Abstract
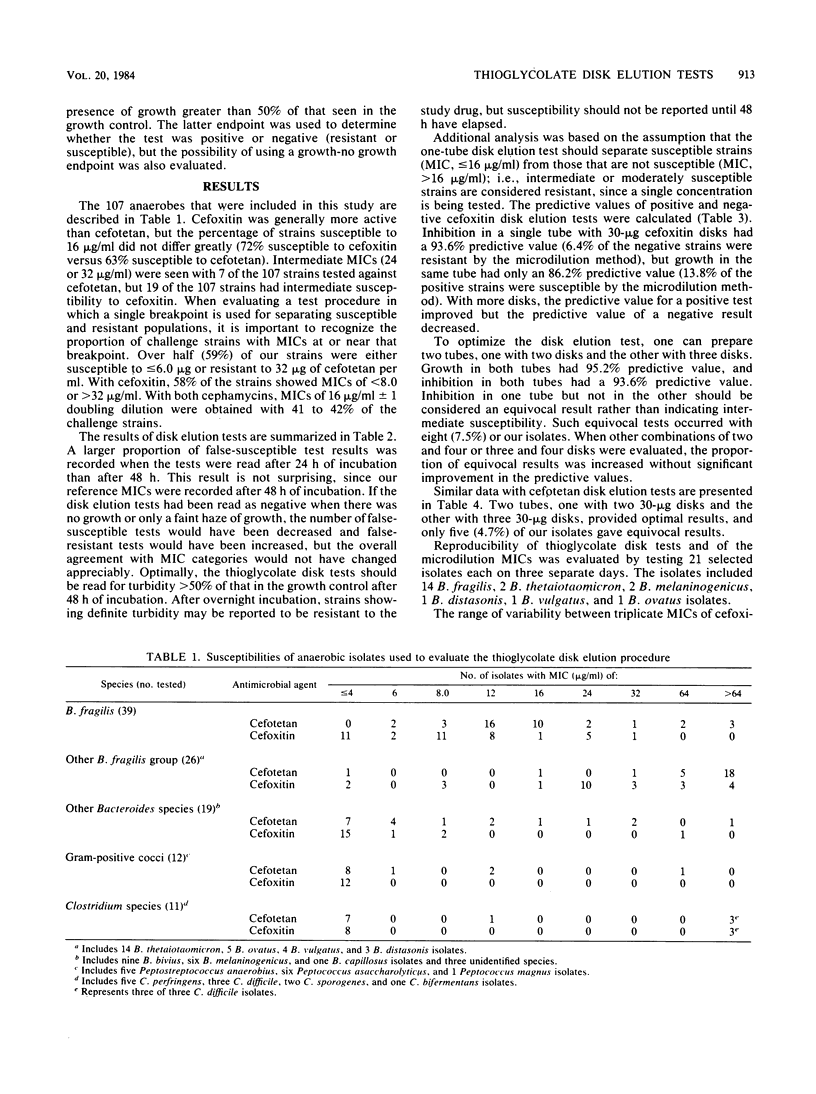
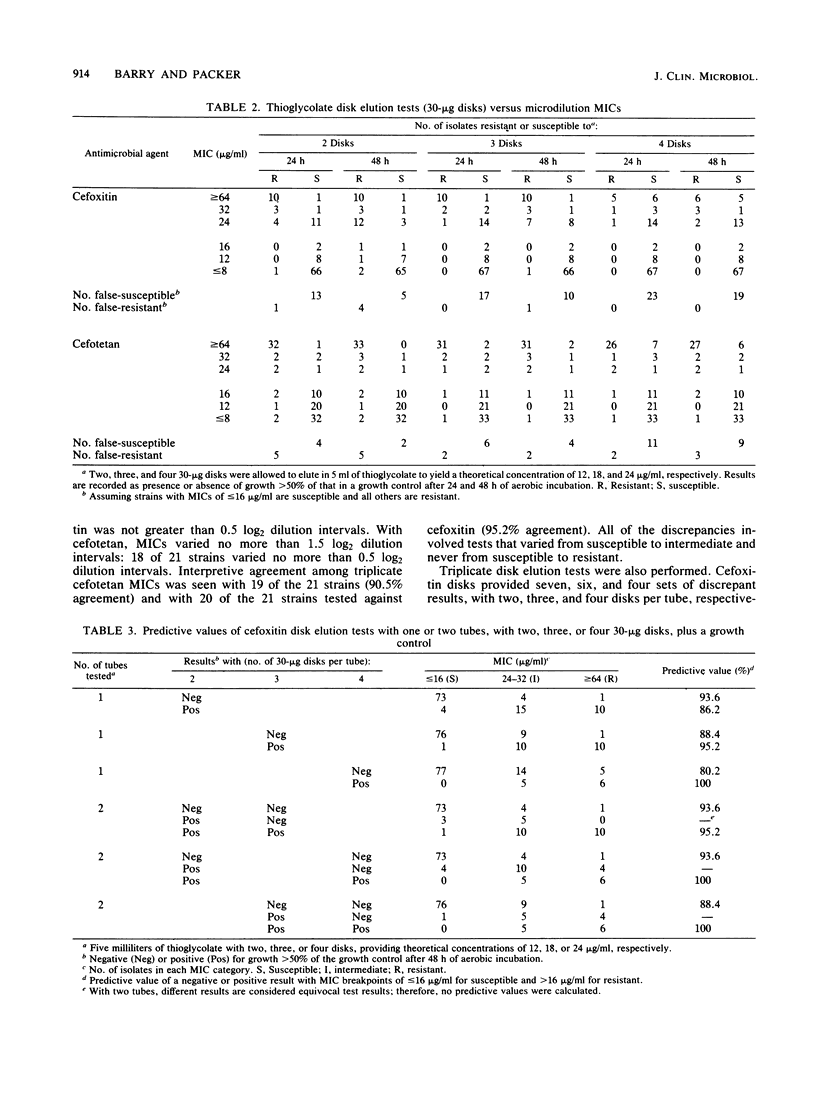
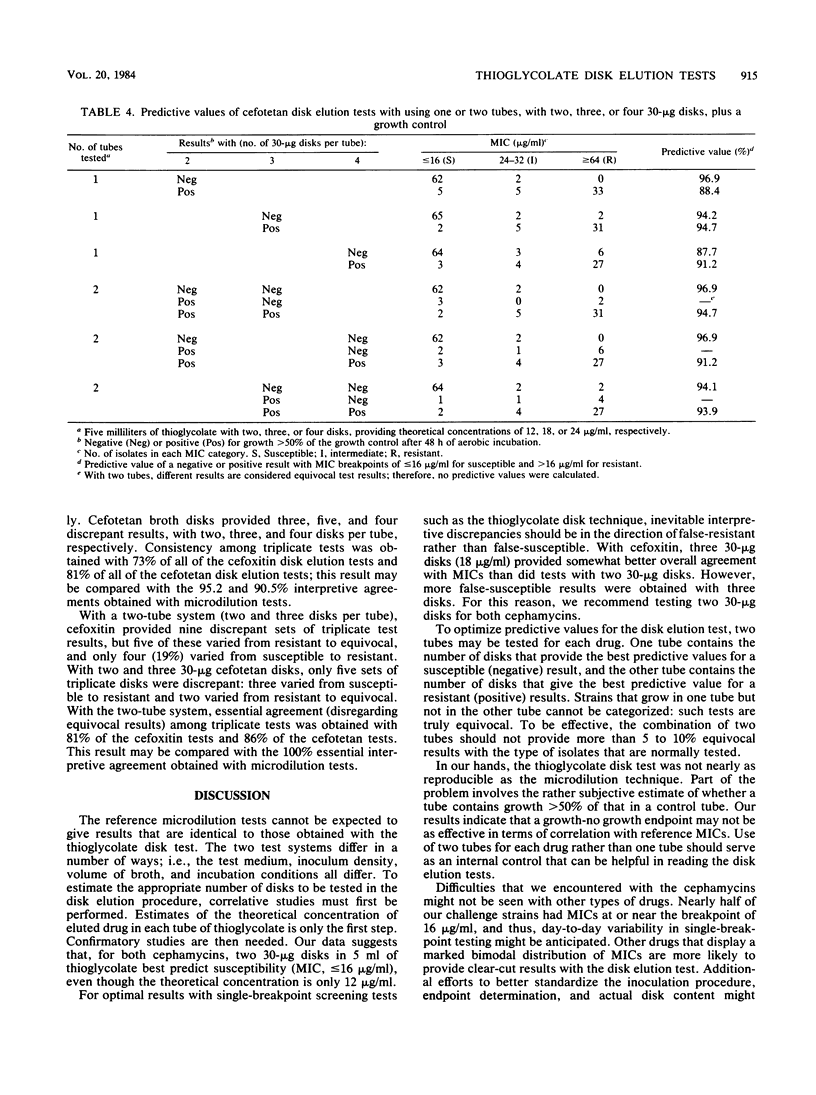
The in vitro activities of two cephamycins, cefotetan and cefoxitin, against 107 anaerobic bacteria were evaluated. The aerobically incubated thioglycolate disk elution technique of Kurzynski et al. (Antimicrob. Agents Chemother. 10:727-732, 1976) was also evaluated to establish the appropriate number of 30-micrograms disks to be added to each tube, assuming that strains with MICs of less than or equal to 16 micrograms/ml are susceptible. Optimal predictive values were obtained when two tubes were prepared for each antimicrobial agent, one with two 30-micrograms disks and the other with three 30-micrograms disks. After 48 h of incubation, resistant strains grew in both tubes and susceptible strains provided no growth or growth less than or equal to 50% of that in the control broth. Growth in one tube but not in the other was considered an equivocal test result indicating the need for additional tests; 5 to 7% of our strains gave equivocal results. Reproducibility studies confirmed that broth microdilution tests were more reproducible than disk elution susceptibility tests.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers L. W., Jones R. N., Barry A. L., Thornsberry C., Fuchs P. C., Gavan T. L., Gerlach E. H., Sommers H. M. Cefotetan, a new cephamycin: comparison of in vitro antimicrobial activity with other cephems, beta-lactamase stability, and preliminary recommendations for disk diffusion testing. Antimicrob Agents Chemother. 1982 Nov;22(5):859–877. doi: 10.1128/aac.22.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum J., Stapley E. O., Miller A. K., Wallick H., Hendlin D., Woodruff H. B. Cefoxitin, a semi-synthetic cephamycin: a microbiological overview. J Antimicrob Chemother. 1978 Jul;4(B):15–32. doi: 10.1093/jac/4.suppl_b.15. [DOI] [PubMed] [Google Scholar]
- Guibert J., Kitzis M. D., Yvelin C., Acar J. F. Pharmacokinetics of single intravenous and intramuscular doses of cefotetan in normal human volunteers. J Antimicrob Chemother. 1983 Jan;11 (Suppl):201–206. doi: 10.1093/jac/11.suppl_a.201. [DOI] [PubMed] [Google Scholar]
- Kurzynski T. A., Yrios J. W., Helstad A. G., Field C. R. Aerobically incubated thioglycolate broth disk method for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1976 Oct;10(4):727–732. doi: 10.1128/aac.10.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosdeen F., Maskell J., Philpott-Howard J., Williams J. D. Cefotetan activity against Gram-negative aerobes and anaerobes. J Antimicrob Chemother. 1983 Jan;11 (Suppl):59–65. doi: 10.1093/jac/11.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Hancox J. In vitro activity of cefotetan, a new cephamycin derivative, compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1982 Mar;21(3):486–491. doi: 10.1128/aac.21.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates R. A., Cockshott I. D., Houghton H. L., Wardleworth A., Adam H., Donnelly R. J. Pharmacokinetics and tolerance of single intramuscular doses of cefotetan in normal Caucasian volunteers. J Antimicrob Chemother. 1983 Jan;11 (Suppl):207–212. doi: 10.1093/jac/11.suppl_a.207. [DOI] [PubMed] [Google Scholar]


