersion instead of clustering of synaptic vesicles. We cannot explain at this time why a subpopulation of MFT (15%) is resistant to Cort treatment.
The decrease in synaptic vesicle density observed in the majority of MFT after Cort treatment is similar to the extent of vesicle depletion in diabetic rats after 7 d of superimposed stress. This suggests that factors common to STZ diabetes and Cort treatment, such as hyperglycemia, may participate in preventing vesicle clustering after stress in MFT, such as occurred in nondiabetic animals after 21 d of stress (28). We have found that stress-induced synaptic vesicle clustering is partially prevented by enhancing the reuptake of serotonin (unpublished results); thus other factors besides Cort may regulate the stress-induced MFT vesicle rearrangement in normoglycemic rats. Indeed, chronic stress increases serotonergic activity in the hippocampus (35, 36), whereas long-term exposure to Cort attenuates serotonin responses in rat hippocampal neurons (37).
If the dispersion of MFT synaptic vesicles is the result of vesicle detachment from the actin cytoskeleton, more vesicles would be available to fuse to the presynaptic membrane and release glutamate. Previous studies revealed that 7 d of Cort treatment increased Tau immunoreactivity and spectrin proteolysis induced by kainic acid injections in the CA3 region (38). The resulting cytoskeletal disruption, regulated by changes in calcium homeostasis (39), could also affect the endocytic recycling of synaptic vesicles (40). Alternatively, an increase in the phosphorylation of vesicle-associated proteins like synapsin I is another possible mechanism, because the phosphorylation of synapsin I is positively correlated with neurotransmitter release from the nerve terminal (41, 42). The fact that GCs induce synapsin I in the hippocampus (43) makes this pathway attractive for further investigation.
In STZ rats, the depletion and dispersion of MFT synaptic vesicles and the Mit hypertrophy in MFT suggest that there may be increases in cellular energy demands (44, 45). When stress was superimposed on diabetic rats, there were signs of neuronal damage such as enlarged glial processes and sporadic terminal and dendritic necrosis. Mit hypertrophy was no longer evident, suggesting that stress may override such a compensatory mechanism and increase further the vulnerability of the energetically compromised CA3 hippocampal neurons. Further studies are necessary to determine whether the astrocytic proliferation is indicative of reactive astrocytosis.
The CA3 region, unique for the presence of recurrent excitatory connections and input from the excitatory mossy fibers, plays a crucial role in the processing of information, specifically the formation of neuronal representations (12). Consequently, changes in hippocampal connectivity resulting from dendritic and synaptic rearrangements induced by diabetes, stress, or the combination of both, could affect the process of perception and adaptation to potential stressors. A link between metabolic disorders and increased susceptibility to stress has been described (20, 46, 47). In particular, insulin-dependent diabetic patients show disruptions in HPA axis responsiveness (32, 48), and a strong correlation among perceived stress, immunological status, and glycemic control has been described (49). In addition, diabetic patients show a higher incidence of affective, depressive, and anxiety disorders (50, 51)
Experimental Diabetes, Hippocampal Vulnerability, and Glucose Homeostasis.
In STZ rats, both hippocampal-mediated spatial learning and synaptic plasticity are affected. For example, diabetic rats show disruptions of open field activity and enhanced retention for passive avoidance training (52, 53). Furthermore, diabetic rodents show impaired water-maze learning associated with decreased expression of long-term potentiation in CA1 hippocampal neurons (54), and the CA1 subregion of hyperglycemic rats is hypersensitive to ischemic insults (55).
Additional evidence of hippocampal vulnerability in diabetes comes from electrophysiological studies on hippocampal slices from STZ rats that reveal an increase in epileptiform activity in the CA3 region (56). Although both hippocampal dendritic and synaptic remodeling is largely reversible in normoglycemic rats after discontinuation of the repeated restraint paradigm (16), this does not seem to be the case for uncontrolled hyperglycemic rats. Preliminary results suggest that STZ diabetes for 21 d causes hippocampal neuronal necrosis, mainly in CA2 and CA3 subregions, as well as sporadic MFT necrosis. Moreover, insulin replacement can prevent the neuronal necrosis observed in the hippocampus (A.M.M., unpublished work). We predict that prolongation of superimposed stress would induce further damage to the already metabolically compromised hippocampus of diabetic rats.
STZ diabetes is associated with deficits in glucose transport into the brain (57–60) and compensatory increases in both the mRNA and protein levels of the neuron-specific glucose transporter GLUT 3 in the hippocampus (61). The decreased utilization of glucose could result in a decline of energy stores and in the disruption of the normal stoichiometry between glucose metabolism and glutamatergic activity (62). The reported decrease in Na,K-ATPase activity, most pronounced in cerebral cortex and hippocampus of diabetic rats (63), provides a mechanism for failure in the energy-dependent glial reuptake of glutamate.
In conclusion, STZ diabetes creates a form of allostatic load (5) involving poor glucose homeostasis, chronically elevated GC levels, increased HPA reactivity, and metabolic imbalance that produces a reorganization of pre- and postsynaptic structures in the hippocampal formation of the brain, which are further potentiated by the superimposition of 7 d of stress. Whereas diabetes and 21-d chronic stress or Cort treatment of nondiabetic animals all produce dendritic remodeling, MFT morphology responds in a unique way to each treatment over and above the common feature of synaptic vesicle depletion. Thus Cort alone does not account for all of the changes in hippocampal structure seen after the three treatments. Further studies are needed to address the factors involved in the increased vulnerability of the hippocampus to diabetes and stress and whether this vulnerability gives rise to permanent damage.
Acknowledgments
This work was supported by National Institutes of Health Grant MH 41256 and by The Health Foundation.
Abbreviations
- Cort
corticosterone
- HPA
hypothalamo–pituitary–adrenal
- Mit
mitochondria
- MFT
mossy fiber terminal
- GC
glucocorticoid
- STZ
streptozotocin
References
- 1.Munck A, Guyre P, Holbrook N. Endocr Rev. 1984;5:25–49. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 2.Keller-Wood M E, Dallman M F. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Sterling P, Eyer J. In: Handbook of Life Stress, Cognition and Health. Fisher S, Reason J, editors. New York: Wiley; 1988. pp. 629–649. [Google Scholar]
- 4.McEwen B S, Stellar E. Arch Int Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 5.McEwen B S. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky R M. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 7.Bremner D J, Randall P, Scott T M, Bronen R A, Seibyl J P, Southwick S M, Delaney R C, McCarthy G, Charney D S, Innis R B. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurvits T V, Shenton M E, Hokama H, Ohta H, Lasko N B, Gilbertson M W, Orr S P, Kikinis R, Jolesz F A, McCarley R W, Pitman R K. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheline Y I, Wang P W, Gado M H, Csernansky J C, Vannier M W. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starkman M N, Gebarsli S S, Berent S, Schteingart D E. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 11.Lupien S J, de Leon M, de Santi S, Convit A, Tarshish C, Nair N P, Thakur M, McEwen B S, Hauger R L, Meaney M J. Nat Neurosci. 1998;1:3–4. [Google Scholar]
- 12.Eichenbaum H. Science. 1997;277:330–332. doi: 10.1126/science.277.5324.330. [DOI] [PubMed] [Google Scholar]
- 13.McEwen B S. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 14.Magariños A M, McEwen B S. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 15.Magariños A M, McEwen B S, Flügge G, Fuchs E. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conrad C D, Magariños A M, LeDoux J E, McEwen B S. Behav Neurosci. 1999;113:1–12. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 17.Magariños A M, Deslandes A, McEwen B S. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 18.Ohl F, Fuchs E. Cognit Brain Res. 1999;7:379–387. doi: 10.1016/s0926-6410(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 19.Popov V I, Bocharova L S. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- 20.Bjorntorp P. Acta Physiol Scand. 1997;640:144–148. [PubMed] [Google Scholar]
- 21.Biessels G J, Kappelle A C, Bravenboer B, Erkelens D W, Gispen W H. Diabetologia. 1994;37:643–650. doi: 10.1007/BF00417687. [DOI] [PubMed] [Google Scholar]
- 22.Junod A, Lambert A E, Stauffacher W, Renold A E. J Clin Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rerup C. Pharmacol Rev. 1970;22:485–518. [PubMed] [Google Scholar]
- 24.Mordes J P, Rossini A A. Am J Med. 1981;70:353–360. doi: 10.1016/0002-9343(81)90772-5. [DOI] [PubMed] [Google Scholar]
- 25.Scribner K A, Walker C D, Cascio C S, Dallman M F. Endocrinology. 1991;129:99–108. doi: 10.1210/endo-129-1-99. [DOI] [PubMed] [Google Scholar]
- 26.Scribner K,A, Akana S F, Walker C D, Dallman M F. Endocrinology. 1993;133:2667–2674. doi: 10.1210/endo.133.6.8243290. [DOI] [PubMed] [Google Scholar]
- 27.Pierce J P, Mendell L M. J Neurosci. 1993;13:4748–4763. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magariños A M, García Verdugo J, McEwen B S. Proc Natl Acad Sci USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabbott P L, Somogy J. J Neurosci Methods. 1984;11:221–230. doi: 10.1016/0165-0270(84)90084-0. [DOI] [PubMed] [Google Scholar]
- 30.Magariños A M, Orchinick M, McEwen B S. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- 31.Lebinger T G, Saenger P, Fukushima D K, Kream J, Wu R, Finkelstein J W. Diabetes Care. 1983;6:506–509. doi: 10.2337/diacare.6.5.506. [DOI] [PubMed] [Google Scholar]
- 32.Oster M H, Castonguay T M, Keen C L, Stern J S. Life Sci. 1988;43:1643–1645. doi: 10.1016/0024-3205(88)90536-x. [DOI] [PubMed] [Google Scholar]
- 33.Bellush L L, Reid S G, North D. Physiol Behav. 1991;50:973–981. doi: 10.1016/0031-9384(91)90424-m. [DOI] [PubMed] [Google Scholar]
- 34.De Nicola A F, Magariños A M, Foglia V G. In: Diabetes. Rifkin H, Colwell J A, Taylor S I, editors. Amsterdam: Elsevier; 1991. pp. 3–7. [Google Scholar]
- 35.Kennet G A, Dickinson S L, Curzon G. Brain Res. 1985;330:253–263. doi: 10.1016/0006-8993(85)90684-5. [DOI] [PubMed] [Google Scholar]
- 36.Ohi K, Mikuni M, Takahashi K. Pharmacol Biochem Behav. 1989;34:603–608. doi: 10.1016/0091-3057(89)90566-2. [DOI] [PubMed] [Google Scholar]
- 37.Karten Y J G, Nair S M, van Essen L, Sibug R, Joëls M. Proc Acad Natl Sci USA. 1999;96:13456–13461. doi: 10.1073/pnas.96.23.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliot E M, Mattson M P, Vankerklish P, Lynch G, Chang I, Sapolsky R M. J Neurochem. 1993;61:57–67. doi: 10.1111/j.1471-4159.1993.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhargava A, Meijer O C, Dallman M F, Pearce D. J Neurosci. 2000;20:3129–3138. doi: 10.1523/JNEUROSCI.20-09-03129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renger J J, Ueda A, Atwood H L, Govind C K, Wu C-F. J Neurosci. 2000;20:3980–3992. doi: 10.1523/JNEUROSCI.20-11-03980.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greengard P, Valtorta F, Czernik A J, Benfenati F. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 42.Pieribone V A, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernic A J, Greengard P. Nature (London) 1996;375:493–496. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 43.Nestler E, Rainbow T, McEwen B S, Greengard P. Science. 1981;212:1162–1164. doi: 10.1126/science.6785886. [DOI] [PubMed] [Google Scholar]
- 44.Lnenicka G A, Atwood H L, Marin L. J Neurosci. 1986;6:2252–2258. doi: 10.1523/JNEUROSCI.06-08-02252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith R, Ord M J. Int Rev Cytol. 1983;83:63–164. doi: 10.1016/s0074-7696(08)61686-1. [DOI] [PubMed] [Google Scholar]
- 46.Natelson B H. Neurosci Biobehav Rev. 1983;7:511–527. doi: 10.1016/0149-7634(83)90031-3. [DOI] [PubMed] [Google Scholar]
- 47.Rosmond R, Dallman M F, Bjorntorp P. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 48.Wurzburger M I, Prelevic G M, Sonksen P H, Peric L A, Till S, Morris L W. Clin Endocrinol. 1990;32:787–797. doi: 10.1111/j.1365-2265.1990.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 49.Linn M W, Linn B S, Skyler J S, Jensen J. Clin Immunol Immunopathol. 1983;27:223–233. doi: 10.1016/0090-1229(83)90072-7. [DOI] [PubMed] [Google Scholar]
- 50.Surridge D, Williams Erdahl D, Lawson J, Donald M, Monga T, Bird C, Letemendia F. Br J Psychiatry. 1984;145:269–276. doi: 10.1192/bjp.145.3.269. [DOI] [PubMed] [Google Scholar]
- 51.Popkin M K, Callies A L, Lentz R D, Colon E A, Sutherland D E. Arch Gen Psychiatry. 1988;45:64–68. doi: 10.1001/archpsyc.1988.01800250078010. [DOI] [PubMed] [Google Scholar]
- 52.Leedom L J, Meehan W P, Zeidler A. Physiol Behav. 1987;40:447–451. doi: 10.1016/0031-9384(87)90029-1. [DOI] [PubMed] [Google Scholar]
- 53.Bellush L L, Rowland N E. Behav Neurosci. 1989;103:144–150. doi: 10.1037//0735-7044.103.1.144. [DOI] [PubMed] [Google Scholar]
- 54.Biessels G J, Kamal A, Urban I J A, Spruijt B M, Erkelens D W, Gispen W H. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 55.Pulsinelli W A, Waldman S, Rawlinson D, Plum F. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- 56.Margineanu D G, Niespodziany I, Wülfert E. Neurosci Lett. 1998;252:183–186. doi: 10.1016/s0304-3940(98)00580-1. [DOI] [PubMed] [Google Scholar]
- 57.Gjedde A, Crone C. Science. 1981;214:456–457. doi: 10.1126/science.7027439. [DOI] [PubMed] [Google Scholar]
- 58.McCall A. Diabetes. 1992;41:557–570. doi: 10.2337/diab.41.5.557. [DOI] [PubMed] [Google Scholar]
- 59.Partridge W M, Castonguay T M, Keen Cl, Stern J S. Life Sci. 1988;43:1643–1645. doi: 10.1016/0024-3205(88)90536-x. [DOI] [PubMed] [Google Scholar]
- 60.Mooradian A D, Morin A M. Am J Med Sci. 1991;301:173–177. doi: 10.1097/00000441-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Reagan L P, Magariños A M, Lucas L R, Van Bueren A, McCall A, McEwen B S. Am J Physiol. 1999;276:E879–E886. doi: 10.1152/ajpendo.1999.276.5.E879. [DOI] [PubMed] [Google Scholar]
- 62.Sibsom N R, Dhankhar A, Mason G F, Rothman D L, Behar K L, Shulman R G. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leong S F, Leung T K C. Neurochem Res. 1991;16:1161–1165. doi: 10.1007/BF00966596. [DOI] [PubMed] [Google Scholar]